Abstract
Many important processes in life take place in or around the cell membranes. Lipids have different properties regarding their membrane-forming capacities, their mobility, shape, size and surface charge, and all of these factors influence the way that proteins and peptides interact with the membrane. In order for us to correctly understand these interactions, we need to be able to study all aspects of the interplay between lipids and peptides and proteins. Solution-state NMR offers a somewhat unique possibility to investigate structure, dynamics and location of proteins and peptides in bilayers. This review focuses on solution NMR as a tool for investigating peptide-lipid interaction, and special attention is given to the various membrane mimetics that are used to model the membrane. Examples from the field of cell-penetrating peptides and their lipid interactions will be given. The importance of studying lipid and peptide dynamics, which reflect on the effect that peptides have on bilayers, is highlighted, and in this respect, also the need for realistic membrane models.
Introduction
The biological membrane, one of the key structural elements in all types of living cells, is involved in a multitude of important functions, including photosynthesis, respiration and signaling with the immediate environment. The presence of the membrane implies that molecules and ions have to be transported across the membrane by various channels and transporters, and that signals have to be transmitted across membranes. The delicate physical chemical properties of the membrane have to be controlled. All of these tasks are performed by membrane proteins: Integral channels, transporters and receptors, as well as peripheral enzymes that sense the lipid composition of the membrane. Despite the fact that maybe more than 30% of known genomes code for membrane proteins, very little is known about their structure and mobility, their interaction with lipids, and hence also about function. Although recent advances using both X-ray crystallography and solution- and solid-state NMR spectroscopy has led to an increased number of structures, they only represent a small fraction in the Protein Data Bank (PDB, www.pdb.org).
The membrane is also the target for many biologically active peptides. Many of them, such as the receptor ligands, often interact with the membrane prior to the receptor-binding event (Sargent and Schwyzer Citation1986). Bacterial antimicrobial peptides target specific membranes, and often their action is to cause membrane disruption (Habermann Citation1972, Lauterwein et al. Citation1979, Dufourc et al. Citation1986, Dufourcq et al. Citation1986, Zasloff Citation1987, Batenburg et al. Citation1988, Dempsey Citation1990, Matsuzaki et al. Citation1995, Kobayashi et al. Citation2000, Takeshima et al. Citation2003, Wimley and Hristova Citation2011). Cell-penetrating peptides are a somewhat heterogeneous class of peptides that are able to translocate cell membranes, and bring cargo with them. These have found use in many applications, and although numerous studies of them are available, the mechanisms behind the translocation are not fully understood. Although not a completely homogeneous group of peptides, CPPs share many features with other membrane interacting peptides, such as antimicrobial peptides, and, in fact, domains of integral membrane proteins. These features are mainly a high cationic charge (high Arg or sometimes Lys content), and a relatively high hydrophobicity (Lindgren et al. Citation2000b, Langel Citation2002, Magzoub and Gräslund Citation2004, Madani et al. Citation2011). Such features are also found in, e.g., the voltage-sensor domains in cation channels, which contain sequences with a high Arg content responsible for the gating of the channels.
The paradigm concerning the structure and dynamics of biological membranes has long been the fluid-mosaic model (Singer and Nicolson Citation1972), which postulates that the membrane is not static, but has many types of motions that are influenced by a variety of bilayer features, such as curvature-induced stress and the packing properties of the lipids. These motions are crucial for the interplay between proteins, peptides and lipids, and are part of the mechanisms by which these molecules are controlled. Real membranes are, however, difficult to study and therefore a good membrane model must be used. The choice of membrane mimetic is very much related to the methodology that is used. For solution NMR studies, size (or motion) is crucial, and hence large lipid particles are not a good choice. Detergents have for a long time been used to solubilize both peptides and membrane proteins, but do not always provide an accurate model for a bilayer. During the last 10 years or so, bicelles have been introduced as a model membrane mimetic, potentially superior to micelles. These mixtures of detergents and lipids are thought to form disc-shaped aggregates, with a central lipid bilayer and a detergent circumference (). The presence of natural lipids and the fact that the surface of the central lipid region is more-or-less flat eliminate some of the limitations associated with micelles.
