Abstract
GltS of Escherichia coli is a secondary transporter that catalyzes Na+-glutamate symport. The structural model of GltS shows two homologous domains with inverted membrane topology that are connected by a central loop that resides in the cytoplasm. Each domain contains a reentrant loop structure. Accessibility of the Cys residues in two GltS mutants in which Pro351 and Asn356 in the reentrant loop in the C-terminal domain were replaced by Cys is demonstrated to be sensitive to the catalytic state supporting a role for the reentrant loops in catalysis. Saturating concentrations of the substrate L-glutamate protected both mutants against inactivation by thiol reagents, while the presence of the co-ion Na+ stimulated the inactivation of both mutants. Insertion of the 10 kDa biotin acceptor domain (BAD) of oxaloacetate decarboxylase of Klebsiella pneumoniae in the central cytoplasmic loop blocked the access pathway from the periplasmic side of the membrane to the cysteine residues in mutants P351C and N356C in the reentrant loop. Kinetically, insertion of BAD increased the maximal rate of uptake 2.7-fold while leaving the apparent affinity constants for L-glutamate and Na+ unaltered. The data suggests that insertion of BAD in the central loop results in conformational changes at the translocation site that lower the activation energy of the translocation step without affecting the access pathway from the periplasmic side for substrate and co-ions. It is concluded that changes in the central loop that connects the two domains may have a regulatory function on the activity of the transporter.
Introduction
Escherichia coli K-12 grows on L-glutamate as a sole carbon and nitrogen source. Transport into the cell is mediated by three glutamate transport systems, an ABC transport system specific for glutamate and aspartate and two secondary transporters, the proton symporter GltP and the Na+ symporter GltS (Schellenberg and Furlong Citation1977, Deguchi et al. Citation1989, Wallace et al. Citation1990, Kalman et al. Citation1991). GltP is a member of the DAACS family (Dicarboxylate/Amino Acid:Cation Symporter; TC 2.A.23) (Saier Citation2000, Slotboom et al. Citation1999), while GltS belongs to the ESS family (Glutamate Sodium Symporter; TC 2.A.27) of secondary transporters. Members of the ESS family are exclusively found in the bacterial domain. GltS has high affinity for L-glutamate, but, with a lower affinity, also transports D-glutamate, α-methylglutamate, and homocysteic acid. Glutamate uptake activity by GltS was observed only in the presence of Na+, irrespective of the pH, suggesting an obligatory coupling of glutamate and Na+ translocation (Tolner et al. Citation1995). The exact physiological function of each of the three transport systems is not known, but it is likely that they function under different conditions which requires some means of regulation of their activity either at the genetic or at the protein level.
Hydropathy profile analysis suggested that transporters of the ESS family share the same structure with transporters of the 2HCT family [2-HydroxyCarboxylate Transporter, TC 2.A.24 (Sobczak and Lolkema Citation2005a)], another family found only in bacteria and specific for 2-hydroxycarboxylates like citrate, malate and lactate (MemGen structural classification, class ST[3]) (Lolkema and Slotboom Citation1998, Citation2003). Transporters from the two families do not share significant sequence identity. Experimental evidence for the structural similarity has been presented (Dobrowolski et al. Citation2007, Dobrowolski and Lolkema Citation2009, ter Horst and Lolkema, Citation2010) and the structural model of the transporters in class ST[3] is largely based on studies of GltS of E. coli and the Na+-citrate symporter CitS of Klebsiella pneumoniae from the ESS and 2HCT families, respectively. The transporters also share a dimeric quaternary structure (Mościcka et al. Citation2009, Krupnik et al. 2011).
represents the structural model of the core of the transporters in structural class ST[3] which is the same as the model for GltS of E. coli (Sobczak and Lolkema Citation2005a). Transporters in other families may have additional transmembrane segments (TMS) at the N- or C-terminus. The core structure consists of two domains sharing the same fold and consisting of 5 TMS each (Lolkema et al. Citation2005, Lolkema Citation2006). The two domains have opposite orientations in the membrane, an architecture that is frequently observed in membrane proteins (Sobczak and Lolkema Citation2005b, Forrest et al. Citation2011). The N-termini of the N- and C-domain are in the periplasm and cytoplasm, respectively, and the two are connected by a large cytoplasmic loop. Strong evidence for the two domain structure was obtained recently by swapping the order of the N- and C-domain encoding parts of the gltS gene. The engineered gene encoded a transporter GltSswap with similar activity as GltS (Dobrowolski and Lolkema Citation2010). In addition, transport activity was recovered by expressing the two domains as separate proteins from an artificial operon (GltSsplit). In the model, the domains contain a reentrant loop structure positioned in between the 4th and 5th TMS that are believed to be involved in catalysis (Dobrowolski and Lolkema Citation2009, Sobczak and Lolkema Citation2003, Citation2004). A mutational study of the GGXG motif found in the reentrant loops of GltS and CitS showed the functional importance of the loops (Dobrowolski and Lolkema Citation2009). When replaced by a Cys residue, only a single residue in the reentrant loops in the C-domains of GltS and CitS (Pro351 and S405, respectively) was accessible from the periplasmic side by a bulky membrane impermeable thiol reagent (Dobrowolski and Lolkema Citation2009). In the 3D-structure, the two domains are believed to fold upon each other with the two reentrant loops forming the translocation pathway (Dobrowolski et al. Citation2010).
Figure 1. Topology model of class ST[3] transporters. The membrane topology of GltS of E. coli is shown which is representative for the core structure of class ST[3] transporters. Cylinders represent transmembrane segments. The homologous N and C domains that have opposite orientation in the membrane were indicated in dashed boxes. VB and XA represent the reentrant loops in the N- and C-domain, respectively. Black dots point at the positions of mutation P351C and N356C introduced by site directed mutagenesis. The open square points at position 206, the insertion site of the BAD domain in the hybrid version of the GltS protein.
