Abstract
The oral and central nervous systems (CNS) present a unique set of barriers to the delivery of important diagnostic and therapeutic agents. Extensive research over the past few years has enabled a better understanding of these physical and biological barriers based on tight cellular junctions and expression of active transporters and metabolizing enzymes at the luminal surfaces of the gastrointestinal (GI) tract and the blood-brain barrier (BBB). This review focuses on the recent understanding of transport across the GI tract and BBB and the development of nanotechnology-based delivery strategies that can enhance bioavailability of drugs. Multifunctional lipid nanosystems, such as oil-in-water nanoemulsions, that integrate enhancement in permeability, tissue and cell targeting, imaging, and therapeutic functions are especially promising. Based on strategic choice of edible oils, surfactants and additional surface modifiers, and different types of payloads, rationale design of multifunctional nanoemulsions can serve as a safe and effective delivery vehicle across oral and CNS barriers.
Introduction
The oral route of drug administration for systemic therapy remains very popular due to ease of administration of various dosage forms, stability, uniformity of dosage, and patient compliance. However, the lack of oral options for many macromolecules (e.g., proteins, peptides and nucleic acid constructs) and some small molecular drugs owing to their poor stability in the gastrointestinal (GI) fluid (due to pH or enzymes) or their inability to cross the various physical and biological barriers results in limited clinical success (Goldberg & Gomez-Orellana Citation2003). For instance, paclitaxel, a potent anticancer agent approved for the treatment of breast and ovarian cancer, is not significantly absorbed after oral administration and the systemic bioavailability in humans is less than 6% (Malingre et al. Citation2001). The most likely reasons for paclitaxel poor oral bioavailability are poor aqueous solubility, the affinity to efflux transporters, and rapid metabolism by cytochrome P450 enzymes in the GI tract (Zhang & Benet Citation2001).
For CNS delivery, the blood-brain barrier (BBB) is a dynamic interface separating the brain from systemic circulation. The BBB prevents 98% of small molecule therapeutic agents and almost all proteins, peptides, and nucleic acid constructs (e.g., genes, oligonucleotides, and small interfering RNA) to reach their targets in the CNS (Pardridge Citation2002). The complex tight junctions between brain endothelial cells, the presence of pericytes and astrocytes, and the expression of a large number of active transporters at the luminal membrane of the brain capillary endothelial cells constitute the BBB (Wolburg & Lippoldt Citation2002). Several review articles, including ours, have discussed at length the BBB and its role in preventing transport of therapeutic molecules into the CNS (Tiwari & Amiji Citation2006b, Pardridge Citation2007, Barchet & Amiji Citation2009, Abbott et al. Citation2010, Jeffrey & Summerfield Citation2010).
To overcome oral and CNS barriers and deliver the payloads to their target sites at concentrations that can achieve therapeutic significance, various strategies have been explored. The use of nanotechnology-based drug delivery approaches that enhance drug solubility, improve permeability, prevent premature hydrolytic or enzymatic degradation, and overcome efflux transporters to facilitate availability at the tissue or cell of interest are especially important for realizing the potential of many different chemical entities with pharmacological significance.
Multifunctional nanosystems, as envisioned by Ferrari (Citation2005), Torchilin (Citation2006), and others (Kim Citation2007, Cho et al. Citation2008, Gindy & Prud'homme, Citation2009), combine the following main structural components: (1) active core material, (2) surface modification for passive or active targeting and intracellular delivery, and (3) variety of payloads that could be encapsulated for imaging, therapeutic, and reporter functions. The core material of the nanocarrier is made from organic or inorganic material with the possibility of using systems that respond to environmental stimuli or afford active interactions with the tissue and cell of interest. Surface modification with poly(ethylene glycol) (PEG) and other hydrophilic polymers imparts long circulation properties that allow the nanoparticles to passively target tumour and other tissues with leaky vasculature. Additionally, inclusion of peptides, antibodies, aptamers, and small molecule targeting ligands can facilitate delivery to tissue and cells of interest and cell penetrating peptides (e.g., HIV Tat-1) can enhance intracellular delivery and subcellular localization in close proximity to the therapeutic target of the drugs (Greish Citation2007, Koning & Krijger Citation2007, Jabr-Milane et al. Citation2008).
Nanoemulsions are oil-in-water formulations made with edible oils, surface-active agent(s), and water where the oil droplet size is reduced to nanometer length scale (Sarker Citation2005). The versatility of nanoemulsions is based on the different types of oils and surface modifiers that can be used. For instance, we have found that oils that are rich in omega-3 polyunsaturated fatty acids (PUFA) can play a very important role in overcoming biological barriers, including the BBB. Phospholipids and other surface modifiers are used to develop a stable nanoemulsion formulation that does not undergo creaming or phase separation. Lastly, hydrophobic payloads and imaging agents can be readily incorporated in the oil phase of the nanoemulsions (Tiwari et al. Citation2006, Vyas et al. Citation2008).
Barriers to oral drug delivery
Although the oral route of administration is one of the most popular methods of drug entry in the body for local and systemic effects, there are several issues that limit effective delivery and bioavailability (Lennernas & Abrahamsson Citation2005). Strategies that can improve drug solubility and stability, enhance permeability, and overcome biological obstacles such as efflux transporters and metabolizing enzymes would provide even greater opportunities for oral drug administration.
Drug dissolution and permeability
The oral drug absorption is the process of drug transport from GI tract to blood compartment. Oral absorption can be hindered by poor aqueous solubility and subsequently the slow dissolution rate in GI fluids. The extent of drug ionization, stability in the acidic environment of the stomach or in the presence of enzymes as well as presence of food and gastric emptying can also reduce pre-systemic bioavailability. The main factors that affect oral absorption, however, are lack of efficient permeability across intestinal epithelial cells due to poor diffusional properties of the drug and biological barriers, and finally first pass metabolism in the liver (Aungst, Citation1999).
Important mechanisms of drug transport in the intestinal epithelium are transcellular and paracellular transport () (Salama et al. Citation2006). In transcellular transport, the drug molecules travel through the cell and need to cross the cell membrane twice on their way in and out of the cell. The possible mechanisms of transcellular transport can be classified as: (1) passive diffusion, (2) facilitated diffusion, (3) active transport, and (4) transcytosis. Hydrophilic and charged molecules require specialized transport systems to facilitate cellular uptake whereas lipophilic drugs can diffuse freely across the plasma membrane. Transcytosis which consist of endocytosis and exocytosis is mainly for macromolecules (proteins and peptides) and small particles (bacteria and viruses). Recent studies suggest that the orally administered nanocarriers can traverse the GI tract membranes through the endoytosis process (Florence, Citation2005, Yang and Benita, Citation2000), and this can be a potential route for transport of macromolecules and for by-passing efflux transporters.
Figure 1. Multiple transport mechanisms for oral absorption of drugs (adapted from Florence, Citation2005).