Figure 1. Cartoon model of three membrane mimetic media. (a) A detergent micelle, (b) a two-component bicelle and (c) a lipid vesicle. The figures were generated with PyMol (W.L. DeLano, The PyMol Molecular graphics system [2002], http://www.pymol.org). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 1. Cartoon model of three membrane mimetic media. (a) A detergent micelle, (b) a two-component bicelle and (c) a lipid vesicle. The figures were generated with PyMol (W.L. DeLano, The PyMol Molecular graphics system [2002], http://www.pymol.org). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/47889384-1595-4ce6-9ba2-dbd30c93245d/imbc_a_683456_f0001_b.jpg)
In this review, the emphasis is on solution NMR studies of peptide-lipid interactions will be presented and selective applications to cell-penetrating peptides will be given. An overview of the advances within the field of membrane protein structure and dynamics by solution NMR will also be given. Special attention is also given to the model membrane systems that are available for solution NMR studies.
Membrane mimetics
Overview
The typical biological membrane is a complex structure composed primarily of lipids and proteins. To investigate the molecular details in peptide or protein structure by NMR, relevant models for the bilayer need to be used (Sanders and Prosser Citation1998, Vinogradova et al. Citation1998, Sanders and Oxenoid Citation2000, Marcotte and Auger Citation2005, Prosser et al. Citation2006, Sanders and Sonnichsen Citation2006, Mäler and Gräslund Citation2009, Citation2011, Popot Citation2010, Gräslund and Mäler Citation2011, Warschawski et al. Citation2011). Typical membrane models in biophysical studies of protein and peptide-lipid interactions include vesicles (or liposomes) (Gabriel and Roberts Citation1984, Hope et al. Citation1986, Mayer et al. Citation1986), bicelles (Vold et al. Citation1997, Sanders and Prosser Citation1998, Marcotte and Auger Citation2005, Prosser et al. Citation2006), and detergent micelles (Sanders and Oxenoid Citation2000, Damberg et al. Citation2001, Sanders and Sonnichsen Citation2006, Warschawski et al. Citation2011) (). The present review focuses on solution-state NMR characterization of the interaction of peptides with model membranes. Compared to integral membrane proteins, the structure, location and dynamics of small peptides depend critically on the choice of membrane mimetic. In addition, it is a strict requirement that the model membrane reorients sufficiently rapid to obtain good spectral resolution. This requirement imposes a rather strong restriction on the types of model membranes that are suitable. Micelles, which have classically been the major model membrane for solution-state NMR studies, suffer from at least two major disadvantages: i.e., they are composed of detergents, rather than the natural lipid components of membranes, and they exhibit a strong curvature, which may have great impact on peptide structure (Chou et al. Citation2002).
The major structural part of a bilayer is a mixture of various lipids, and a typical eukaryotic zwitterionic phospholipid is 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (). This lipid is commonly used to produce vesicles, or liposomes. By changing the head-group from a choline to a glycerol, a negatively charged lipid is obtained (POPG). Vesicles can, however, be produced by a large variety of phospholipids, where head-group properties (size and charge), acyl chain-length and unsaturations can be varied. In addition, sterols such as cholesterol can be incorporated. Unfortunately, vesicles are far from ideal when it comes to solution NMR studies, simply due to their large size. A large unilamellar vesicle (LUV), produced by the extrusion technique (Mayer et al. Citation1986), typically has a diameter of 100 nm, which for most solution-state NMR applications is too large. Small unilamellar vesicles (SUVs), produced by sonication, are on the other hand smaller, but have the problem of having a very large curvature. Vesicles have, however, been used successfully to investigate lipid dynamics in bilayers by, e.g., spin relaxation where the influence of the lipid mobility from various peptides have been determined (Brown et al. Citation1983, Korstanje et al. Citation1989, Lepore et al. Citation1992, Ellena et al. Citation1993).
Figure 2. Molecular structures of selected phospholipids used for membrane mimetic media such as vesicles, fast-tumbling bicelles and nanodiscs.
It is not the aim of this review to give a thorough overview of all membrane mimetic media, but a review of the commonly used membrane models in solution NMR. For a detailed review on membrane mimetics in general, the reader is referred to a recent review by Warschawski et al. (Citation2011).