![Figure 1. Topology model of class ST[3] transporters. The membrane topology of GltS of E. coli is shown which is representative for the core structure of class ST[3] transporters. Cylinders represent transmembrane segments. The homologous N and C domains that have opposite orientation in the membrane were indicated in dashed boxes. VB and XA represent the reentrant loops in the N- and C-domain, respectively. Black dots point at the positions of mutation P351C and N356C introduced by site directed mutagenesis. The open square points at position 206, the insertion site of the BAD domain in the hybrid version of the GltS protein.](/cms/asset/b7faf126-2dc0-4ddc-a48c-ed872c3491ec/imbc_a_624989_f0001_b.jpg)
Translocation of substrate and co-ions by this class of transporters is believed to follow an alternate access mechanism in which movement of the two domains relative to one another results in alternate access of the binding sites positioned at the domain interface, similarly as observed for other classes of transporters (Forrest et al. Citation2011). Here, a potential role of the cytoplasmic loop that connects the two domains in regulation of the activity of the transporters is suggested by demonstrating that an insertion of a 10 kDa protein in the loop increases the transport rate and effects the accessibility of Pro351 at the vertex of the reentrant loop and of the nearby residue Asn356 that in the model is deeper inside the translocation pore.
Methods
Growth conditions and membrane preparation
GltS and GltS derived transporters were expressed in Escherichia coli strain DH5α under control of the arabinose promoter using pBAD24 (Invitrogen) derived plasmids. Freshly transformed bacteria were used to inoculate an overnight preculture. A total of 25 ml of preculture was added to 1 l of Luria Broth (LB) medium containing 50 μg/ml ampicilin in a 5 l flask at 37°C that was under continuous shaking at 200 rpm. At an optical density of OD660 = 0.6 0.1% w/v arabinose (Sigma-Aldrich GmbH, Steinheim, Germany) was added to induce expression of the transporters after which the culture was allowed to grow for an additional hour. Cells were harvested by spinning at 8000 rpm at 4°C for 10 min, washed with 50 ml 50 mM KPi, pH 7 at 4°C and resuspended in the same buffer.
Cytoplasmic membranes were isolated by breaking cells resuspended at high density in 100 μl and on ice with a Soniprep 150 sonicator operated at an amplitude of 10 μm and using 15 cycles of 15 s ON and 15 s OFF. Cell debris and unbroken cells were removed by spinning for 5 min at 13,000 rpm at 4°C. Membranes were collected from the supernatant by ultracentrifugation for 25 min at 80,000 rpm at 4°C.
Right-side-out (RSO) membranes were prepared from a 1 l culture by the osmotic shock procedure as described (Kaback Citation1974). RSO membranes were resuspended in 50 mM KPi pH 7.0 and aliquoted in 150 μl portions that were stored at −80°C. Membrane protein concentration in the samples was determined by DC protein assay kit (BioRad Laboratories, Hercules, CA, USA).
Construction of plasmids
All genetic manipulations were done in E. coli DH5α using standard techniques. Plasmids pGltS, pGltS P351C and pGltS N356C encode the wild type GltS protein and the GltS mutants P351C and N356C, respectively. The constructs that encode GltS variants extended with an N-terminal His6-tag were described before (Dobrowolski et al. Citation2007, Dobrowolski and Lolkema Citation2009). Plasmid pGltS-BAD206 encodes the GltS protein with the Biotin Acceptor Domain (BAD) of oxaloacetate decarboxylase of K. pneumoniae inserted at position 206 of wild type GltS as described before (Krupnik et al. 2011).
Plasmid pGltS-BAD206 was mutated to introduce mutations P351C and N356C in the BAD insertion variant of GltS yielding plasmids pGltS-BAD206 P351C and pGltS-BAD206 N356C. The mutants were constructed by PCR using the QuickChange site-directed mutagenesis kit (Stratgane, La Jolla, CA, USA). The presence of the mutations was confirmed by DNA sequencing (Service SX, Leiden, The Netherlands).
Expression levels of GltS and GltS derivatives
Expression levels of GltS and GltS derivatives were estimated from the staining intensity of the partial purified proteins after SDS-PAGE. Samples of RSO membranes or cytoplasmic membranes obtained by sonication and containing between 0.5 and 1 mg of membrane protein were solubilized in 1 ml of 50 mM KPi pH 7 buffer containing 400 mM NaCl, 10% glycerol and 1% Triton X-100 for 1 h at 4°C while shaking gently. Solubilized proteins were recovered by spinning for 25 min at 4°C in a Beckman TLA 100.4 rotor at 80,000 rpm. The supernatant was applied to a 50 μl bed volume Ni-NTA resin equilibrated with 50 mM KPi pH 8 buffer containing 600 mM NaCl, 10% glycerol, 0.1% Triton X100, and 30 mM imidazole and left overnight at 4°C while shaking. Next, the resin was spun down in a table centrifuge for 1 min at 3,500 rpm and the supernatant was removed. Subsequently the resin was washed twice with equilibration buffer and once with the same buffer but containing 40 mM imidazole. Proteins were eluted with 50 μl of 50 mM KPi pH 7 buffer containing 600 mM NaCl, 10% glycerol, 0.1% TritonX, 500 mM imidazole and analyzed by SDS-PAGE. Staining intensity of the appropriate bands was visualized and quantified using a Fujifilm LAS-4000 image analyzer (Fuji).