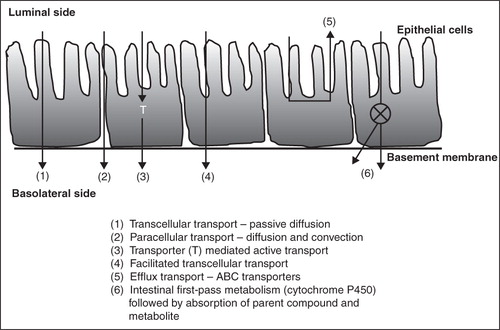
On the other hand, paracellular transport involves diffusion of hydrophilic drug molecules between the cells of epithelial or endothelial membrane. This passive diffusion process occurs due to concentration, electrochemical, or hydrostatic gradients. Molecules smaller in size than the cell junction can freely pass through paracellular transport irrespective of polarity, for example, ions and water. The joining of the paracellular cleft between cells of epithelial and endothelial forms the tight junctions (TJ). This tight junction between cells strictly limits the paracellular drug transport. Recent research has led to better understanding of the signaling pathways for the tight junction regulations and identification of the trans-membrane proteins (Salama et al. Citation2006). Structurally, the tight junctions are composed of integral membrane proteins, linker or adaptor proteins connecting them to the actin cytoskeleton and signaling molecules enabling the dynamic regulation of paracellular transport (Stevenson Citation1998). Controlled and reversible opening of the tight junctions to enhance drug delivery by safe and effective absorption enhancer or paracellular enhancers is the ultimate goal in pharmaceutical research. Using physical and chemical approaches, temporary disruptions of the tight junctions can facilitate drug absorption. However, there is also a safety issue due to lack of selectivity that may lead to enhance transport of toxic agents or pathogens (Hochman & Artursson Citation1994). Significant research is focused on understanding the nature of the tight junctions and the signaling molecules involved in maintaining the barrier function in order to find effective strategies to facilitate oral drug permeability and bioavailability.
Efflux drug transporters
The ATP-binding cassette (ABC) family of efflux transporters are a large family of structurally diverse integral membrane proteins expressed at various tissue sites within the body (Jones & George Citation2004). About 49 human genes coding for ABC transporters have been identified and they are further categorized into subfamilies A through G (Dean & Annilo Citation2005). These transporters use ATP as a source of energy and transport molecules across the lipid cell membranes (Loscher & Potschka Citation2005a). Among them, P-glycoprotein (P-gp) is highly expressed in columnar epithelial cells (enterocytes) of the small intestine (), the primary site of absorption for the majority of orally-administered drugs (Zhang & Benet Citation2001). All these transporter proteins show a broad overlap in substrate and inhibitor specificities and act as a concerted barrier to drug absorption (Zhang & Benet Citation2001). The expression level is influenced by the age of the individual, the location in the GI tract and, in certain segments of the population, may be affected by polymorphic transformations (Zhang & Benet Citation2001).
Among the ABC transporters, P-gp, the encoded product of the human multidrug resistance gene (ABCB1/MDR1) family is the most widely studied transporter (Gottesman et al. Citation2002). P-gp is expressed in a broad spectrum of tissues including the GI tract. Structurally diverse compounds act as substrates for P-gp, it is suggested that the two or three electron donor groups separated by a fixed space is a representative common structural feature of P-gp substrates (Seelig Citation1998). Another commonly expressed efflux transporter in the GI tract is multi-drug resistant protein (MRP). The substrate specificity of MRP and P-gp are distinct even though they overlap each other (Seelig Citation1998). Substrates and inhibitors of MRP are anionic, whereas most of the P-gp substrates are usually hydrophobic cationic molecules (Aungst Citation1999).
The effect of transporters on bioavailability brings up the concept of ‘active excipient’ as several excipients such as PEG-300, Pluronic® P85, and Tween® 20 have been shown to have an effect on efflux transporters. A recent review by Buggins et al. (Citation2007) on excipients raises the question of how many so-called inactive formulation ingredients are having an effect on transporters, enzymes, and ultimately on oral drug bioavailability.
Metabolizing enzymes
Drug metabolism in humans is catalyzed by both hepatic and intestinal enzymes. Drugs are metabolized by various sequential and competitive chemical processes involving oxidation, reduction, hydrolysis (phase I reactions) and glucouronidation, sulfation, acetylation and methylation (phase II reactions). Oxidation reactions catalyzed by cytochromes P450 are the most common first pass biotransformation occurring in humans limiting the oral bioavailability of pharmacologically effective drugs (Thummel et al. Citation1997). Cytochrome P4503A4 (CYP3A4) is the major phase I drug metabolizing enzyme in humans because of its abundance in the intestinal epithelium and the ability to metabolize a multitude of chemically unrelated drugs from major drug therapeutic classes (Thummel et al. Citation1997). Notable for the diversity of reactions catalyzed as well as chemically dissimilar substrates, these cytochrome P450s are a superfamily of heme thiolate proteins believed to have originated from an ancesteral gene existing 3 billion years ago (Danielson Citation2002). Another potential barrier against absorption of drugs and xenobiotics is produced due to coordinated action of CYP3A4 metabolizing enzymes and P-glycoprotein owing to the considerable overlap in their substrate selectivity, tissue localization and co-inducibility (Benet et al. Citation2004).
Barriers to CNS delivery
The recent data show that CNS disorders account for 11% of the global burden of disease, which is likely to rise to 14% in 2020, mainly due to the aging of population (Tiwari & Amiji Citation2006b, Pardridge Citation2007). Advances in biological understanding of CNS diseases, such as neurodegenerative diseases, are leading to the development of effective therapeutic strategies. However, a large number of newly-discovered small molecule and macromolecular therapeutics do not cross into the CNS after systemic administration (Pardridge Citation2002, Pardridge Citation2007). The following sections will describe the important factors that limit drug availability in the CNS.
Peripheral drug distribution and clearance
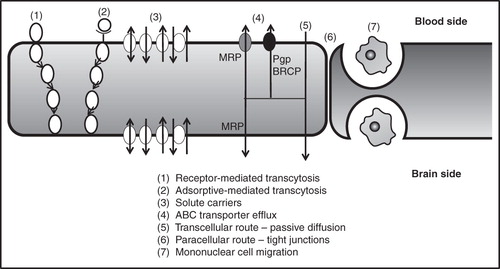
Based on in vitro permeability studies, a number of hydrophobic molecules are identified for CNS therapy. Unfortunately, one of the most neglected aspects of CNS drug administration is the fact that hydrophobic drugs administered systemically will undergo extensive dilution due to plasma protein binding and distribution in peripheral tissue (e.g., adipose tissue). Although the brain is a highly perfused organ, the drug distribution in peripheral tissues as well as premature metabolism and excretion limits the total amount that can reach the CNS. Once at the brain capillary interface, hydrophobic drugs generally do pass through lipid cell membranes by passive diffusion, whereas hydrophilic molecules cannot permeate efficiently as there is very poor paracellular transport through the tight junctions at the BBB ().
Figure 2. Transport mechanism at the blood-brain barrier (adapted from Abbott, Citation2005).
Increased CNS penetration of hydrophobic molecules, however, does not guarantee that there will be adequate availability at the site of action, especially in the intracellular compartments of specialized neuronal structures. For instance, to promote paclitaxel transport into the brain, the plasma paclitaxel concentration has to be maintained at higher levels for prolonged periods of time, resulting in increased systemic toxicity (Fellner et al. Citation2002, Hubensack et al. Citation2008). Thus, research should be focused on improving overall pharmacokinetic profile of potential CNS drug molecules.