Detergents
For NMR studies, the micellar membrane model is preferred for a number of reasons. Micelles are relatively small () which means that they rotate rapidly, on the time-scale required for NMR. Theses micelles consist of detergent molecules that aggregate above a certain threshold concentration called the critical micelle concentration (CMC) and the size of a micelle is defined by the aggregation number, i.e., the average number of detergent molecules present. An increase in the detergent concentration to above the CMC results in the formation of more micelles, rather than larger micelles (Evans and Wennerström Citation1999, Rosen and Kunjappu Citation2012). Examples of detergents that form micelles commonly used in NMR studies of small peptides, in particular, but also of proteins (Tanford and Reynolds Citation1976) are dihexanoyl phosphatidylcholine (DHPC), dodecylphosphocholine (DPC), sodium dodecylsulfate (SDS) and CHAPS (), which are sometimes mixed with phospholipids. Lyso-myristoylphosphatidylglycerol (LMPG) and lyso-myristoylphosphatidylcholine (LMPC) have recently been used extensively for NMR studies of integral membrane proteins (Arora and Tamm Citation2001, Patching Citation2011). SDS, DPC and DHPC have been used successfully to determine NMR solution structures of peptides, integral membrane proteins as well as of protein domains (Papavoine et al. Citation1994, Citation1998, Jarvet et al. Citation1997, Arora et al. Citation2001, Fernández et al. Citation2001, Lindberg et al. Citation2001, Citation2003, Lind et al. Citation2007, Unnerståle et al. Citation2009). A list of commonly used detergents and some of their physical properties is given in .
Figure 3. Molecular structure of selected detergents commonly used in solution NMR studies of peptides.
Table 1. Physical properties of some selected detergents.
As mentioned, micelles are suitable for solution NMR work because of their limited size and because they allow extensive dynamics. There are two main problems associated with the use of micelles, both related to this fact. First, it is evident that many proteins are not active in a micelle solution, and it has been demonstrated many times that protein structures in micelles and other membrane models differ. One example is the crystal structure of the KvAP potassium channel, which was solved by MacKinnons group (Jiang et al. Citation2003a, Citation2003b). Later, they solved the structure of this protein using a more native-like lipid mixture, which produced significant differences in the location of parts of the voltage-sensor domain (VSD) (Long et al. Citation2007). This has led to some controversy concerning the mechanism behind the voltage-gating (Hristova and Wimley Citation2011). Another example is the structure of the Influenza A proton channel, for which a solution NMR structure in DHPC was reported by Schnell and Chou (Citation2008), which differed in some respects to the solid-state NMR structure determined later by Sharma et al. (Citation2010). The second problem has to do with smaller peptides in micelles. These short peptides are often seen to readily adapt to the environment, and the detergent solubilizes the peptide in a way that may be different from a more native-like lipid bilayer. It is often observed that peptide structures are more helical than in detergent than in other media (Damberg et al. Citation2001), and that the peptide structure may be distorted, indicating that for small membrane proteins and short peptides, the membrane mimetic plays a crucial role for the properties of the peptides.
Fast-tumbling bicelles
It has been recognized since the 1980s that mixtures of phospholipids and bile salts or detergents form soluble bilayers, termed bicelles, that at appropriate relative concentrations and conditions (such as temperature) align in the magnetic field (Ram and Prestegard Citation1988, Prestegard Citation1990, Lin et al. Citation1991, Sanders and Schwonek Citation1992, Sanders et al. Citation1994, Sanders and Prosser Citation1998). They were first used to characterize the interaction between peptides, proteins and the bilayer, including structural studies of integral membrane proteins by solid-state NMR (De Angelis et al. Citation2005), and later to align soluble proteins for measurements of residual dipolar couplings (RDCs) for structural biology work (Tjandra and Bax Citation1997). Bicelles are mixtures of various phospholipids and a suitable detergent (Vold et al. Citation1997, Sanders and Prosser Citation1998) and they can be produced with varying amounts of lipids and detergents (known as the q-ratio, q = [lipids]/[detergents]), which controls the size and phase properties of them.