Transport studies
RSO membrane vesicles were diluted to 1 mg/ml in 50 mM KPi pH 6 containing 10 or 100 mM NaCl as indicated. Aliquotes of 100 μl were incubated for 2 min at 30°C with 10 mM K-ascorbate and 100 μM phenazine methosulfate (PMS) under a flow of water-saturated air and continuous stirring with a magnetic bar. At t = 0, [14C]-proline or L-[14C]glutamate was added to final concentrations of 2.2 and 2.9 μM, respectively. Uptake was stopped at 5, 10, 20, 40 s by the addition of 2 ml of ice-cold 0.1 M LiCl followed by immediate filtering over cellulose nitrate filters (0.45 μm pore size) and washing of the filter with an additional 2 ml of the LiCl solution. Filters were collected and their radioactivity was measured in a liquid scintillation counter. Background activities due to the chromosomal copy of the gltS gene were determined in parallel experiments by the uptake activity of membranes treated with an excess of NEM (GltS-P351, GltS-N356C and BAD206-N356C) or by the uptake of membrane vesicles derived from cells containing the empty vector (BAD206-P351).
Labeling studies
RSO vesicles were incubated for 5 min at room temperature in 50 mM KPi pH 7 buffer with or without the indicated concentrations of L-glutamate (K+ salt) and Na+ (chloride salt). The samples were treated with 1 mM of NEM or 0.25 mM of AmdiS for 10 min unless otherwise stated. The thiol reagent solutions were prepared freshly. The treatment was stopped by addition of 10 mM DTT. Subsequently, the membranes were collected by spinning at 7,000 rpm for 5 min at 4°C, followed by resuspension and three times washing in 50 mM KPi pH 7 buffer. RSO membranes were resuspended in 50 mM KPi pH 6 containing 100 mM NaCl. Control samples were included that were not treated with the thiol reagents but with and without L-glutamate. Rates of uptake in these samples were comparable, indicating efficient removal of L-glutamate by the washing procedure.
Cells expressing the GltS variants were treated in the same way, followed by a second treatment with 0.1 mM fluorescent maleimide (FM) for 1 min at 20°C. The reaction was stopped with 1 mM DTT and the cells were washed twice with 50 mM KPi pH 7 buffer. Direct labeling of the proteins by FM was performed in the same way but the first labeling step with NEM or AmdiS was omitted. Following purification and SDS-PAGE, the fluorescence intensity of the bands was visualized using a Fujifilm LAS-4000 image analyzer (Fuji).
Materials
Ampicilin was obtained from Roche Diagnostic GmbH, Mannheim, Germany, arabinose from Sigma-Aldrich GmbH, Steinheim, Germany, and [1,5-14C]-citrate and L-[14C]-glutamate from Amersham Pharmacia, Roosendaal, The Netherlands.
Results
Kinetics of L-glutamate uptake by RSO membranes containing GltS and GltS-BAD206
Before (Krupnik et al. 2011), the biotin acceptor domain (BAD) of the oxaloacetate decarboxylase of Klebsiella pneumoniae, a 10 kDa protein, was inserted at position 206 in the central loop of the Na+-dependent glutamate transporter GltS (see ) for cross-linking studies. Transport activity of GltS-BAD206 and the wild type transporter was measured by the rate of uptake of radiolabeled L-glutamate by right-side-out (RSO) membrane vesicles derived from E. coli DH5α producing the proteins with six histidine residues added at the N-terminus (His6-tag). RSO membrane vesicles rather than whole cells were used to prevent further metabolism of the substrate following uptake. L-glutamate accumulation was driven by a proton motive force that was generated using the artificial electron donor system ascorbate/PMS (Konings et al. Citation1971). Membrane vesicles prepared from the host cells contain a low level of Na+-dependent L-glutamate transport activity due to expression of the gltS gene encoded on the chromosome. Background activity was approximately 20–25% of membranes containing in addition plasmid encoded GltS () (Dobrowolski et al. Citation2007, Dobrowolski and Lolkema Citation2009).
Figure 2. Effect of insertion of the BAD domain in the central loop on L-glutamate uptake activity of GltS. A,B. RSO vesicles expressing GltS (▪), GltS BAD206 (□) or control vesicles (▴) were assayed for L-glutamate (A) and proline (B) uptake activity. (C) SDS-PAGE of GltS (lane 1) and GltS BAD206 (lane 2) partially purified from the same batch of vesicles used in the uptake experiments shown in panels A and B. Molecular masses of marker proteins (lane M), masses were indicated in kDa. (D) Relation between uptake activity and expression of GltS (▪) and GltS BAD206 (□) in RSO membranes. L-glutamate uptake was measured in RSO membranes isolated from cells induced with 0, 0.001 and 0.1% (w/v) of arabinose. Expression levels were determined densitometrically after partial purification of the transporter proteins from the same RSO membranes followed by SDS-PAGE. The insert shows the Coomasie Brilliant Blue (CBB) stained gel.
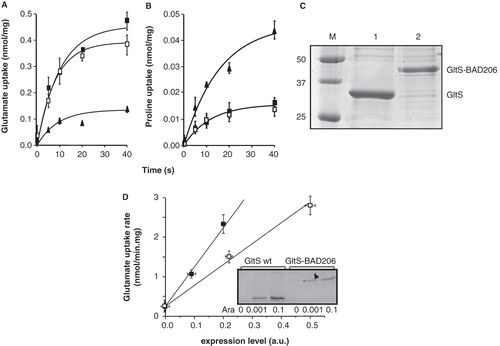
RSO membranes containing GltS-BAD206 took up L-glutamate with an initial rate that was within experimental error the same as the rate observed with membranes containing wild-type GltS ().