The blood-brain barrier
The BBB is a mechanical and extremely specialized capillary network system that acts as a protective layer across the CNS. The capillary system mainly consists of endothelial cells held together by the continuous adherens junctions and the tight intracellular junctions (Abbott et al. Citation2010). The tight junctions are made up of the self interacting protein molecules, claudins and occludins, that inhibit permeation across the BBB (Cornford Citation1985). In the case of the adherens junctions, cadherin proteins are the functional units that span between the intracellular cleft and the cytoplasm and provide structural support to the capillary network system. Any injury to the adherens junctions damages the tight junctions and ultimately ruptures the BBB (Wolburg & Lippoldt Citation2002). Surrounding the endothelial cells is a dense protective layer consisting of the basal lamina and the astrocytes (Abbott, Citation2005). Occasional pericytes are found dispersed between the endothelium and the astrocytes. Other cellular units found within the BBB include the perivascular macrophages (Begley & Brightman, Citation2003), the smooth muscle cells enriched with collagenous matrix and the microglia (Luurtsema et al. Citation2004). The perivascular astoglia release certain growth factors that are involved in the development of the BBB phenotype (Abbott Citation2002). Initially, the BBB was considered to be a static region but recent advances have projected the BBB as a dynamic regulatory interface protecting the brain (Cornford Citation1985). The main function of the BBB is to protect the CNS from toxic molecules. In addition, the BBB also maintains homeostasis in the CNS and instills protection against compositional fluctuations in blood concentration (Luurtsema et al. Citation2004). The function of the capillary system is induced, maintained and tightly regulated by complex interactions involving other cells as well as the extracellular matrix of the brain (Hawkins & Egleton Citation2008). Usually, transport across the BBB takes place by different mechanisms () which include paracellular movement (between endothelial cells), transcellular movement (diffusion through endothelial cells), receptor-mediated transport (endocytosis) and carrier-mediated active transport (Demeule et al. Citation2002, Varatharajana & Thomas Citation2009). However, the presence of the tight junctions in the capillary system, lower number of intracellular fenestration in the brain endothelium, wide range of transport pumps and metabolizing enzymes, and the lack of sufficient endocytic vesicles limit the transport of drugs across the BBB (Varatharajana & Thomas Citation2009).
Blood-cerebrospinal fluid barrier
Drug permeation across the brain is also restricted by the blood-cerebrospinal fluid (blood-CSF) barrier. Capillary endothelium surrounding the choroid plexus has several intracellular fenestrations and allows diffusion of small molecules (Beduneau et al. Citation2007). It is the epithelial cells of the choroid plexus that show the presence of several tight junctions that act as a barrier and inhibit drug permeation (Wolburg et al. Citation2001). The epithelium lining the arachnoid completely seals the CNS from the rest of the body and also acts as a barrier to drug penetration. However, due to its avascular nature and relatively small surface area, it is the least significant barrier to CNS drug delivery (Abbott et al. Citation2010).
Efflux transporters at the blood-brain interface
The extent of drug transport across the BBB is minimal due to the presence of the tight junctions and several efflux transporters. The efflux transporters mainly protect the CNS from several toxins and drugs by preventing their entry into the CNS by pumping out the entire payload entering the brain endothelium (Gray et al. Citation2004). These transporters can be classified into two main categories which include the ABC transporters and the solute-carrier (SLC) super family (Loscher & Potschka Citation2005b, Varatharajana & Thomas Citation2009).
ABC efflux transporters
Among the several subfamilies of ABC transporters expressed in different tissues, the P-gp (ABCB1) subfamily, breast cancer-related protein subfamily (ABCG2/BCRP) and multidrug resistance-associated protein subfamily (ABCC/MRP) is found at the blood brain interface (Potschka Citation2010). Typically, ABC transporters consist of two membrane spanning domains, where each domain is made up of many transmembrane helices that contain a cytoplasmic nucleotide binding domain (NBD) (Nies Citation2007). An additional family of transporter proteins known as half ABC proteins has also been identified where the transporter proteins are made up of either a single (dimer/heteromer) or three membrane spanning domains leading to a different core structure (Dean & Annilo Citation2005).
P-glycoprotein
Among the two types of human P-gp, MDR1 P-gp encoded by the multi-drug resistance gene 1 (MDR-1) is found at the BBB and it weighs around 150–180 kDa (Loscher & Potschka Citation2005a, Varatharajana & Thomas Citation2009). Structurally, P-gp transporters consist of 12 transmembrane helices with substrate binding sites located within internal pockets (Seelig & Landwojtowicz Citation2000). These transporters identify drug molecules based on electron donor groups available for binding (Golden & Pollack Citation2003). Thus, drug molecules with weak binding affinity for P-gp receptor sites may penetrate across the BBB and reach the CNS (Potschka Citation2010). The P-gp mainly protects the brain from hyrdrophobic molecules and uncharged weakly basic molecules (Varatharajana & Thomas Citation2009). Located at the luminal side of the endothelial cells within the caveolae (Loscher & Potschka Citation2005a), the P-gp transporters pump out the drugs into the systemic circulation (Demeule et al. Citation2002). This efflux of drug molecules by the P-gp pumps helps maintain low concentration of the drugs within the endothelium. This leads to an extremely low concentration gradient across the capillary endothelium and the brain and any diffusion of drugs into the CNS is prevented (Pardridge Citation1999). Traces of P-gp transporters have also been observed in astrocytes found at the BBB (Loscher & Potschka Citation2005a). The primary location of the P-gp is the cell membrane, however, recent studies indicate that the P-gp is also found in certain intracellular compartments where it sequesters drug molecules and prevents subcellular target binding of the drugs (Rajagopal & Simon Citation2003). Many studies show that the extent of P-gp expression at the cell surface is also dependent on intracellular P-gp concentration and endocytic trafficking between the surface and the intracellular P-gp transporters.
Multidrug resistance proteins
The MRP are located at the luminal membrane of the endothelial cells, this subfamily of ABC transporter proteins consists of nine isoforms that are involved in the efflux of conjugated anionic drugs (Nies Citation2007, Varatharajana & Thomas Citation2009). Studies indicate the presence of four isoforms (MRP-1, MRP-2, MRP-4 and MRP-5) in the abluminal and cytoplasmic regions of the capillary endothelium at the BBB and two isoforms (MRP-1 and MRP-3) at the blood-CSF barrier (Demeule et al. Citation2002, Varatharajana & Thomas Citation2009). These proteins usually transport anionic and neutral anionic compounds and utilize ATP as well as certain co-factors for the efflux of these molecules (Deeley et al. Citation2006). Conjugates of glucoronide, glutathione, phosphate, and sulfate are ideal substrates for the MRP transporters (Nies Citation2007).
Breast cancer resistance protein (BCRP)
The BCRP transporter is found at the BBB, along the luminal surface of the endothelium and has expression levels similar to the P-gp (Varatharajana & Thomas Citation2009). The BCRP transporters are also known as ABCG2 and MXR and compared to the P-gp and MRP protein subfamily, the BCRP family has a stronger expression at the BBB. The exact substrate specificity for the BCRP class of proteins is not yet known. Other sub-families of the ABC transporters include ABCC2, ABCC3, ABCC4, ABCC5, ABCC6, ABCC10, ABCC11 AND ABCC12 where ABCC4 and ABCC5 are expressed by the endothelium at the BBB interface (Nies Citation2007). However, MDR1 P-gp, MRP and BCRP are the key transporters involved in the efflux of molecules across the BBB (Nies Citation2007).
