Abstract
We have shown previously that in T cells, LAT co-immunoprecipitates with the active but not the inactive-‘closed’ form of Lck, and that this interaction impacts negatively on Lck activity. Here we confirm that activation of T cells induced a transient LAT/Lck association within 4 min after stimulation, returning to basal levels by 30 min. Interestingly, autoimmune T cells isolated from patients with systemic lupus erythematosus, which contain a larger pool of active Lck and LAT, exhibited increased LAT/Lck association compared to healthy controls. To identify the domain of LAT responsible for its interaction with active Lck, a series of LAT truncation mutants were constructed and tested in co-immunoprecipitation experiments. We found that the segment comprising residues 112–126 of human LAT is required for its interaction with Lck. This domain is rich in negatively charged amino acids and is conserved among different species. Therefore, in addition to the conserved tyrosines, the 112–126 domain identified here could be important for certain functions of LAT in T cells.
Introduction
Engagement of the T cell antigen receptor (TCR) initiates intracellular signalling that leads to T cell activation and development of effector functions (reviewed in Smith-Garvin et al. Citation2009). The Src family tyrosine kinase Lck is required for the transduction of signals from the TCR (Salmond et al. Citation2009). Upon antigenic stimulation, Lck phosphorylates defined motifs present within subunits of the TCR, called Immunoreceptor Tyrosine-based Activation Motifs (ITAMs). Following their phosphorylation, ITAMs recruit the cytosolic tyrosine kinase ZAP-70, which in turn phosphorylates the transmembrane adaptor protein LAT at multiple tyrosine residues (Zhang et al. Citation1998). Phosphorylated LAT nucleates protein complexes at the plasma membrane forming the point of origin for multiple intracellular signalling cascades culminating in gene transcription. Signalling proteins participating in LAT-induced complexes include the enzymes phospholypase Cγ1 (PLCγ1) and phosphoinositide-3 kinase (PI3K), and adaptor proteins Grb2, Gads and SLP-76 (Zhang et al. Citation2000, Sommers et al. Citation2004, Acuto et al. Citation2008). Therefore, both Lck and LAT have critical roles during T cell activation and it is important to fully understand the mechanisms that regulate their action.
Lck can adopt an ‘open’-active or ‘closed’-inactive configuration, which is largely regulated by the phosphorylation-dephosphorylation cycle of a tyrosine residue close to the C-terminus of the protein (Y505 in the mouse protein). Phosphorylation of this residue induces the ‘closed’ conformation while its dephosphorylation removes structural constraints and primes Lck for action. This structure of Lck is modelled according to the crystal structure reported for the Hck member of the Src family kinases (Sicheri and Kuriyan Citation1997). In accordance with this model, the Lck Y505F point mutant is constitutively active presumably because it is locked in the extended configuration. However, no discernible changes in the level of 505Y dephosphorylation have been observed in TCR-stimulated T cells. Recent reports show that changes in the localization of an existing pool of active Lck might initiate TCR signalling upon antigenic challenge (Paster et al. Citation2009, Dong et al. Citation2010, Nika et al. Citation2010). Previously, we have shown that endogenous LAT co-immunoprecipitated with Y505F-Lck from transfected Jurkat cells (Kabouridis, Citation2003). Interestingly, the Y505F-Lck/LAT interaction was detectable in the detergent-resistant fraction but not in the detergent-soluble fraction. Interaction of LAT with the active form of Lck could point to an important, as yet uncharacterized, step in the process of TCR signalling and may play a significant role in T cell biology.
TCR stimulation was shown to lead to co-precipitation of Lck with LAT (Dong et al. Citation2010). Here we confirm this finding and further demonstrate that T cells isolated from patients suffering from the autoimmune disease systemic lupus erythematosus (SLE) show increased association of LAT with Lck as compared to healthy controls, most likely due to the larger pool of active Lck and LAT as we reported previously (Jury et al. Citation2004). Furthermore, we map the region of LAT which mediates its interaction with active Lck to a species-conserved domain rich in negatively charged amino acids.