Small (q ≤ 0.5) isotropic bicelles have been used to investigate structure and membrane interaction of several peptides and as well as membrane proteins (Sanders and Landis Citation1995, Whiles et al. Citation2001, Citation2002). The morphology of small isotropic bicelles has been studied in detail, including determination of geometry, dynamics as well as phase behaviour and such bicelle mixtures with a very high content of detergent (q < 1) have been found to resemble disc-like aggregates (), composed of a bilayered phospholipid surface surrounded by detergent molecules (Struppe et al. Citation2000, Glover et al. Citation2001, Luchette et al. Citation2001, Chou et al. Citation2004, van Dam et al. Citation2004, Andersson and Mäler Citation2005, Triba et al. Citation2005). Common lipids to produce such bicelles are dimyristoylphosphatidylcholine (DMPC), and the negatively charged dimyristoylphosphatidylglycerol (DMPG), together with the detergent dihexanoylphosphatidylcholine (DHPC). The molecular structures of DMPC, DMPG and DHPC are shown in . Bicelles are produced by mixing the appropriate lipids in a suitable buffer with a solution of detergent. This mixture is vortexed until a clear solution is obtained. Peptides or proteins can be added to this mixture, or reconstituted into the bicelles by using a co-solvent, such as chloroform, TFE, methanol or mixture of them. This solution is lyophilized end re-dissolved in water (Mäler and Gräslund Citation2011). One drawback of the bicelle solution is that that a high lipid concentration is needed to maintain the bicelle disc-like structure. Recent studies have, however, indicated that by choosing an appropriate detergent molecule, one may reduce the total concentration significantly (Lu et al. Citation2012), which is beneficial for NMR purposes.
Small fast-tumbling (isotropic) bicelles are ideal for combining studies of structure and membrane interactions of peptides, since their tumbling is isotropic and the reorientational diffusion of the lipids is fast enough to give reasonable solution-state NMR spectra. These versatile membrane models are disc-like with a diameter between 5 and 15 nm (depending on the composition), and the properties of the lipids constituting the bilayered surface can be varied. Bicelles with different chain lengths (Triba et al. Citation2006, Lind et al. Citation2008), and head-group properties (charge and size) have been reported (Struppe et al. Citation2000). Cholesterol has also been incorporated into bicelles, and these were used to study the influence of cholesterol on melittin-lipid interactions (Andersson et al. Citation2007).
The early applications of the lipid bicelles focused on high q-value mixtures, which align in the magnetic field of the NMR spectrometer to form a lyotropic liquid crystalline phase. These were used as model membranes in solid-state NMR (Sanders et al. Citation1994), and later for aligning soluble proteins enabling measurements of residual dipolar couplings, RDCs, for structural studies (Tjandra and Bax Citation1997). It is now believed that these bicelles form a different phase altogether than the small fast-tumbling bicelles. Based on a number of different experimental observations using various techniques, it has been demonstrated that the bicelles may adopt different morphologies dependent on concentration, temperature and composition (Prosser et al. Citation1998, Gaemers and Bax, Citation2001, Nieh et al. Citation2001, Rowe and Neal, Citation2003), and it has been suggested that the magnetically aligned bicelles adopt a lamellar structure of perforated bilayers, also known as the Swiss cheese model (Prosser et al. Citation1998). It has been reported that the disc-like bilayered particles persist down to a q-ratio of q = 0.25 (Chou et al. Citation2002, Andersson et al. Citation2004), and below this q-value the particles are more mixed micelle-like. The effect of the peptides on the phospholipids has more recently become the focus of several studies. In this case, the size of the bicelle is not crucial, and typically q = 0.5 bicelles have been used. Carbon-13 NMR spin relaxation of the lipid molecules has been used to determine local lipid mobility in the bicelles.
There are several reviews discussing bicelles, especially the mixture forming lyotropic liquid crystalline phases (Sanders et al. Citation1994, Sanders and Prosser Citation1998, De Angelis et al. Citation2005, Katsaras et al. Citation2005, Marcotte and Auger Citation2005, Prosser et al. Citation2006, De Angelis and Opella Citation2007, Kim et al. Citation2009, Raschle et al. Citation2010, Warschawski et al. Citation2011).