Solute carrier transporters
The solute carrier (SLC) superfamily of protein transporters is found at both the BBB and blood-CSF interface. The human genome contains about 360 SLC transporters that have been classified into 46 subfamilies (Hediger et al. Citation2004). These transporters are mainly involved in efflux of organic molecules, especially anions through an ATP and sodium independent pathway (Soontornmalai et al. Citation2006). The organic anion transporting polypeptides (OATPs), organic cation transporters (OCTs) and organic anion transporters (OATs) are members of the SLC superfamily (Varatharajana & Thomas Citation2009). The transport of molecules by these transporters is gradient based (ions, concentration etc.) and unlike ABC transporters, their exact location within the brain has not been clearly identified (Loscher & Potschka Citation2005b).
Several isoforms of this class have been studied of which the OATP1A2 and OATP2B1 is expressed at the BBB (Varatharajana & Thomas Citation2009), while OATP3A1 is found at the blood-CSF barrier. Anionic amphipathic molecules that are greater than 450 daltons and have high binding affinity for albumin are ideal substrates for OATP transporters (Hagenbuch & Meier Citation2004, Eyal et al. Citation2009). The isoforms of these transporters include OAT1, OAT2, OAT3, OAT4, and sequentially similar RST and URAT1 isoforms (Kusuhara & Sugiyama Citation2005, Varatharajana & Thomas Citation2009). The exact location of the OATs is not clearly understood. However, the OAT3 and OAT1 are expressed by the epithelial cells at the blood-CSF interface (Eyal et al. Citation2009).
The presence and role of OCTs at the BBB still needs to be elucidated. However, five isoforms of this class have been identified so far in rat brains which include OCT1, OCT2, OCT3, OCTN1 and OCTN2. In humans, the OCTS play a vital role in hepatic and renal clearance of cationic molecules (Kusuhara & Sugiyama Citation2005, Varatharajana & Thomas, Citation2009). OCTs are also found within dendritic cells, macrophages, monocytes and lymphocytes and lymphocyte activation is known to up regulate the expression of OCTs (Eraly et al. Citation2004). In general, small, hydrophilic cationic molecules are ideal substrates for the OCT's and the transport is potential sensitive and driven by a proton gradient (Eyal et al. Citation2009).
Nucleoside transporters
This sub family of membrane proteins is mainly involved in transport of nucleosides across cellular membranes. They are further divided into two classes, equilibrative nucleoside transporters (ENTs) and concentrative nucleoside transporters (CNTs). The ENT family is also termed as the SLC29 family and is expressed as four different isoforms which are further classified as equilibrative sensitive and equilibrative insensitive with respect to nitrobenzythioinosine (Minuesa et al. Citation2008, Varatharajana & Thomas Citation2009). The human BBB shows the presence of hENT1 that is involved in the movement of nucleoside and nucleobases across the endothelial membrane and the transport is Na+ independent (Eyal et al. Citation2009, Varatharajana & Thomas Citation2009). Intriguingly, it has been reported that activation of primary lymphocytes leads to increased expression and activity of the ENTs across CD4+ T-cells and peripheral blood mononuclear cells (Eraly et al. Citation2004).
The CNTs belong to the SLC28 family and are expressed as three different isoforms. The CNTs are found in several regions including the epithelium of the choroid plexus (Baldwin et al. Citation2004). While the CNT1s (pyrimidine specific) and CNT2 (purine specific) are more substrate specific transporters, CNT3s transport a broader range of nucleoside molecules and the transport by the CNTs is Na+ dependent (Redzic et al. Citation2005, Eyal et al. Citation2009).
Miscellaneous transporters
Besides the ABC transporters and the SLC superfamily of proteins, a few other protein families are also involved in efflux transport of drugs and development of multidrug resistance (Loscher & Potschka Citation2005b). One such example includes the major vault proteins (MVP) that are primary constituents of the multimeric vault particles (Scheffer et al. Citation2000). These cytoplasmic ribonucleoproteins are found in the human brain endothelium and are over expressed in case of certain disease conditions (Mossink et al. Citation2003). Another such protein subfamily found at the BBB interface includes the RLIP76 which is actively involved in the efflux of several xenobiotics (Loscher & Potschka Citation2005b). System L transporters have also been found at the BBB and are involved in the bidirectional transport of neutral amino acids (Eyal et al. Citation2009). Lastly, monocarboxylate transporters have also been identified at the BBB and are involved in transport of molecules containing carboxylic moieties (Pierre & Pellerin Citation2005).
Thus, several transporters across the BBB and the blood-CSF interface inhibit the access of drug molecules and xenobiotics to the CNS. Also, certain disease states or drug molecules induce over expression/activation of the efflux pumps that can further restrict the entry of drug molecules into the CNS (Potschka Citation2010).
Metabolizing enzymes
The entry of drug molecules within the brain is also prevented by several enzymes located at the BBB interface as well as the blood-CSF barrier. These include, alkaline phosphatase, aminopeptidase A and N, aromatic acid decarboxylase, catechol-O-methyl transferase, dipeptidyl (amino) peptidase IV, epoxide hydrolase, γ-glutamyl transpeptidases, glutathione-S-transferase, monoamine oxidase and UDP-glucuronosyl transferase (Brasnjevica et al. Citation2009, Eyal et al. Citation2009). Usually the enzymes are found in the cytosolic fraction of the capillary endothelium and choroid epithelium. These enzymes structurally modify the drug molecules and render them to be therapeutically inactive.
Nanoemulsion systems to improve oral and CNS delivery
In order to improve oral and CNS bioavailability of therapeutic agents, a number of strategies have been tested over the years. Some examples include use of prodrugs to improve solubility or permeability, transient openings of the tight junctions using chemical or physical enhancers, or direct administration to the site and, as such, completing bypassing the barrier. With nanotechnology-based delivery strategies, the pharmacokinetic properties are now dictated by the formulation rather than the individual agents.
Multifunctional nanoemulsion formulations
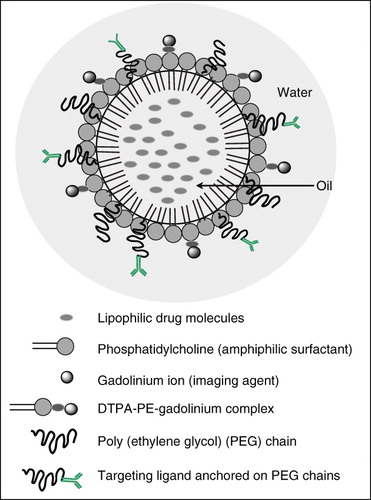
Nanoemulsions are heterogenous dispersions of oil in water, where the effective size of the oil droplets is reduced to less than 500 nm in diameter. The oil core can successfully incorporate poorly soluble drugs (Ganta et al. Citation2008b, Vyas et al. Citation2008). The use of omega-3 and omega-6 PUFA containing oils like flax-seed, pine-nut, hemp, fish oils as well as safflower and wheat-germ oils can be used in the formulations of nanoemulsions. These nanoemulsion systems are usually stabilized with amphiphilic surfactant, such as egg phosphatidylcholine, which is one of the components of cell membrane lipids. The co-surfactant molecules like stearylamine, deoxycholic acid, dioleoyltrimethylammoniumpropane (DOTAP) are also used for stabilizing the nanoemulsion droplets and imparting desired surface charge (Tiwari et al. Citation2006). For imaging capabilities, paramagnetic contrast agents like gadolinium ions (Gd3+) can be added to the nanoemulsions, using suitable ligand complexes like diethylenetriaminepentaaceticacid (DTPA) – phosphatidylethanolamine (PE) (DTPA-PE) (Levchenko et al. Citation2002, Torchilin Citation2002). Nanoemulsions can be produced using a variety of high energy emulsification techniques such as microfluidization and ultrasonication (Ganta et al. Citation2008a, Patlolla & Vobalaboina Citation2008, Ganta & Amiji Citation2009). In addition, the phase inversion temperature method can be used to produce self-assembled nanoemulsion delivery systems. Self-assembled nanoemulsions are particularly advantageous due to the relatively simple processing conditions and low energy requirements as compared to high-energy emulsification methods.