Methods
Cells lines and T cell isolation
Jurkat T cells (clone E6.1) were cultured in RPMI-1640 medium supplemented with 5% foetal calf serum (FCS), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. COS-7 cells were cultured in DMEM (Dulbecco's modified Eagle's medium) containing 10% FCS and antibiotics as above. T cells from four patients with SLE and four healthy volunteers were obtained as described before (Jury et al. Citation2003). Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from approximately 30 ml of heparinized venous blood following centrifugation over Ficoll-Hypaque (Pharmacia Biotech, Buckinghamshire, UK). Purified CD3+ T cells were negatively selected using magnetic beads (Miltenyi Biotech, Surry, UK). The purity of T lymphocyte preparations was consistently above 97% as assessed by flow cytometry. Informed consent was obtained from patients and healthy donors before recruitment. The Ethics Committee of University College London Hospitals NHS Trust approved the study.
Antibodies and other reagents
Anti-Lck mAb (clone 3A5) and polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), anti-V5 mAb from Invitrogen (Carlsbad, CA, USA), anti-GFP from Roche, and anti-LAT polyclonal, either unconjugated or conjugated to horseradish peroxidase (HRP), from Upstate Biotechnology (Lake Placid, NY, USA). Beads coated with anti-CD3/CD28 antibodies were from Invitrogen (Carlsbad, CA). Methyl-β-cyclodextrin (MβCD) and phytohaemaglutinin (PHA) were purchased from Sigma-Aldrich (Dorset, UK).
T cell stimulation
Jurkat T cells were stimulated with 5 μg/ml PHA or anti-CD3/CD28 coated beads for the times indicated, after which cells were lysed and Lck immunoprecipitated. 5 × 106 purified human T cells from healthy volunteers or SLE patients were left unstimulated or stimulated with anti-CD3/CD28-coated beads (Invitrogen) for 10 min after which cells were lysed and Lck was immunoprecipitated.
In vitro transcription/translation
Cell-free protein expression was achieved with the TNT® T7 Quick coupled transcription/translation kit from Promega (Madison, WI, USA). This system allows for eukaryotic protein expression from circular plasmid in which the gene of interest is cloned downstream of the T7 RNA polymerase promoter. The system can be used to probe for protein-protein interactions of co-expressed proteins in co-immunoprecipitation experiments. LAT and Lck-Y505F proteins were expressed in a single reaction from their corresponding plasmids. A small aliquot of the reaction was tested for successful expression of the two proteins by Western blotting while the reminder was divided into two equal portions that were used for immunoprecipitations with anti-Lck and anti-LAT antibodies, respectively.
Immunoprecipitation and Western blotting
Cells were disrupted in ice-cold RIPA lysis buffer (150 mM NaCl, 50 mM Tris pH 8.0, 1% NP-40, 0.5% deoxycholic acid and 0.1% SDS) containing protease inhibitors (5 μg/ml of each Chymostatin, Pepstatin A, Leupeptin, Aprotinin and 1 mM of AEBSF hydrochloride, all from Calbiochem, La Jolla, CA, USA) and phosphatase inhibitors (10 mM of sodium orthovanadate from Sigma-Aldrich, Dorset, UK and 0.3 μM okadaic acid from Calbiochem) plus 20 mM MβCD to disrupt detergent-insoluble microdomains. Pre-cleared lysates were used for anti-Lck immunoprecipitations. Immune complexes or total cell lysates were resolved by 10% SDS-PAGE, transferred onto PVDF membrane and immunoblotted with the indicated antibodies. Westerns were developed with enhanced chemi-luminescence (ECL) reagents.
Construction of C-terminal truncation mutants of LAT
The human LAT cDNA (a kind gift from L. Samelson, NIH) was used as template for PCR amplification to generate C-terminally truncated products. The truncated constructs were digested with the KpnI/XhoI restriction enzymes and ligated to the corresponding sites of the pcDNA6/V5-His(B) vector (Invitrogen, Carslbad, CA) in frame with the V5 and 6XHis tags. The oligonucleotide 5′-GAGGTACCATGGAGGAGGCCATCCTGGTC-3′ was used as a forward primer for all the PCR reactions. The reverse primers were as follows:
Full-length (FL)-V5-His 5′-GTCCTCGAGACGTTCAGCTCCTGCAGATT-3′;
169-V5-His 5′-GTCCTCGAGACAATGGACTCCATGGAGAA-3′;
136-V5-His 5′-GTCCTCGAGACAAGCACCACCAGGTAGCC-3′;
129-V5-His 5′-GTCCTCGAGACGTTGTGATAGTCGTCCTC-3′;
Y127F-V5-His 5′-GTCCTCGAGACGTTGTGAAAGTCGTCCTC-3′;
126-V5-His 5′-GTCCTCGAGACGTCCTCCTCATCCTCATC-3′;
112-V5-His 5′-GTCCTCGAGACGTTCTCGTAGCTCGCCAC-3′;
103-V5-His 5′-GTCCTCGAGTCAACCATCAGAATCCCGCCG-3′.