To determine the specific activity of the insertion mutant relative to wild-type GltS, the expression levels and quality of the membranes were determined. The latter was inferred from pmf driven uptake of radiolabeled proline by an endogenous transporter using the same batch of membranes under the same conditions (). Initial rates of uptake of proline by the membranes containing the plasmid encoded GltS and GltS-BAD206 transporters were not significantly different, while the control membranes took up proline at a 3-fold higher rate. It follows that the contribution of the endogenous GltS transporter to the L-glutamate uptake rates () by the former two types of membranes is largely overestimated and amounts to less than 10%. The latter result is in agreement with experiments where the background rate was estimated by inactivating a mutant GltS (Krupnik et al. 2011). More importantly, it follows that membranes derived from cells overexpressing GltS or GltS-BAD206 are of comparable quality allowing for a direct comparison of the activity of the transporters in the two types of membranes. The His-tagged GltS proteins were partially purified by Ni2+-NTA affinity chromatography from the same batch of RSO membranes used in the uptake experiments. Densitometric analysis of the intensity of the Coomassie Brilliant Blue stained bands after SDS-PAGE revealed an intensity ratio of GltS over GltS-BAD206 of 2.2 (). Assuming the same staining efficiency of GltS and the BAD domain and taking into account the different molecular masses of GltS and GltS-BAD206 (43.5 and 52.9 kDa, respectively), the expression level of the insertion mutant in the membranes would be a factor of 2.7 lower. The relation between uptake activity and amount of transporter protein in the membranes was determined by preparing RSO membranes from cells harboring the appropriate plasmid that were induced with 0, 0.001 and 0.1% arabinose in the medium (). Induction with 0.1% arabinose corresponds to the standard growth condition. No protein was detected with the uninduced cells. For both GltS and GltS-BAD206, linear relations were obtained that extrapolated back to an initial rate of 0.25 nmol/min.mg, which corresponds to the uptake activity of the uninduced cells. It follows that for both types of membranes, uptake activity was proportional to the amount of plasmid encoded protein in the membrane. In conclusion, the specific activity of GltS-BAD206 was 2.7 times higher than observed for wild type GltS at concentrations of L-glutamate and Na+ of 2.9 μM and 10 mM, respectively.
In the presence of 10 mM Na+, the total initial rate of L-glutamate uptake at increasing L-glutamate concentrations in the range between 2.9 and 52.9 μM followed a saturation curve with an affinity constant KM of 13.5 ± 3.5 μM and a maximal rate of 15.0 ± 1.6 nmol/min.mg of membrane protein for membranes containing wild type GltS (, insert).
Figure 3. Kinetic parameters of GltS in RSO membranes. The initial rate of uptake of L-glutamate at a concentration of 2.9 μM in RSO membranes expressing GltS was measured at the indicated Na+ concentrations. The K0.5 for Na+ was 5.8 ± 0.5 mM. Inset, L-glutamate dependent kinetics of the same membranes at a concentration of 10 mM of Na+. The affinity for glutamate was KM = 13.5 ± 3.5 μM.
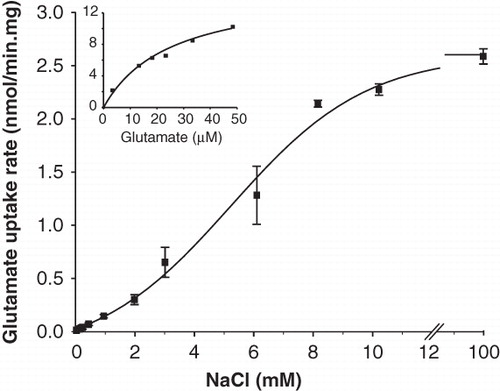
At a constant L-glutamate concentration of 2.9 μM, initial rates increased sigmoidal with increasing concentrations of Na+ covering a range between 0.1 and 100 mM (). The rate was half the maximal rate at a Na+ concentration of 5.8 ± 0.5 mM. The sigmoidal curve indicates that more than one Na+ ions are symported with the L-glutamate anion, in agreement with the electrogenic nature of transport reported before (Tolner et al. Citation1995). The same analysis with membranes containing the GltS-BAD206 insertion mutant yielded an affinity constant for L-glutamate that was not significantly different than observed for the wild type, KM = 15.0 ± 3.0 μM (). Also, the Na+ concentration giving half the maximum rate was within the error observed for the wild type, K0.5 = 5.6 ± 0.3 mM. For both analyses, no correction for the background activity was applied because the difference between the parameters was not significant. Since the kinetic parameters of wild type GltS and the insertion mutant GltS-BAD206 are similar within experimental error, it follows that insertion of the BAD domain in the cytoplasmic central loop of GltS results in a 2.7-fold increase of the maximal turnover rate per GltS molecule ()
Table I. Kinetic parameters of GltS and GltS-BAD206 for pmf driven L-glutamate uptake in RSO membranes.
Inactivation of reentrant loop mutants P351C and N356C by thiol reagents
Residues Pro351 and Asn356 of GltS were replaced with Cys residues before (Dobrowolski and Lolkema Citation2009) as part of a cysteine scanning mutagenesis study of the putative cytoplasmic reentrant loop in the C-terminal domain of GltS (see ). In a stretch of 18 residues, mutation of residues Pro351 and Asn356 to Cys rendered the transporter sensitive to inactivation by the membrane permeable thiol reagent N-ethylmaleimide (NEM). In addition, P351C was also inactivated by membrane impermeable 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AmdiS) indicating accessibility of the thiol from the periplasmic side of the membrane. Pretreatment of RSO membranes containing the mutants in 50 mM KPi buffer pH 7 for 10 min at increasing concentrations of the thiol reagents followed by measurement of the residual activity revealed a much higher sensitivity of the P351C mutant than the N356C mutant for inactivation by NEM (, , open bars).