Nanodiscs
More recently, nanodiscs have emerged as an alternative for both solution NMR, solid-state NMR and crystallization of membrane proteins. Nanodiscs are protein-containing lipid aggregates, containing 100–200 lipid molecules, surrounded by a membrane scaffold protein (MSP) or amphipathic helical peptides typically derived from apolipoprotein A-1 (Bayburt et al. Citation2002, Denisov et al. Citation2004, Nath et al. Citation2007, Lyukmanova et al. Citation2008, Nakano et al. Citation2009, Ritchie et al. Citation2009). Nanodiscs are on the order of 10 nm in diameter, comparable to the small fast-tumbling bicelles, but their size can be varied through variations in the MSP (Denisov et al. Citation2004, Chromy et al. Citation2007, Borch and Hamann Citation2009, Nakano et al. Citation2009). As with vesicles and bicelles, the properties of nanodiscs can be varied, through variation in the phospholipid content. Typically, acyl chain length, head-group properties (charge and size) and the amount of unsaturation in the acyl chain can be varied. Lipids such as cholesterol can also readily be incorporated into the nanodiscs. It should also be noted that the nanodiscs do not contain detergent, which is favorable compared to bicelles, since the detergent may interact with the peptide or protein instead of the lipids. There are other advantages with nanodiscs; one is that their size can be varied without the addition of large amounts of detergent. Since the number of proteins or peptides per particle is limited, the sample is monodisperse and because of this, the solution can be diluted, which is not the case with small fast-tumbling bicelles. For bicelles, it has been shown that the disc-like properties persist down to a total [lipid + detergent] concentration of 1–5% w/v, and that below this concentration a mixture of free detergent and lipid assemblies are obtained (Sanders and Schwonek Citation1992, Glover et al. Citation2001). As noted earlier though, by choosing a detergent with lower cmc, however, lower total concentrations can be used also for bicelles (Lu et al. Citation2012).
Nanodiscs can be produced in several ways, but in short, a MSP is mixed with phospholipid vesicles that contain the desired lipids. This can be done by sonication or by dissolving the protein in a detergent, which can be removed by the use of biobeads (Jonas Citation1986, Bayburt et al. Citation2002, Bayburt and Sligar Citation2002, Borch and Hamann Citation2009). Several NMR studies of proteins in nanodiscs have been reported (Bayburt and Sligar Citation2002, Citation2003, Baas et al. Citation2004, Glück et al. Citation2009, Raschle et al. Citation2009, Shenkarev et al. Citation2010a). An example is of the voltage-sensor domain (VSD) from the potassium channel KvAP, which gave broader signals than in micelles but still a tractable NMR spectrum (Shenkarev et al. Citation2010a). Recently, larger nanodiscs, macrodiscs, have been proposed as a means for producing discs that become magnetically aligned, suitable for solid-state NMR studies of membrane proteins (Park et al. Citation2011). These discs are simply created by increasing the lipid/helical peptide ratio (in a manner very similar to the fast-tumbling bicelles), whereby the particles become larger leading to magnetic alignment.
Structure
Overview
Solution NMR has over the past 30 years or so contributed to the field of structural biology by solution structure determinations of small globular proteins. The success of structural studies of protein and membrane-bound peptides by solution NMR relies critically on the dynamics of the complex that is to be studied (Kowalewski and Mäler Citation2006). The overall tumbling of a protein or a peptide-lipid complex should not exceed a critical correlation time, implying that the complex cannot be too large. The quality of the NMR spectrum, in terms of resolution and line-broadening, is governed by the mobility of the molecules, which for a membrane protein or peptide is given at least partly by the size of the membrane mimetic.