The LAT-GFP chimera was generated by amplifying full length LAT with PCR using the forward primer 5′-GAGGTACCATGGAGGAGGCCATCCTGGTC-3′ and the reverse primer 5′-CTCCCGGGGTTCAGCTCCTGCAGATTCTC-3′. The PCR product was digested with the KpnI/XmaI restriction enzymes and subcloned in frame into the corresponding sites of the pEGFP-N2 vector (BD Biosciences, Palo Alto, CA, USA), which incorporates the EGFP gene downstream of the multiple cloning site. Construction of the Y505F-Lck expression vector used for co-transfections has been described before (Jury et al. Citation2003).
Transient cell transfection
COS-7 cells grown to approximately 75% confluence were collected by trypsinization, washed and 5 × 106 cells and resuspended in 300 μl of DMEM medium without FCS. 2 μg of each of Y505F-Lck or the indicated LAT expression construct were added and cells were electroporated in a 4 mm gap cuvette (BioRad, Hercules, CA, USA) at 270V/960mF after which they were cultured in 10% FCS-containing medium for 48 h before harvest.
Results
The association of LAT with Lck is induced by T cell activation
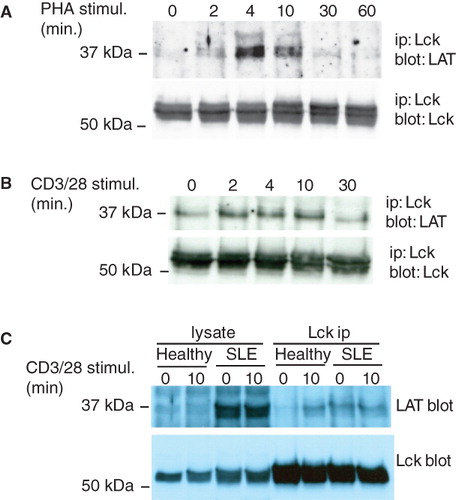
We have previously reported that LAT co-immunoprecipitates with the Y505F active mutant of Lck from transfected Jurkat T cells (Kabouridis Citation2003). To investigate whether this association is regulated by cell activation, Jurkat T cells were stimulated with PHA for different times and Lck was immunoprecipitated. Western blot analysis of the immunoprecipitates showed that the association of LAT with Lck is induced by cell activation (). This result confirms a previous report by Dong et al (Citation2010). Interaction was transient peaking at approximately 4 min and returning to basal levels by 30 min (). Similarly, a transient increase in the level of LAT co-immunoprecipitating with Lck was seen when Jurkat T cells were stimulated with anti-CD3/CD28 antibodies ().
Figure 1. LAT associates with the active form of Lck. (A) Jurkat T cells (107) were stimulated with 5 μg/ml PHA for the times indicated and Lck was immunoprecipitated. Immune complexes were analyzed with anti-LAT (upper panel) and anti-Lck (lower panel) antibodies. (B) Lck immunoprecipitations from Jurkat T cells stimulated with beads coated with anti-CD3/CD28 antibodies for the indicated times. (C) 5 × 106 purified T cells from a healthy volunteer and a patient with active SLE were left unstimulated or stimulated with anti-CD3/CD28. Immunoprecipitated Lck and associated LAT were detected with specific antibodies. The experiment shown is one out of three performed with T cells from different donors with similar result.