Nanoemulsions can also be made into multifunctional systems to allow for simultaneous targeting, imaging and drug delivery as shown in . The surface presence of targeting ligands could facilitate uptake of nanoemulsion droplets along with its encapsulated drugs through receptor-mediated endocytosis (Patlolla & Vobalaboina Citation2008). In addition, incorporating contrast agents in the oil phase or on the surface of the oil droplets improves visualization properties (Tiwari et al. Citation2006). Multifunctional nanoemulsions have several advantages over other nanocarrier systems. The PUFA oils are generally regarded as safe (GRAS) excipients, and also possess several biological properties owing to their rich omega-3 and omega-6 fatty acid content (La Guardia et al. Citation2005). The use of PUFA oils and egg phosphatidylcholine allows permeation of the nanoemulsion across cellular membranes making the nanoemulsions ideal for in vivo drug delivery. In case of oral drug delivery, several absorption barriers across the GI tract limit the extent of drug absorption and hence the bioavailability of drugs. The lack of in situ PUFA generation in the body leads to preferential absorption of the oils across the GI tract. Studies have shown that oral bioavailability of paclitaxel increased significantly when the drug is incorporated in a nanoemulsion system containing pine-nut oil (Tiwari & Amiji Citation2006a). Similarly, these nanoemulsion systems have shown a significant increase in oral bioavailability as well as brain delivery of the antiretroviral drug (Vyas et al. Citation2008). Orally administered nanoemulsions are stabilized using deoxycholic acid which helps bypass the first-pass metabolism while the presence of PUFA oils leads to rapid distribution of the drug to peripheral sites, especially the brain (Taogoshi et al. Citation2005, Vyas et al. Citation2008). PUFA oil-containing nanoemulsions are hypothesized to be ideal carriers for the delivery of drugs across the brain (Tiwari et al. Citation2006). For instance, pine-nut oil with a high gamma linolenic acid content is expected to rapidly penetrate across the BBB and thus, incorporating P-gp substrates like paclitaxel in pine-nut oil-containing nanoemulsions is hypothesized to increase the drug availability in the brain (Tiwari et al. Citation2006).
Enhancing drug solubility and permeability
The main permeation barriers in the human body to be crossed by drugs are epithelia and endothelia. Epithelial cells cover the surface of the body and line up various cavities (Ganta et al. Citation2008c). Endothelial cells line the blood capillaries so as to regulate the transport of molecules between the blood and interstitial fluid compartments (Ganta et al. Citation2008c). Physicochemical properties (solubility – hydrophilic and liphophilic, molecular size, hydrogen bonding property and pKa) of drugs influence their permeability through these physiological membranes (Ganta et al. Citation2008c). If the drug is not a substrate to efflux transporters and metabolizing enzymes, then the solubility is a major determining factor in drug permeability through physiological membranes. Biopharmaceutical Classification System (BCS) is derived based on the solubility and permeability properties of the drugs (Amidon et al. Citation1995). By knowing these properties, BCS classifies drugs into one of the four classes. Class I drugs are highly soluble and highly permeable through the GI tract, therefore, oral bioavailability is not a problem with these drugs. Class II drugs are highly lipophilic and well permeable across the GI tract, but the bioavailability is likely to be dissolution rate-limited due to low aqueous solubility. In such cases, drug delivery systems play a significant role in overcoming drug solubility issues. Class III drugs are highly soluble but have low permeability due to their low lipophilicity. Class IV drugs show low solubility and low permeability, and exhibit low and variable oral bioavailability. Methods to improve both solubility and permeability should be established for these compounds.
Nanoemulsions are lipid-based systems, which can allow the encapsulation of lipophilic drugs like BCS class II drugs, by solubilizing them in the oil phase. Many recent studies have shown that nanoemulsion could allow encapsulation of lipophilic drugs at therapeutically relevant doses (Tiwari & Amiji Citation2006a, Ganta et al. Citation2008a, Ganta et al. Citation2008b, Vyas et al. Citation2008, Ganta et al. Citation2009, Ganta et al. Citation2010). The drug encapsulation in nanoemulsion can facilitate direct transfer of drug to the GI tract membranes and excluding the dissolution of drugs in aqueous fluids in GI tract. In one study, we have incorporated highly lipophilic anticancer drug, paclitaxel into deoxycholic acid-modified pine-nut oil nanoemulsion (Tiwari & Amiji Citation2006a). Increased bioavailability was noted when paclitaxel was given in nanoemulsion compared to paclitaxel alone to mice (Tiwari & Amiji Citation2006a). In another study, we noted that saquinavir, an anti-HIV protease inhibitor, could be efficiently absorbed orally when given in safflower and flax seed oil containing nanoemulsions (Vyas et al. Citation2008). These nanoemulsions were made specifically with oils rich in PUFA, such as omega-3 and omega-6 fatty acids (Vyas et al. Citation2008). As well as the fact that nanoemulsions contribute to the increased permeability of drugs in the GI tract, the oil phase consisting of omega-3 and omega-6 fatty acids can produce interesting biological properties, such as a neuroprotective function and chemoprevention (La Guardia et al. Citation2005, Shimazawa et al. Citation2009).
Unlike in the GI tract, the paracellular permeability is very negligible in the CNS. The brain capillary endothelial cells form complex tight junctions that cause severe restriction of the paracellular pathway for transport of hydrophilic drugs (Abbott Citation2002, Deli Citation2009). So that the permeation across the endothelial cell barrier is effectively confined to the transcellular route (Abbott Citation2002). Lipophilic drugs can diffuse across the lipid membranes of the endothelium (Abbott Citation2002, Deli Citation2009). However, the lipid-mediated diffusion of a lipophilic drug through the BBB depends on the molecular mass threshold of 400–500 Da (Pardridge Citation2002). The lipid-based nanocarriers, such as liposomes, nanoemulsions and solid lipid or polymeric nanoparticles may be useful over the current strategies to deliver the lipophilic molecules across the BBB (Vyas et al. Citation2008). These carriers can not only cover the BBB limiting properties of drug molecules, but may also protect the drug from chemical/enzymatic degradation, besides providing the opportunity for sustained release of drug (Tiwari & Amiji Citation2006b). Due to the high oil core fraction, nanoemulsions can serve as excellent vehicles for incorporation of lipophilic compounds. Nanoemulsions have the ability to enhance the drug delivery to the brain by both parenteral and oral route of administrations (Vyas et al. Citation2008). Previous studies from our lab have shown an enhancement in oral and CNS bioavailability when saquinavir-containing nanoemulsion formulation, with an average oil droplet size of 100–200 nm, was given orally and intravenously to the male Balb/C mice relative to control formulation (Vyas et al. Citation2008).