Provided that the membrane mimetic medium allows for reasonable NMR spectra, standard 2D and 3D techniques for determining solution structures can be used. For an overview of such techniques, the reader is directed to several reviews covering these (Wüthrich Citation1986, Clore and Gronenborn Citation1994, Cavanagh et al. Citation2007). In short, geometrical constraints are collected from a set of NMR spectra that are converted into an ensemble of structures using a minimization algorithm. These geometrical constraints include interproton distances, derived from cross-relaxation, which can be measured through quantification of NOESY (Jeener et al. Citation1979) cross-peak intensities. Further, dihedral angles can be derived from J-coupling measurements, and residual dipolar couplings defining the orientation of entire segments in the structure are obtained by dissolving the protein (soluble) in a liquid crystalline solvent (Tolman et al. Citation1995, Tjandra and Bax Citation1997). Cell-free protein expression systems and the use of the SAIL (Stereo-Array Isotope Labeling) isotope-labeling scheme (Kainosho et al. Citation2006), have contributed to the advances in determining membrane protein solution structures.
For membrane-interacting peptides, the structural constraints are usually few, since typically one peptide forms a single helix. In this case it is easy to find constraints that define the helical structure, but not the orientation or shape. Hence, the focus of peptide studies is more often connected with determining the location of the peptide in the bilayer, rather than the three-dimensional structure by itself. This will be dealt with in more detail in a later section.
Integral membrane proteins – brief overview
The introduction of the TROSY experiment (Pervushin et al. Citation1997) and the development in protein production using cell-free expression methods have led to an increase in the number of structures of integral membrane proteins. Recent progress includes structures of two seven-transmembrane (7-TM) helical proteins, sensory rhodopsin (Gautier et al. Citation2010) and proteorhodopsin (Reckel et al. Citation2011). For reviews see (Sanders and Sonnichsen Citation2006, Kim et al. Citation2009, Patching Citation2011). Selected structures that are discussed in this section are shown in . The first structures of proteins larger than those containing one or two membrane-spanning helices were of E. coli β-barrel proteins, the outer membrane protein X (OmpX, 8 β strands) in DHPC (Fernández et al. Citation2001, Citation2004) and the very similar outer membrane protein A (OmpA) in DPC micelles, both reported in 2001 (Arora et al. Citation2001). Since then a few more structures of β-barrel proteins have been determined by NMR. More recently, the solution NMR structure of the human voltage-dependent anion channel 1 (VDAC1), which is significantly larger, was obtained in LDAO micelles (Hiller et al. Citation2008), but studies have also been performed in nanodiscs (Raschle et al. Citation2009) which showed that the properties of the protein appears to be very similar to those in the in micelles.
Figure 4. NMR-derived solution structures of three β-barrel proteins and two helical proteins with three or more unique helices. The coordinates were taken from the PDB (www.rcsb.org). (a) OmpX in DHPC (PDB accession code 1Q9F, (b) OmpA in DPC (PDB accession code 1G90), (c) VDAC-1 in LDAO (PDB accession code 2K4T), (d) DAGK in DPC (PDB accession code 2kdc), (e) the VSD domain of KvAP in D7PC (PDB accession code 2KYH) (f) sensory rhodopsin II (residues 1–221) in D7PC (PDB accession code 2KSY), and (g) proteorhodopsin in D7PC (PDB accession code 2L6X). The figures were generated with PyMol (W.L. DeLano, The PyMol Molecular graphics system [2002], http://www.pymol.org). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 4. NMR-derived solution structures of three β-barrel proteins and two helical proteins with three or more unique helices. The coordinates were taken from the PDB (www.rcsb.org). (a) OmpX in DHPC (PDB accession code 1Q9F, (b) OmpA in DPC (PDB accession code 1G90), (c) VDAC-1 in LDAO (PDB accession code 2K4T), (d) DAGK in DPC (PDB accession code 2kdc), (e) the VSD domain of KvAP in D7PC (PDB accession code 2KYH) (f) sensory rhodopsin II (residues 1–221) in D7PC (PDB accession code 2KSY), and (g) proteorhodopsin in D7PC (PDB accession code 2L6X). The figures were generated with PyMol (W.L. DeLano, The PyMol Molecular graphics system [2002], http://www.pymol.org). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/512beafa-026c-4468-a833-c2f4726328a9/imbc_a_683456_f0004_b.jpg)