Autoimmune T cells with a higher content of active Lck show increased level of LAT/Lck complexes
We have previously shown that T cells from SLE patients with active disease contain increased levels of active Lck which also differs in its localization compared to healthy T cells, even though they do not significantly differ with regard to activation or memory marker expression (Jury et al. Citation2003, Citation2004). To investigate the interaction of LAT with Lck in primary cells, CD3+ T lymphocytes from healthy volunteers and patients with SLE were left unstimulated or stimulated with anti-CD3/CD28 and Lck was immunoprecipitated. Low levels of LAT co-immunoprecipitated with Lck from non-stimulated healthy T cells, which however were increased following anti-CD3/CD28 stimulation (). In contrast, the amount of LAT co-immunoprecipitated with Lck from non-stimulated SLE T cells was substantially higher compared to the healthy controls, and did not increase after in vitro stimulation (). T cells from two additional lupus patients also showed higher levels of LAT associating with Lck in the absence of in vitro stimulation. We found higher expression of LAT in active SLE T cells, confirming a previous report (Januchowski et al. Citation2008), although the significance of this is unclear at present. This result is further evidence demonstrating that conditions and stimuli that result in higher levels or redistribution of active Lck bring about its association with LAT.
A negatively charged domain of LAT mediates its interaction with active Lck
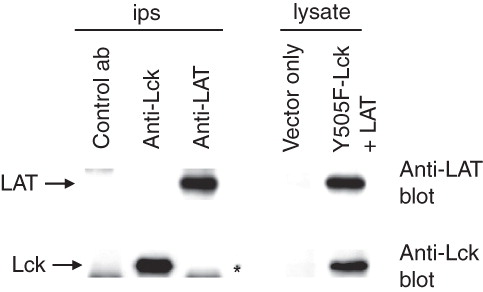
To investigate whether the interaction of LAT with the active form of Lck is direct, a coupled in vitro transcription/translation system was employed to generate LAT and Y505F-Lck proteins. Both proteins were successfully produced (, lysate samples). However, no LAT was detected in anti-Lck immunoprecipitates and similarly no Lck was detected in anti-LAT immunoprecipitates (, ip samples) raising the possibility that the interaction between LAT and Lck in cells is indirect, bridged by intervening proteins.
Figure 2. In vitro produced LAT and Y505F-Lck do not interact. LAT and Y505F-Lck proteins were produced in an in vitro transcription/translation system and successful production was established by Western blotting (lysate). Aliquots of the mix were used for Lck and LAT immonoprecipitations and immune complexes were analysed for the presence of associated proteins (ips). The star in the immunoprecipitations panel indicates the migration distance of the heavy chain of the immunoprecipitating antibody.
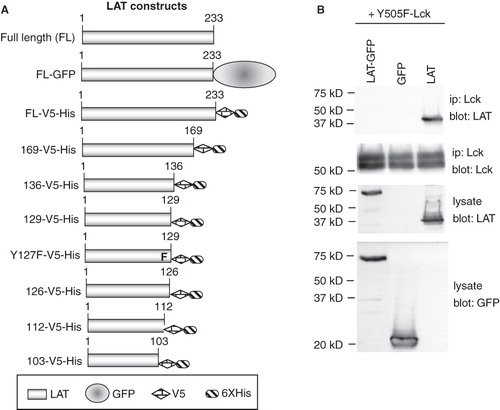
In light of the above result, we proceeded to dissect the LAT/Lck interaction by co-expression in COS cells. The association is preserved in COS cells as we reported previously (Kabouridis Citation2003) and because they are easily transfectable they are a convenient system for testing large numbers of mutants. To characterize the area of LAT responsible for its association with the active form of Lck, we generated a series of LAT mutants incrementally truncated at the C-terminus (). To safeguard against possible loss in reactivity with anti-LAT antibodies, the truncation mutants were fused either to GFP or to V5 and 6xHis tags. Nevertheless, all truncation mutants reacted with the commercially available anti-LAT polyclonal antibodies (from Upstate Biotechnology) and therefore all the immunoblots shown are with these antibodies. Initially we generated LAT mutants fused to GFP. As controls we first generated the full length LAT-GFP chimera and tested it for interaction with Y505F-Lck. Wild type LAT and GFP expressing plasmids were used as positive and negative controls, respectively. The LAT-GFP chimera completely failed to interact with Y505F-Lck, while wt-LAT reacted as expected (). Since the GFP protein, presumably due to its large size, interferes with the LAT/Lck association, we resorted to adding the smaller V5 and 6xHis epitopes of the pcDNA6 vector to the C-terminus of the truncated mutants ().