Figure 4. Sensitivity of P351C and N356C mutants of GltS to inactivation by NEM and AmdiS. RSO membranes containing mutants GltS-N356C and BAD206-N356C (A) and GltS-P351C and BAD206-P351C (B,C) were treated in 50 mM KPi pH 7 buffer for 10 min with NEM (A,B) and AmdiS (C) at the indicated concentrations. Following quenching of the remaining thiol reagent by access DTT, washing of the membranes, and resuspension in 50 mM KPi pH 6 buffer, residual L-glutamate uptake activity was measured relative to membranes containing the same mutant that was treated in exactly the same way, except that the thiol reagents were omitted during the treatment. Open bars, GltS-N356C and GltS-P351C. Shaded bars, BAD206-N356C and BAD206-P351C. Back ground activities amounted to 30, 25, 20 and 50% for the membranes containing mutants GltS-N356C, BAD206-N356C, GltS-P351C and BAD206-P351C, respectively.
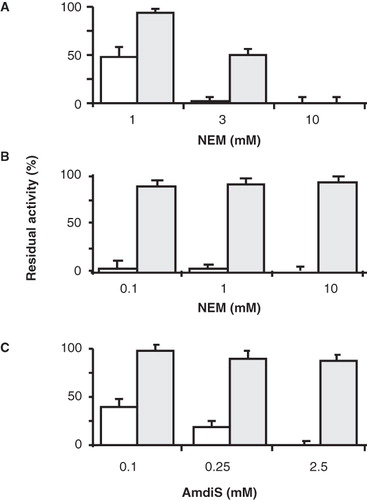
Concentrations of NEM as low as 0.1 mM completely inactivated the former while a 30-fold higher concentration was needed for complete inactivation of the latter. Uptake activities by the membranes were corrected for the background activity of the chromosomally encoded wild type GltS that is not sensitive to thiol reagents (Dobrowolski et al. Citation2007). The sensitivity of the P351C mutant was slightly higher for NEM than for AmdiS (, ). Mutant N356C was not inactivated by concentrations of AmdiS as high as 3 mM under the conditions of the experiments (not shown).
Remarkably, insertion of the BAD domain in the central loop of GltS mutants P351C and N356C significantly affected the reactivity of the mutants with the thiol reagents NEM and AmdiS. Treatment of RSO membranes containing the BAD206 variant of the N356C mutant in KPi pH 7 buffer with 1 mM of NEM for 10 min which resulted in 50% inactivation of the wt variant had no significant effect on the activity of BAD206-N356C (, open and shaded bars). At 3 mM NEM, the residual activity of the BAD206-N356C mutant was still about 50% and only at 10 mM full inactivation was obtained. The protective effect of the BAD domain was even more pronounced in case of the P351C mutant. In fact, no significant inactivation was observed after treatment of the BAD variant with concentrations up to 10 mM of NEM, while the wild type version was inactivated completely at a 100-fold lower concentration (). Similarly, BAD206-P351C was only marginally inactivated by concentrations of AmdiS that were 10 times higher than the concentration required to inactivate the wild type version (). It follows that insertion of the BAD domain in the cytoplasmic central loop of GltS makes the residue at the vertex of the putative reentrant loop positioned at the periplasmic side () virtually inaccessible for thiol reagents NEM and AmdiS.
Effect of substrate L-glutamate and co-ion Na+ on inactivation of the P351C and N356C mutants
The presence of a saturating concentration of 1 mM L-glutamate (KM = 13.5 μM; see , insert) protected both the P351C and N356C mutants against inactivation by the thiol reagents. Treatment of RSO membranes containing the P351C mutant with 0.25 mM of AmdiS for 10 min resulted in 78% of inactivation of uptake activity, while the presence of 1 mM of L-glutamate during the inactivation essentially completely protected the transporter ().
Figure 5. Protection of GltS-P351C (A) and GltS-N356C (B) mutants by L-glutamate. RSO vesicles containing GltS-P351C (A) or GltS-N356C (B) were treated in 50 mM KPi pH 7 buffer without (▪) and with 0.25 mM AmdiS (A) or 1 mM NEM (B) for 10 min in the presence (▴) or absence (•) of 1 mM L-glutamate. The treatment was stopped by the addition of 1 mM DTT. L-glutamate uptake was measured after extensive washing of the membranes to remove unlabeled L-glutamate and resuspension in 50 mM KPi pH 6 buffer.
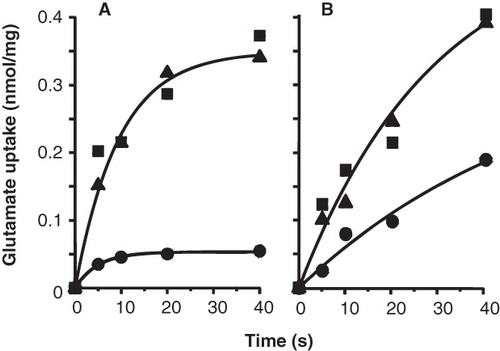
Full protection was also observed when NEM instead of AmdiS was used as the thiol reagent (not shown). NEM inhibited the N356C mutant catalyzed uptake activity by 62% when used at a concentration of 1 mM of NEM for 10 min, while no inactivation was observed in the presence of 1 mM L-glutamate (). In the BAD inserted variant of the N356C mutant a similar protection was observed with a concentration of 3 mM NEM (not shown).
The effect of the co-ion Na+ on the inactivation of the mutants was measured by treating the membranes with the thiol reagents in the presence of 10 mM Na+ and a saturating concentration of 100 mM of Na+ (K0.5 = 5.8 mM; ). For both mutants, the presence of Na+ had the opposite effect than the presence of L-glutamate discussed above. Under the conditions of the experiment and within experimental error, AmdiS completely inhibited the P351C mutant in the presence of Na+ and the same was observed for the inhibition of the N356C mutant by NEM treatment (, open bars).