Improving membrane interactions
Many studies have proven that nanoemulsions are effective drug delivery carriers for lipophilic drugs (Ganta et al. Citation2008b, Patlolla & Vobalaboina Citation2008, Vyas et al. Citation2008, Ganta et al. Citation2010). Lipophilic drug is either associated with the oil core or amphiphilic surfactant of the nanoemulsion system. The transfer of the drug molecules into the cells may take place by fusion of the oil droplet with the cell membranes through lipid exchange or by endocytosis of the oil droplets (Wickline & Lanza Citation2003). If targeting moieties (e.g., folate, thiamine, etc.) with corresponding cell surface receptors are anchored to the nanoemulsion to adapt multifunctional property, they can directly target a tissue of interest and remain longer at the disease site to allow for total transfer of the drug molecules (Oyewumi et al. Citation2003, Patlolla & Vobalaboina Citation2008).
Many studies have demonstrated that nanoemulsions can enhance oral drug absorption (Yang & Benita Citation2000, Tiwari & Amiji Citation2006a, Vyas et al. Citation2008, Bali et al. Citation2010). The mechanisms that account for improved oral drug absorption are due to: (1) lipophilic drug being present in soluble form in nanoemulsion oil droplets and excluding the dissolution step required in aqueous GI tract media, (2) direct transfer of drug molecules by fusion of oil droplets to the biological membrane (Wickline & Lanza Citation2003), and (3) nanoemulsion oil droplets can be taken up by lymphatic transport (Nishioka & Yoshino Citation2001). The recent studies show that nanoemulsion can also promote CNS drug delivery (Vyas et al. Citation2008). This is to some extent attributed to the presence of essential PUFA such as omega-3 and omega-6 in oil phase of nanoemulsion formulations. Essential PUFA transporters are present on the abluminal membrane of the endothelial cells of BBB to allow for the entry of PUFA into the brain (Edmond Citation2001). For example the flax seed oil contains up to 57% by weight linolenic acid, an example of omega-3 fatty acid, and 17% by weight linoleic acid, an example of omega-6 fatty acid with 18 carbons and 2 double bonds. Safflower oil, on the other hand, contains up to 73% by weight of linoleic acid. It is demonstrated that the 18 carbon-monocarboxylic fatty acids, linoleic acid with 2 cis-double bonds entered brain via selective mechanism, whereas the other fatty acids (e.g., oleic acid) with 1 cis-double bond did not enter the brain (Edmond Citation2001).
Surface charge on the nanoemulsion oil droplets can also play a role in in vivo disposition and clearance, and improving the membrane interactions. Surface charge is expressed in terms of zeta potential and is the net result of the ionization of the components forming interfacial monolayer at the oil and water interface (Tiwari Citation2007). In case of negatively charged nanoemulsion, the interfacial layer is formed by adsorption of lecithin (e.g., egg lecithin, soy lecithin) or lecithin and other phospholipids (e.g., cholesterol, PEG2000DSPE). In case of cationic nanoemulsions, the interfacial layer is formed of lecithin and cationic lipids (e.g., DOTAP, stearyl amine), resulting in a positive charge on the surface of the oil droplets.
Negatively charged nanoemulsion is removed faster from the systemic blood circulation and showed higher RES organ uptake than positively charged nanoemulsions (Tiwari Citation2007). On the other hand, positively charged nanoemulsions exhibited an early accumulation in the lungs followed by redistribution to the spleen and liver (Tiwari Citation2007). In addition, positively charged nanoemulsions could promote enhanced membrane interactions compared to negatively charged nanoemulsions.
Besides their role in oral and CNS drug delivery, nanoemulsions can be made into multifunctional systems to allow for drug delivery, targeting and imaging as described in . Presence of targeting ligands can facilitate the uptake of nanoemulsion droplets along with its encapsulated drugs into the brain. In addition, a multifunctional nanoemulsion can be designed to incorporate lipophilic drug in the oil core and imaging agent (e.g., gadolinium ions for magnetic resonance imaging contrast enhancement) on the surface of the oil droplets (Tiwari et al. Citation2006).
Modulation of drug efflux transporters
Expression of drug efflux transporters in intestines and CNS can potentially limit the oral absorption and brain entry of many lipophilic drugs. Thus, inhibition of drug efflux transporters can be used to enhance oral and CNS permeability of a wide range of drugs. For example, paclitaxel has shown potent activity in vitro against glioblastomas, but infective against brain tumours in vivo, due to its limited entry through BBB. However, the use of P-gp inhibitor, PSC833, enhanced paclitaxel accumulation in brain by several folds and paclitaxel levels were continued for at least 24 h (Fellner et al. Citation2002). Similarly, paclitaxel permeability in intestines is restricted by efflux transporters (Zhang & Benet Citation2001), and its oral bioavailability is shown to be increased with co-administration of P-gp inhibitors, such as cyclosporine A or its analogs or verapamil analogs (van Asperen et al. Citation1998, Malingre et al. Citation2001). In mice co-administered with cyclosporine A this resulted in increased oral bioavailability of paclitaxel from 9.3% up to 67% (van Asperen et al. Citation1998). A herbal compound, curcumin has also shown potential inhibitory activity against P-gp and cytochrome P450 in the GI tract, thus increasing the absorption of orally administered drugs (Zhang et al. Citation2007). Recently, we employed the curcumin to enhance the oral bioavailability of paclitaxel in nude mice (Ganta et al. Citation2010). In this study, paclitaxel and curcumin were administered in nanoemulsion to the mice, resulting in significantly enhanced oral bioavailability, as a consequence of enhanced drug delivery efficiency and effective P-gp and cytochrome P450 inhibition in the mouse intestines.
However, several of these inhibitors have led to unpredictable pharmacokinetic interactions with cytotoxic agents by inhibiting cytochrome P450 enzymes, resulting in reduced drug clearance with consequent toxicity (Loscher & Potschka Citation2005b). It is also uncertain whether prolonged use of such inhibitors is feasible given the defensive role of efflux transporters in different organs and cell types (Loscher & Potschka Citation2005b). Therefore, another potentially promising strategy to improving the permeability of otherwise non-penetrating drugs through the intestines and BBB is to by-pass efflux transporters present at these barriers without their direct inhibition.
Several approaches can be used to by-pass efflux transporters. Drug-containing nanoparticles can pass through the biological membranes by the endocytosis process (Yang & Benita Citation2000, Fricker & Miller Citation2004, Florence Citation2005). Nanoparticles loaded with the P-gp substrate and loperamide allowed a remarkable transport of these drugs to the brain possibly by involving endocytosis of particles through the luminal membranes of the BBB (Gulyaev et al. Citation1999). Incorporation of drugs into liposomes appears to enhance their therapeutic effects (Straubinger et al. Citation2004). However, liposomal poor penetration through the BBB is a problem, and this can overcome by attaching targeting ligands (Fricker & Miller Citation2004). Nanoemulsions also appear to enhance drug permeability by involving fusion of oil droplets with biological membrane and transport, thus by-passing the drug efflux mechanisms in the GI tract. Nanoemulsions made up of oils rich in PUFA can traverse the BBB by involving discrete mechanisms of essential PUFA transporters at the BBB (Edmond Citation2001), thus facilitating improved drug delivery (Vyas et al. Citation2008). In addition, stabilization of nanoemulsion with 1-O-alkylglycerol which has a prominent effect on permeability of BBB, enhanced the brain uptake of drugs (Madhusudhan et al. Citation2007). As noted with immune-liposomes (Fricker & Miller Citation2004), the nanoemulsions can also made into immune-nanoemulsions by attaching target-specific antibody ligands that could facilitate brain uptake.