Figure 3. Generation of LAT mutants. (A) Schematic representation of the LAT mutants generated for this study. (B) COS cells were transfected with the indicated plasmids and potential association of expressed proteins was assessed in co-immunoprecipitation experiments. Equivalent expression of proteins was determined by Western blotting of cell lysate aliquots.
Initially, three V5-His-tagged mutants were constructed terminating at residues 169, 136 and 103 respectively. Co-transfection with Lck-Y505F showed that similarly to full length FL-LAT-V5-His, 169-V5-His and 136-V5-His mutants co-immunoprecipitated with Y505F-Lck (). In contrast, 103-V5-His failed to co-immunoprecipitate although it was expressed equally well in transfected cells (). For reasons that are not clear at present, transfection of the FL-LAT-V5-His plasmid produces two discernable bands, only the larger of which reacted with anti-tag antibodies (data not shown). To narrow down on the area of interest, two additional truncated constructs, 129-V5-His and 112-V5-His were tested. 129-V5-His co-immunoprecipitated with Y505F-Lck, although less efficiently compared to 136-V5-His, while 112-V5-His did not (). Therefore, the domain of LAT responsible for its interaction with Y505F-Lck resides between residues 112 and 129. Conserved residues in this area of LAT among different species are a string of acidic amino acids and tyrosine 127. To investigate the importance of Y127, two additional mutants were generated; a truncation at 126 (126-V5-His) and a point mutant of Y127F-V5-His (terminates at residue 129). Both mutants co-immunoprecipitated with Y505F-Lck demonstrating that Y127 is not involved (). A summary of the results from the co-immunoprecipitation experiments is shown in .
Figure 4. A string of acidic residues mediate the interaction of LAT with active Lck. (A), (B), and (C) COS cells were singly transfected or co-transfected with the indicated LAT mutants and Y505F-Lck. Comparable expression of proteins was assessed by Western blotting equivalent aliquots of cell lysates with anti-Lck and anti-LAT antibodies (lysate). Association of the various LAT mutants with Y505F-Lck was determined by Western blotting Lck immunoprecipitations with anti-LAT antibodies (Lck ip). The levels of immunoprecipitated Lck from each sample were determined by immunoblotting the same membrane with anti-Lck antibodies.
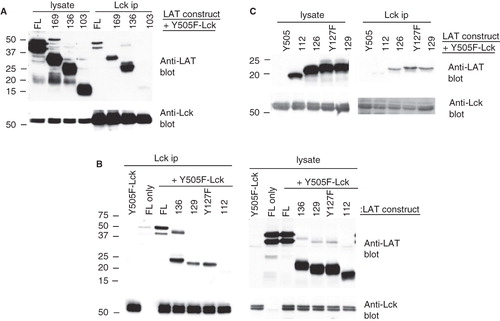
Table I. Summary of LAT mutant interaction with Y505F Lck.
Discussion
The results presented show that an area rich in acidic amino acids between residues 112 and 126 of LAT is important for its interaction with the active form of Lck. Curiously, the 136 truncation mutant seems to co-immunoprecipitate more efficiently with Y505F-Lck compared to FL-LAT. It is possible that in this truncation the acidic domain is better exposed leading to stronger association with the co-expressed Y505F-Lck. Although the mechanism behind this association is unknown at present, it is conceivable that adoption of the extended conformation by active Lck exposes a cryptic domain which attracts other protein(s) that bridge active Lck with LAT. Alternatively, active Lck could phosphorylate a downstream target(s) which then mediates its association with LAT in a larger protein complex. Comparison of the various LAT sequences deposited in NCBI databanks shows that the negatively charged amino acids between residues 112 and 126 are conserved between species (). Interestingly, alternative transcripts have been reported (NCBI) for human, Callithrix jacchus (a primate) and Bos Taurus (cow), which contain an additional exon that interrupts the continuity of this segment although it is not known whether alternatively spliced LAT retains its ability to interact with active Lck.
Table II. Amino acid sequence of the corresponding LAT domains from various species deposited in the NCBI databank. Negatively charged amino acids are in bold.