Figure 6. Effect of the co-ion Na+ on the sensitvity of the P351C and N356C mutants to thiol reagents. RSO membranes containing GltS-P351C and BAD206-P351C (A) and GltS-N356C and BAD206-N356C mutants were treated with AmdiS or NEM in 50 mM KPi pH 7 buffer containing 0, 10 and 100 mM NaCl as indicated. GltS-P351C (open bars) and the BAD206-P351C (shaded bars) were treated with 0.25 mM AmdiS for 10 min. GltS-N356C (open bars) was treated with 1 mM NEM for 10 min and the BAD206-N356C (shaded bars) with 3 mM NEM for 20 min. Following the treatment, the membranes were washed and resuspended in 50 mM KPi pH 6 buffer containing 10 mM Na+. Bars indicate the L-glutamate uptake activity relative to a sample that was not treated with the thiol reagents.
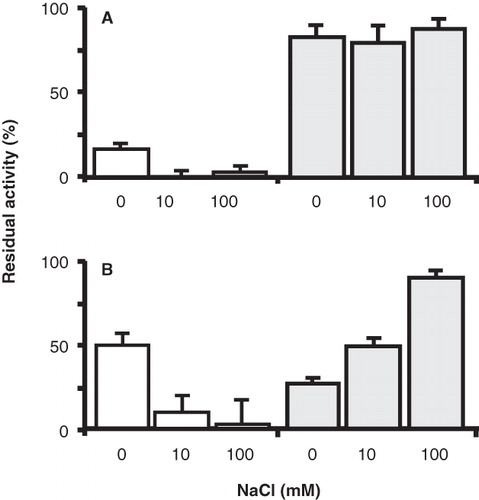
Apparently, the presence of Na+ increased the sensitivity to the thiol reagents. Insertion of the BAD domain in the P351C mutant rendered the mutant insensitive to inactivation, also in the presence of Na+. The residual activity was down to 80–90% under all conditions tested (, shaded bars). This level of inactivation is unspecific and due to a small inactivating effect on the pmf generating system by the treatment (Dobrowolski et al. Citation2007, Dobrowolski and Lolkema Citation2010). Treatment of the RSO membranes containing the BAD206-N356C mutant with 3 mM NEM for 20 min reduced the initial rate of uptake down to 26%. In contrast to what was observed with the N356C mutant, the presence of Na+ protected the insertion mutant against inactivation resulting in 48% and 92% residual activity in the presence of 10 and 100 mM of Na+, respectively (, shaded bars). It follows that where the presence of Na+ increases the reactivity of the thiol in the putative reentrant loop in the N356C mutant, the opposite effect was observed when the BAD domain is inserted in the central loop.
Accessibility of reentrant loop mutants P351C and N356C in whole cells
E. coli DH5α cells producing GltS and the reentrant loop mutants P351C and N356C were treated with the fluorescent thiol reagent fluorescein maleimide to detect thiols accessible at the outer cell surface. Following treatment with the label for 10 min in KPi pH 7 buffer, the proteins were purified from the cells and labeling was analyzed by fluorescence imaging of the gel after SDS-PAGE. The three GltS alleles showed more or less the same intensity after protein staining ().
Figure 7. Labeling of GltS and the GltS-P351C and GltS-N356C mutants by fluorescein maleimide in whole cells. A. Whole cells expressing GltS, GltS-P351C, and GltS-N356C were treated for 1 min with 0.1 mM fluorescein maleimide. B. Cells expressing GltS-P351C were treated for the indicated times with 0.25 mM AmdiS and in the presence or absence of 1 mM L-glutamate as indicated. Following quenching and removal of the excess thiol reagent, the cells were treated with fluorescein maleimide as described under A. Following the treatment, the transporter proteins were isolated from the cells and analyzed by SDS-PAGE. The lower panels (CBB) show the Coomasie Brilliant Blue stained protein bands, the top panels the fluorescence image of the same parts of the gels (FM).
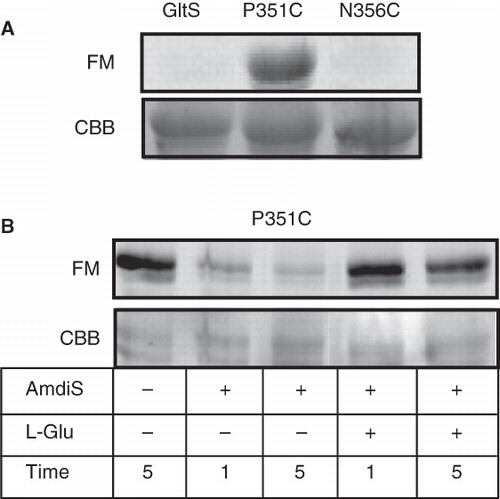
Wild type GltS showed no fluorescence indicating that under the conditions of the experiment none of the endogenous Cys residues were accessible for fluorescein maleimide added to the outside of the cells (ter Horst and Lolkema Citation2010). The same was observed for the N356C mutant showing that the Cys residue at the position of Asn356 was not accessible as well by this reagent. In contrast, the P351C protein was clearly fluorescent indicating that the Cys residue at the position of Pro351 was exposed at the external cell surface. Pretreatment of cells containing the P351C mutant with membrane impermeable AmdiS prevented subsequent labeling with the fluorescent label demonstrating that position 351 was also accessible for the bulky and charged AmdiS in whole cells (). The presence of 1 mM of L-glutamate during the treatment with AmdiS followed by washing of the cells to remove L-glutamate did result in fluorescent labeling indicating that the substrate protected against AmdiS labeling.
The results show the same behavior of GltS and the mutants P351C and N356C in whole cells and RSO membranes. Thiols of the wild type and N356C mutant are not accessible from the outside, in contrast to the thiol at position 351 of the P351C mutant. The latter is protected from labeling in the presence of glutamate.