Conclusions
Oral and CNS delivery challenges continue to be a significant issue in the development of therapeutic agents for chronic diseases. The physical and biological barriers present in the GI tract and at the BBB interface have to be overcome in order to enhance drug transport and availability in the systemic circulation and in the brain. In this review, we have discussed the role of oil-in-water nanoemulsion formulations that are specifically engineered to overcome these barriers. The opportunity to impart multifunctional properties in nanoemulsion formulations through selection of the edible oils from the PUFA-rich family, surface modification of the oil droplets with surfactants and imaging moieties, as well as selection of payloads that can be encapsulated in the oil phase allow for the development of a versatile platform that can help in improving oral and CNS bioavailability of therapeutic agents.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbott NJ. 2002. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200:629–638.
- Abbott NJ. 2005. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25:5–23.
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. 2010. Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25.
- Amidon GL, Lennernas H, Shah VP, Crison JR. 1995. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res 12:413–420.
- Aungst BJ. 1999. P-glycoprotein, secretory transport, and other barriers to the oral delivery of anti-HIV drugs. Adv Drug Deliv Rev 39:105–116.
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. 2004. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch 447:735–743.
- Bali V, Ali M, Ali J. 2010. Novel nanoemulsion for minimizing variations in bioavailability of ezetimibe. J Drug Target Article online in advance of print - DOI 10.3109/10611860903548362.
- Barchet TM, Amiji MM. 2009. Challenges and opportunities in CNS delivery of therapeutics for neurodegenerative diseases. Expert Opin Drug Deliv 6:211–225.
- Beduneau A, Saulnier P, Benoit JP. 2007. Active targeting of brain tumors using nanocarriers. Biomaterials 28:4947–4967.
- Begley DJ, Brightman MW. 2003. Structural and functional aspects of the blood-brain barrier. Prog Drug Res 61:39–78.
- Benet LZ, Cummins CL, Wu CY. 2004. Unmasking the dynamic interplay between efflux transporters and metabolic enzymes. Int J Pharm 277:3–9.
- Brasnjevica I, Steinbuscha H, Schmitza C, Martinez-Martinez P. 2009. Delivery of peptide and protein drugs over the blood brain barrier. Prog Neurobiol 87:212–251.
- Buggins T, Dickinson P, Taylor G. 2007. The effects of pharmaceutical excipients on drug disposition. Adv Drug Deliv Rev 59:1482–1503.
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. 2008. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res 14:1310–1316.
- Cornford EM. 1985. The blood brain barrier, a dynamic regulatory interface. Mol Physiol 7:219–259.
- Danielson PB. 2002. The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr Drug Metab 3:561–597.
- Dean M, Annilo T. 2005. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet 6:123–142.
- Deeley RG, Westlake C, Cole SP. 2006. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86:849–899.
- Deli MA. 2009. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta 1788:892–910.
- Demeule M, Regina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, Moghrabi A, Beliveau R. 2002. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul Pharmacol 38:339–348.
- Edmond J. 2001. Essential polyunsaturated fatty acids and the barrier to the brain: the components of a model for transport. J Mol Neurosci 16:181–193; discussion 215–221.
- Eraly SA, Bush KT, Sampogna RV, Bhatnagar V, Nigam SK. 2004. The molecular pharmacology of organic anion transporters: from DNA to FDA? Mol Pharmacol 65:479–487.
- Eyal S, Hsiao P, Unadkat JD. 2009. Drug interactions at the blood-brain barrier: fact or fantasy? Pharmacol Ther 123:80–104.
- Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, Bernhardt G, Graeff C, Farber L, Gschaidmeier H, Buschauer A, Fricker G. 2002. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest 110:1309–1318.
- Ferrari M. 2005. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5:161–171.
- Florence AT. 2005. Nanoparticle uptake by the oral route: fulfilling its potential? Drug Discov Today 2:75–81.
- Fricker G, Miller DS. 2004. Modulation of drug transporters at the blood-brain barrier. Pharmacology 70:169–176.
- Ganta S, Amiji M. 2009. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm 6:928–939.
- Ganta S, Devalapally H, Amiji M. 2010. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. J Pharm Sci Article online in advance of print - DOI: 10.1002/jps.22157.
- Ganta S, Devalapally H, Baguley B, Garg S, Amiji M. 2008a. Microfluidic preparation of chlorambucil nanoemulsion formulations and evaluation of cytotoxicity and pro-apoptotic activity in tumor cells. J Biomed Nanotechnol 4:165–173.
- Ganta S, Paxton JW, Baguley BC, Garg S. 2008b. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in parenteral emulsion. Int J Pharm 360:115–121.
- Ganta S, Sharma P, Garg S. 2008c. Permeability assessment. New York: Wiley-Interscience.
- Ganta S, Sharma P, Paxton JW, Baguley BC, Garg S. 2010. Pharmacokinetics and pharmacodynamics of chlorambucil delivered in long-circulating nanoemulsion. J Drug Target 18(2): 125–133.
- Gindy ME, Prud'homme RK. 2009. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin Drug Deliv 6:865–878.
- Goldberg M, Gomez-Orellana I. 2003. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov 2:289–295.
- Golden PL, Pollack GM. 2003. Blood-brain barrier efflux transport. J Pharm Sci 92:1739–1753.
- Gottesman MM, Fojo T, Bates SE. 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58.
- Gray JH, Owen RP, Giacomini KM. 2004. The concentrative nucleoside transporter family, SLC28. Pflugers Arch 447:728–734.
- Greish K. 2007. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target 15:457–464.
- Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. 1999. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res 16:1564–1569.
- Hagenbuch B, Meier PJ. 2004. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665.
- Hawkins BT, Egleton RD. 2008. Pathophysiology of the blood-brain barrier: animal models and methods. Curr Top Dev Biol 80:277–309.
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. 2004. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch 447:465–468.
- Hochman J, Artursson P. 1994. Mechanism of absorption enhancement and tight junction regulation. J Control Release 29:253–267.
- Hubensack M, Muller C, Hocherl P, Fellner S, Spruss T, Bernhardt G, Buschauer A. 2008. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol 134:597–607.
- Jabr-Milane LS, Van Vlerken LE, Yadav S, Amiji MM. 2008. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev 34:592–602.
- Jeffrey P, Summerfield S. 2010. Assessment of the blood-brain barrier in CNS drug discovery. Neurobiol Dis 37:33–37.
- Jones PM, George AM. 2004. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol Life Sci 61:682–699.
- Kim KY. 2007. Nanotechnology platforms and physiological challenges for cancer therapeutics. Nanomedicine 3:103–110.
- Koning GA, Krijger GC. 2007. Targeted multifunctional lipid-based nanocarriers for image-guided drug delivery. Anticancer Agents Med Chem 7:425–440.
- Kusuhara H, Sugiyama Y. 2005. Active efflux across the blood-brain barrier: role of the solute carrier family. NeuroRx 2:73–85.
- La Guardia M, Giammanco S, Di Majo D, Tabacchi G, Tripoli E, Giammanco M. 2005. Omega 3 fatty acids: biological activity and effects on human health. Panminerva Med 47:245–257.