The critical importance of conserved tyrosine residues (Y226, Y191, Y171, and Y132) in LAT for communicating signals from the TCR is well established (Zhang et al. Citation1998). However, apart from these tyrosines, the potential functions of other conserved areas of LAT remain largely unexplored. It has been reported that a juxtamembrane region (amino acids 32–104) has a role in the partition of a pool of LAT to intracellular compartments rather than to the plasma membrane (Bonello et al. Citation2004), while the section of LAT downstream from residue 104 contains signals for the ubiquitination and degradation of the protein (Brignatz et al. Citation2005). Recently, the conserved lysine residue at position 54 was found to be a target for poly-ubiquitination and its substitution produced a LAT mutant that enhanced T cell activation (Balagopalan et al. Citation2011). We previously proposed that the interaction of LAT with the active form of Lck in the detergent-resistant fraction of Jurkat T cells negatively regulates the activity of Lck (Kabouridis Citation2003, Kabouridis and Jury Citation2008). Here we mapped the region of LAT responsible for this interaction to the negatively charged domain 112–126. Negatively charged domains often regulate trafficking of proteins to appropriate sorting complexes. For example phosphofurin acidic cluster sorting protein-1 or -2 (PACS-1 and -2) are two chaperones recently identified to interact with acidic domains in certain proteins and to regulate their endocytosis and trafficking (Youker et al. Citation2009). Co-localization of PACS-1 with LAT has been reported in Jurkat T cells (Brignatz et al. Citation2005). One possibility is that LAT and active Lck are part of a larger protein complex the role of which could be to maintain cell homeostasis by regulating their endocytosis/recycling from the plasma membrane. Such interaction to occur might require the localization of LAT and Lck into appropriate membrane compartments. Indeed, we have shown that the LAT/active Lck interaction is detected only in detergent-resistant microdomains, and a recent publication has shown that TCR stimulation does not increase the levels of active Lck per se, but rather T cell activation depends on an existing constitutively active pool of Lck, the localization of which might change at the plasma membrane following TCR triggering (Nika et al. Citation2010). Furthermore, the association and co-dependence between LAT and Lck during T cell activation is highlighted by another report that investigated low-grade TCR stimulation (Dong et al. Citation2010).
A homeostatic role for LAT has been proposed based on the phenotype of mice transgenic for a point mutant of LAT; tyrosine 132 which recruits PLCγ1 (Aguado et al. Citation2002, Sommers et al. Citation2002). These mice developed a Th2-like lymphoproliferative disorder. Recently, complete absence of LAT in mature T cells was reported to induce a similar phenotype (Mingueneau et al. Citation2009). Collectively these data suggest that absent or defective LAT compromises a negative regulatory mechanism which is important for T cell homeostasis (Roncagalli et al. Citation2010). Further reports have provided additional evidence arguing for the participation of LAT in negative regulatory signalling in T cells (Matsuda et al. Citation2004, Dong et al. Citation2006) and mast cells (Malbec et al. Citation2004). Whether the active Lck interaction with LAT is important for this homeostatic control is not known and it will be an important future investigation.
Conclusion
Here we confirm that the active form of Lck transiently and rapidly associates with LAT upon TCR stimulation and subsequently quickly returns to basal levels. We identify that a conserved segment of human LAT (residues 112–126) is required for this interaction suggesting it could be important for certain functions of LAT in T cells. Interestingly, SLE T cells, which contain a larger pool of active Lck and LAT (Jury et al. Citation2004), exhibited increased LAT/Lck association compared to healthy controls. Thus, these results point to an important, as yet uncharacterized, step in the process of TCR signalling and/or T cell homeostasis that is worthy of further exploration.
Acknowledgements
This work was supported by an Arthritis Research UK Career Development Award (18106) to ECJ and an Arthritis Research UK grant (16018) to PSK.
Declaration of interest : The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Acuto O, Di Bartolo V, Michel F. 2008. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol 8:699–712.
- Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura AM, Richelme M, Guo XJ, Sainty D, He HT, Malissen B, Malissen M. 2002. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science 296:2036–2040.