Discussion
The two domains of the transporters in the different families of structural class ST[3] in the MemGen classification are connected by a large cytoplasmic loop that shows up as a typical dip in the hydropathy profile of the proteins as is seen for two-domain transporters in other structural classes as well. The GltS and CitS proteins as well as the H+/lactose symporter LacY, a major facilitator superfamily (MFS) transporter (class ST[1]), have been produced as active ‘split’ permeases consisting of the two domains as separate proteins which shows that a covalent connection between the domains is not essential (Bibi and Kaback Citation1990, Dobrowolski et al. Citation2010, Dobrowolski and Lolkema Citation2010). Nevertheless, in nature, such constructs are not observed, suggesting that a loop linking the two domains did have some advantage during evolution. This is in contrast to, for instance, ABC transporters or phosphoenolpyruvate dependent phosphotransferase system transporters where the functional units consist of either a single polypeptide containing multiple domains or a complex built from different subunits. In some families of class ST[3] secondary transporters, the connecting loop is extended and probably folds into a cytoplasmic domain, suggesting a possible function of the loop in the activity of the transporter (Lolkema et al. Citation2005, Barabote et al. Citation2006, Nanatani et al. Citation2007). In a previous study we noticed that insertion of the 10 kDa biotin acceptor domain of K. pneumoniae in the central cytoplasmic loop of GltS, while not significantly affecting the uptake activity in RSO membranes under the conditions employed, seemed to result in less of the hybrid protein in the membranes (Krupnik et al. 2011). A detailed analysis presented here, taking into account different effects that might contribute to the observed uptake rates showed that the maximal rate catalyzed by the GltS-BAD206 transporter protein was a factor of 2.7 higher than observed for GltS while the kinetic affinities for L-glutamate and the Na+-ions were unaltered. This suggests that the wild type transporter protein may not be in the ‘optimal’ conformation when embedded in the membrane of isolated RSO membrane vesicles. It also suggests that changes in conformation of the central loop may be used to modulate the activity of the transporter thereby providing a post-translational regulatory mechanism for glutamate uptake activity to the cell. Possible effectors might be cytoplasmic pH affecting the conformation of the central loop or a cellular metabolite that binds to the loop.
The structural model of GltS shows putative reentrant loops in the N- and C-terminal domains () that are believed to form (part of) the translocation site in the 3D structure. Close vicinity of the two reentrant loops was demonstrated by cross-linking studies (Dobrowolski et al. Citation2010). The present data demonstrates that this part of the protein is likely to be involved in the substrate and co-ion binding pocket and supports the involvement of the reentrant loops in catalysis. Cysteine scanning mutagenesis of the putative reentrant loop region in the C-domain of GltS identified only position 351 in the P351C mutant being accessible to membrane impermeable AmdiS suggesting that the residue at this position would be the one most exposed to the periplasm and, therefore, be at the vertex of the reentrant loop (Dobrowolski et al. Citation2007). Consequently, Asn356 would be in the ingoing stretch, inside the pore (see ). The relative reactivity of the P351C and N356C mutants with thiol reagents reported here are consistent with this view. The reactivity of the P351C mutant with NEM was found to be at least one order of magnitude higher than observed for the N356C mutant, while the latter does not react with AmdiS at all. The observations are in line with a lower accessibility of the cysteine residue at position 356. The accessibility of the cysteines at the position of Pro351 and Asn356 was strongly reduced in the presence of a saturating concentration of L-glutamate and enhanced in the presence of the co-ion. The effect of the presence of L-glutamate and Na+ on the reactivity of the two mutants was similar suggesting that for both membrane impermeable AmdiS and membrane permeable NEM the access pathway to the cysteine residues was from the periplasmic side of the membrane. Binding of L-glutamate or Na+ added at the periplasmic side recruits the transporter molecules to the outward-facing conformation. Protection by L-glutamate most likely arises from steric hindrance in the access pathway or from direct involvement of the target residue in substrate binding. In this respect it may be noted that both Pro351 and Asn356 are highly conserved residues in the ESS family. Enhancement of the reactivity in the presence of Na+ would be a direct effect of locking the transporters in the outward-facing conformation without the need of recruiting (part of) the proteins from the inward-facing conformation during inactivation. Because of its small size, the Na+ ions are unlikely to restrict the access pathway.
The change in turnover rate of GltS by the insertion of the BAD domain in the loop that connects the two domains parallels changes in the accessibility of residues in the cytoplasmic reentrant loop in the C-domain that may shed some light on the mechanism of the activation. Insertion of the domain in the central loop reduces the accessibility of positions 351 and 356 at the periplasmic side of the membrane. The P351C insertion mutant appears to be insensitive to AmdiS as well as NEM, irrespective of the catalytic state of the transporter ( and ). The N356 insertion mutant showed a reduced reactivity with NEM, but at higher concentrations of the thiol reagent, inactivation was clearly observed (). Importantly, binding of Na+ protected the N356C-BAD206 mutant against NEM, while it enhanced the inactivation of N356C strongly suggesting different access pathways. If the enhanced reactivity of N356C is explained by recruitment in the outward facing state (see above), it follows that the N356C-BAD206 mutant reacts in the inward facing conformation with membrane permeable NEM coming from the cytoplasm. Summarizing, the dominant effect of the insertion of the BAD domain is a strong restriction of the access pathway to positions 351 and 356 in the reentrant loop for thiol reagents from the periplasmic side of the membrane. At the same time, position 356, but not position 351, appears to become more accessible from the cytoplasmic side. The equilibrium of the transition between the inward and outward facing conformations appears to be shifted to former conformation.
Conclusion
The Na+-glutamate transporter GltS is believed to consist of two homologous domains that are connected by a large hydrophilic cytoplasmic loop. The translocation site is believed to be formed at the interface of the two domains where two reentrant loops interact. Cysteine residues engineered in the reentrant loop in the C-terminal domain that enters the membrane from the cytoplasmic face of the membrane are accessible from the periplasm and their reactivity is modulated by the presence of substrate and co-ion. Insertion of the 10 kDa BAD polypeptide in the loop that connects the two domains in the cytoplasm blocks the accessibility of the cysteine residues in the reentrant loop from the periplasm. Kinetically, the insertion results in an increase of the maximal rate of transport by a factor of 2.7. It follows that changes in the connecting loop affect turnover and may have a function in regulation of transporter activity.