- Lennernas H, Abrahamsson B. 2005. The use of biopharmaceutic classification of drugs in drug discovery and development: current status and future extension. J Pharm Pharmacol 57:273–285.
- Levchenko TS, Rammohan R, Lukyanov AN, Whiteman KR, Torchilin VP. 2002. Liposome clearance in mice: the effect of a separate and combined presence of surface charge and polymer coating. Int J Pharm 240:95–102.
- Loscher W, Potschka H. 2005a. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2:86–98.
- Loscher W, Potschka H. 2005b. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol 76:22–76.
- Luurtsema G, De Lange EC, Lammertsma AA, Franssen EJ. 2004. Transport across the blood-brain barrier: stereoselectivity and PET-tracers. Mol Imaging Biol 6:306–318.
- Madhusudhan B, Rambhau D, Apte SS, Gopinath D. 2007. 1-O-alkylglycerol stabilized carbamazepine intravenous o/w nanoemulsions for drug targeting in mice. J Drug Target 15:154–161.
- Malingre MM, Beijnen JH, Schellens JH. 2001. Oral delivery of taxanes. Invest New Drugs 19:155–162.
- Minuesa G, Purcet S, Erkizia I, Molina-Arcas M, Bofill M, Izquierdo-Useros N, Casado FJ, Clotet B, Pastor-Anglada M, Martinez-Picado J. 2008. Expression and functionality of anti-human immunodeficiency virus and anticancer drug uptake transporters in immune cells. J Pharmacol Exp Ther 324:558–567.
- Mossink MH, Van Zon A, Scheper RJ, Sonneveld P, Wiemer EA. 2003. Vaults: a ribonucleoprotein particle involved in drug resistance? Oncogene 22:7458–7467.
- Nies AT. 2007. The role of membrane transporters in drug delivery to brain tumors. Cancer Lett 254:11–29.
- Nishioka Y, Yoshino H. 2001. Lymphatic targeting with nanoparticulate system. Adv Drug Deliv Rev 47:55–64.
- Oyewumi MO, Liu S, Moscow JA, Mumper RJ. 2003. Specific association of thiamine-coated gadolinium nanoparticles with human breast cancer cells expressing thiamine transporters. Bioconjug Chem 14:404–11.
- Pardridge WM. 1999. Blood-brain barrier biology and methodology. J Neurovirol 5:556–569.
- Pardridge WM. 2002. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov 1:131–139.
- Pardridge WM. 2007. Blood-brain barrier delivery. Drug Discov Today 12:54–61.
- Patlolla RR, Vobalaboina V. 2008. Folate-targeted etoposide-encapsulated lipid nanospheres. J Drug Target 16:269–275.
- Pierre K, Pellerin L. 2005. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem 94:1–14.
- Potschka H. 2010. Targeting regulation of ABC efflux transporters in brain diseases: a novel therapeutic approach. Pharmacol Ther 125:118–127.
- Rajagopal A, Simon SM. 2003. Subcellular localization and activity of multidrug resistance proteins. Mol Biol Cell 14:3389–3399.
- Redzic ZB, Biringer J, Barnes K, Baldwin SA, Al-Sarraf H, Nicola PA, Young JD, Cass CE, Barrand MA, Hladky SB. 2005. Polarized distribution of nucleoside transporters in rat brain endothelial and choroid plexus epithelial cells. J Neurochem 94:1420–1426.
- Salama N, Eddington N, Fasano A. 2006. Tight junction modulation and its relationship to drug delivery. Adv Drug Deliv Rev 58:15–28.
- Sarker DK. 2005. Engineering of nanoemulsions for drug delivery. Curr Drug Deliv 2:297–310.
- Scheffer GL, Schroeijers AB, Izquierdo MA, Wiemer EA, Scheper RJ. 2000. Lung resistance-related protein/major vault protein and vaults in multidrug-resistant cancer. Curr Opin Oncol 12:550–556.
- Seelig A. 1998. A general pattern for substrate recognition by P-glycoprotein. Eur J Biochem 251:252–261.
- Seelig A, Landwojtowicz E. 2000. Structure-activity relationship of P-glycoprotein substrates and modifiers. Eur J Pharm Sci 12:31–40.
- Shimazawa M, Nakajima Y, Mashima Y, Hara H. 2009. Docosahexaenoic acid (DHA) has neuroprotective effects against oxidative stress in retinal ganglion cells. Brain Res 1251:269–275.
- Soontornmalai A, Vlaming ML, Fritschy JM. 2006. Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier. Neuroscience 138:159–169.
- Stevenson BR. 1998. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol 14:89–109.
- Straubinger RM, Arnold RD, Zhou R, Mazurchuk R, Slack JE. 2004. Antivascular and antitumor activities of liposome-associated drugs. Anticancer Res 24:397–404.
- Taogoshi T, Nomura A, Murakami T, Nagai J, Takano M. 2005. Transport of prostaglandin E1 across the blood-brain barrier in rats. J Pharm Pharmacol 57:61–66.
- Thummel K, Kunze K, Shen D. 1997. Enzyme catalyzed processes of first pass hepatic and intestinal drug extraction. Adv Drug Deliv Rev 27:99–127.
- Tiwari S. 2007. Nanoemulsion formulations for tumor-targeted delivery. In: Mansoor M. Amiji, editor. Nanotechnology for cancer therapy. New York: CRC Press.
- Tiwari S, Amiji M. 2006a. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol 9:3215–3221.
- Tiwari S, Tan Y-M, Amiji M. 2006. Preparation and in vitro characterization of multifunctional nanoemulsions for simultaneous MR imaging and targeted drug delivery. J Biomed Nanotechnol 2:217–224.
- Tiwari SB, Amiji MM. 2006b. A review of nanocarrier-based CNS delivery systems. Curr Drug Deliv 3:219–232.
- Torchilin VP. 2002. PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv Drug Deliv Rev 54:235–252.
- Torchilin VP. 2006. Multifunctional nanocarriers. Adv Drug Deliv Rev 58:1532–1555.
- Van Asperen J, Van Tellingen O, Van Der Valk MA, Rozenhart M, Beijnen JH. 1998. Enhanced oral absorption and decreased elimination of paclitaxel in mice cotreated with cyclosporin A. Clin Cancer Res 4:2293–2297.
- Varatharajana L, Thomas S. 2009. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recomendations for further research. Antiviral Res 82:A99–A109.
- Vyas TK, Shahiwala A, Amiji MM. 2008. Improved oral bioavailability and brain transport of saquinavir upon administration in novel nanoemulsion formulations. Int J Pharm 347:93–101.
- Wickline SA, Lanza GM. 2003. Nanotechnology for molecular imaging and targeted therapy. Circulation 107:1092–1095.
- Wolburg H, Lippoldt A. 2002. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol 38:323–337.
- Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B. 2001. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett 307:77–80.
- Yang SC, Benita S. 2000. Enhanced absorption and drug targeting by positively charged submicron emulsions. Drug Develop Res 50:476–486.
- Zhang W, Tan TM, Lim LY. 2007. Impact of curcumin-induced changes in P-glycoprotein and CYP3A expression on the pharmacokinetics of peroral celiprolol and midazolam in rats. Drug Metab Dispos 35:110–115.
- Zhang Y, Benet LZ. 2001. The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin Pharmacokinet 40:159–168.