- Balagopalan L, Ashwell BA, Bernot KM, Akpan IO, Quasba N, Barr VA, Samelson LE. 2011. Enhanced T-cell signaling in cells bearing linker for activation of T-cell (LAT) molecules resistant to ubiquitylation. Proc Natl Acad Sci USA 108:2885–2890.
- Bonello G, Blanchard N, Montoya MC, Aguado E, Langlet C, He HT, Nunez-Cruz S, Malissen M, Sanchez-Madrid F, Olive D, Hivroz C, Collette Y. 2004. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J Cell Sci 117:1009–1016.
- Brignatz C, Restouin A, Bonello G, Olive D, Collette Y. 2005. Evidences for ubiquitination and intracellular trafficking of LAT, the linker of activated T cells. Biochim Biophys Acta 1746:108–115.
- Dong S, Corre B, Foulon E, Dufour E, Veillette A, Acuto O, Michel F. 2006. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J Exp Med 203:2509–2518.
- Dong S, Corre B, Nika K, Pellegrini S, Michel F. 2010. T cell receptor signal initiation induced by low-grade stimulation requires the cooperation of LAT in human T cells. PLoS One 5:e15114.
- Januchowski R, Wudarski M, Chwalinska-Sadowska H, Jagodzinski PP. 2008. Prevalence of ZAP-70, LAT, SLP-76, and DNA methyltransferase 1 expression in CD4+ T cells of patients with systemic lupus erythematosus. Clin Rheumatol 27:21–27.
- Jury EC, Kabouridis PS, Abba A, Mageed RA, Isenberg DA. 2003. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum 48:1343–1354.
- Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. 2004. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest 113:1176–1187.
- Kabouridis PS. 2003. Selective interaction of LAT (linker of activated T cells) with the open-active form of Lck in lipid rafts reveals a new mechanism for the regulation of Lck in T cells. Biochem J 371:907–915.
- Kabouridis PS, Jury EC. 2008. Lipid rafts and T-lymphocyte function: implications for autoimmunity. FEBS Lett 582:3711–3718.
- Malbec O, Malissen M, Isnardi I, Lesourne R, Mura AM, Fridman WH, Malissen B, Daeron M. 2004. Linker for activation of T cells integrates positive and negative signaling in mast cells. J Immunol 173:5086–5094.
- Matsuda S, Miwa Y, Hirata Y, Minowa A, Tanaka J, Nishida E, Koyasu S. 2004. Negative feedback loop in T-cell activation through MAPK-catalyzed threonine phosphorylation of LAT. EMBO J 23:2577–2585.
- Mingueneau M, Roncagalli R, Gregoire C, Kissenpfennig A, Miazek A, Archambaud C, Wang Y, Perrin P, Bertosio E, Sansoni A, Richelme S, Locksley RM, Aguado E, Malissen M, Malissen B. 2009. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity 31:197–208.
- Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Hofer T, Viola A, Acuto O. 2010. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32:766–777.
- Paster W, Paar C, Eckerstorfer P, Jakober A, Drbal K, Schutz GJ, Sonnleitner A, Stockinger H. 2009. Genetically encoded Forster resonance energy transfer sensors for the conformation of the Src family kinase Lck. J Immunol 182:2160–2167.
- Roncagalli R, Mingueneau M, Gregoire C, Malissen M, Malissen B. 2010. LAT signaling pathology: An ‘autoimmune’ condition without T cell self-reactivity. Trends Immunol 31:253–259.
- Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. 2009. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev 228:9–22.
- Sicheri F, Kuriyan J. 1997. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol 7:777–785.
- Smith-Garvin JE, Koretzky GA, Jordan MS. 2009. T cell activation. Annu Rev Immunol 27:591–619.
- Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacana E, Menon RK, Shores EW, Samelson LE, Love PE. 2002. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science 296:2040–2043.
- Sommers CL, Samelson LE, Love PE. 2004. LAT: A T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. Bioessays 26:61–67.
- Youker RT, Shinde U, Day R, Thomas G. 2009. At the crossroads of homoeostasis and disease: Roles of the PACS proteins in membrane traffic and apoptosis. Biochem J 421:1–15.
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. 1998. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92:83–92.
- Zhang W, Trible RP, Zhu M, Liu SK, Mcglade CJ, Samelson LE. 2000. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J Biol Chem 275:23355–23361.