Acknowledgements
This work was supported by a grant from the Dutch Organization for Scientific Research (NWO-CW).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Barabote RD, Tamang DG, Abeywardena SN, Fallah NS, Fu JY, Lio JK, Mirhosseini P, Pezeshk R, Podell S, Salampessy ML, Thever MD, Saier MH Jr. 2006. Extra domains in secondary transport carriers and channel proteins. Biochim Biophys Acta 1758:1557–1579.
- Bibi E, Kaback HR. 1990. In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc Natl Acad Sci USA 87:4325–4329.
- Deguchi Y, Yamato I, Anraku Y. 1989. Molecular cloning of gltS and gltP, which encode glutamate carriers of Escherichia coli B. J Bacteriol 171:1314–1319.
- Dobrowolski A, Sobczak-Elbourne I, Lolkema JS. 2007. Membrane topology prediction by hydropathy profile alignment: membrane topology of the Na(+)-glutamate transporter GltS. Biochemistry 46:2326–2332.
- Dobrowolski A, Lolkema JS. 2009. Functional importance of GGXG sequence motifs in putative reentrant loops of 2HCT and ESS transport proteins. Biochemistry 48:7448–7456.
- Dobrowolski A, Lolkema JS. 2010. Evolution of antiparallel two-domain membrane proteins. Swapping domains in the glutamate transporter GltS. Biochemistry 49:5972–5974.
- Dobrowolski A, Fusetti F, Lolkema JS. 2010. Cross-linking of trans reentrant loops in the Na+-citrate transporter CitS of Klebsiella pneumoniae. Biochemistry 49:4509–4515. Erratum: Biochemistry 49:10349 2010.
- Forrest LR, Krämer R, Ziegler C. 2011. The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807:167–188.
- Kaback HR. 1974. Transport in isolated bacterial membrane vesicles. Methods Enzymol 31:698–709.
- Kalman M, Gentry DR, Cashel M. 1991. Characterization of the Escherichia coli K12 gltS glutamate permease gene. Mol Gen Genet 225:379–386.
- Konings WN, Barnes EN Jr, Kaback HR. 1971. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of β-galactosides and amino acids. J Biol Chem 246:5857–5861.
- Krupnik T, Dobrowolski A, Lolkema JS. 2011. Cross-linking of dimeric CitS and GltS transport proteins. Mol Membr Biol 28:337–347.
- Lolkema JS, Slotboom DJ. 1998. Estimation of structural similarity of membrane proteins by hydropathy profile alignment. Mol Membr Biol 15:33–42.
- Lolkema JS, Slotboom DJ. 2003. Classification of 29 families of secondary transport proteins into a single structural class using hydropathy profile analysis. J Mol Biol 327:901–909.
- Lolkema JS, Sobczak I, Slotboom DJ. 2005. Secondary transporters of the 2HCT family contain two homologous domains with inverted membrane topology and trans re-entrant loops. FEBS J 272:2334–2344.
- Lolkema JS. 2006. Domain structure and pore-loops in the 2-hydroxycarboxylate transporter family. J Mol Microbiol Biotechnol 11:318–325.
- Mościcka KB, Krupnik T, Boekema EJ, Lolkema JS. 2009. Projection structure by single-particle electron microscopy of secondary transport proteins GltT, CitS, and GltS. Biochemistry 48:6618–6623.
- Nanatani K, Fujiki T, Kanou K, Takeda-Shitaka M, Umeyama H, Ye L, Wang X, Nakajima T, Uchida T, Maloney PC, Abe K. 2007. Topology of AspT, the aspartate:alanine antiporter of Tetragenococcus halophilus, determined by site-directed fluorescence labeling. J Bacteriol 189:7089–7097.
- Saier MH Jr. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol Mol Rev 64:354–411.
- Schellenberg GD, Furlong CE. 1977. Resolution of the multiplicity of the glutamate and aspartate transport systems of Escherichia coli. J Biol Chem 252:9055–9064.
- Slotboom DJ, Konings WN, Lolkema JS. 1999. Structural features of the glutamate transporter family. Microbiol Molec Biol Rev 63:293–307.
- Sobczak I, Lolkema JS. 2003. Accessibility of cysteine residues in a cytoplasmic loop of CitS of Klebsiella pneumoniae is controlled by the catalytic state of the transporter. Biochemistry 42:9789–9796.
- Sobczak I, Lolkema JS. 2004. Alternating access and a pore-loop structure in the Na+-citrate transporter CitS of Klebsiella pneumoniae. J Biol Chem 279:31113–31120.
- Sobczak I, Lolkema JS. 2005a. The 2-hydroxycarboxylate transporter family: Physiology, structure, and mechanism. Microbiol Molec Biol Rev 69:665–695.
- Sobczak I, Lolkema JS. 2005b. Structural and mechanistic diversity of secondary transporters. Curr Opin Microbiol 8:161–167.
- ter Horst R, Lolkema JS. 2010. Rapid screening of membrane topology of secondary transport proteins. Biochim Biophys Acta 1798:672–680.
- Tolner B, Ubbink-Kok T, Poolman B, Konings WN. 1995. Cation-selectivity of the L-glutamate transporters of Escherichia coli, Bacillus stearothermophilus and Bacillus caldotenax: Dependence on the environment in which the proteins are expressed. Mol Microbiol 18:123–133.
- Wallace B, Yang YJ, Hong JS, Lum D. 1990. Cloning and sequencing of a gene encoding a glutamate and aspartate carrier of Escherichia coli K-12. J Bacteriol 172:3214–3220.