Helical structures were for a long time limited to single-helix spanning proteins, but more recently structures of proteins with more than one or two unique helices have been reported. One of those is of the E. coli diacylglycerol kinase (DAGK), a homotrimer of subunits consisting of three transmembrane helices, which has been studied in several membrane mimetic media (Oxenoid et al. Citation2002, Citation2004). The structure was ultimately determined in DPC (Van Horn et al. Citation2009), and the authors also used NMR to map out the substrate-binding sites in the enzyme, and concluded that a part of the structure termed portico included the lipid-binding site. A second example is of the voltage-sensor domain (VSD) in the potassium channel KvAP, which contains four transmembrane helices for which there is a solution structure in diheptanoylphosphatidylcholine (D7PC) micelles (Butterwick and MacKinnon Citation2010). At the same time, a structural study of the same VSD was performed in DPC/LDAO mixed micelles but no final structure was reported (Shenkarev et al. Citation2010b). Both studies were found to agree fairly well with the previous crystal structure in detergent (Jiang et al. Citation2003a). Due to the difficulties associated with sample preparation, NMR solution structures of 7-TM proteins were not reported until a few years ago. In 2010, Nietlispach and co-workers reported the solution structure of sensory rhodopsin II, a seven-helix receptor dissolved in DHPC (Gautier et al. Citation2010). They obtained high-quality NMR data by adjusting pH and temperature, and the structure is remarkably well-defined, with low RMSD values for the position of backbone atoms. The structure of proteorhodopsin in D7PC was reported in 2011 (Reckel et al. Citation2011). In this case selective labeling techniques together with cell-free expression using SAIL amino acids (Kainosho et al. Citation2006) were used to facilitate NMR assignments. The reader who is interested further in membrane protein solution structures is directed to reviews on membrane protein structure (Kim et al. Citation2009, Kang and Li Citation2011, Patching Citation2011).
Membrane-interacting peptides
There are numerous reports in the literature of studies of membrane-interacting peptides that interact with lipids, and especially the structures of peptides have been determined by solution NMR (as well as by other techniques). Many of these studies have been conducted in the presence of organic solvents, such as TFE, HFP, methanol, chloroform, or mixtures of these and water. These studies often produce structures that are helical in character, despite the fact that the peptides are not helical in the presence of, e.g., real lipids (vesicles or bicelles).
Many structures of a wide range of biologically active peptides that interact with membranes have also been determined in the presence of detergent micelles (Papavoine et al. Citation1994, Citation1995, Citation1997, Citation1998, Jarvet et al. Citation1997, Öhman et al. Citation1998, Lindberg et al. Citation2001, Citation2003). It is believed that the individual detergent molecules interact with the protein or peptide in such a way as to cover the hydrophobic part of the peptide sequence, and that only one protein or peptide is bound to each micelles.
Several structures of antimicrobial peptides have been reported, and recently a structure of the cationic peptide maximin-4 (27 aa's) from the Chinese red-belly toad was reported in both SDS and 50% methanol (Toke et al. Citation2011). Although the structure was largely helical in both solvents, significant differences between the structures were observed. In SDS, several non-sequential NOEs indicated helix-helix interactions, and a kink in the structure, while no such features was observed in methanol solution. In a related study of the shorter (20 aa's) antimicrobial peptide maximin H6, which was shown to be helical in both DPC and SDS, no indication of a kinked structure was observed (Kosol and Zangger Citation2010). The structure of LL-23, one of many active fragments of LL-37 in human skin, has been determined in DPC (Wang et al. Citation2011). A helical slightly bent structure was observed, that largely resembled that of LL-37 in SDS (Wang Citation2008). Although most of the reported structures of antimicrobial peptides are helical, several structures of β-sheet forming peptides also exist. Shenkarev et al. (Citation2011) found that the β-hairpin peptide aranicin oligomerizes into dimers in DPC, and this structure was used to explain the formation of membrane pores.
Diffusion NMR has been used to confirm that well-defined complexes between a peptide and a detergent micelle are obtained (Biverståhl et al. Citation2004, Papadopoulos et al. Citation2006, Unnerståle et al. Citation2009). In the study of the Arg-rich sequence from the voltage sensor domain in the human BK channel in the presence of DPC (Unnerståle et al. Citation2009), it was demonstrated by pulsed field gradient NMR (Stejskal and Tanner Citation1965, Von Meerwall and Kamat Citation1989, Callaghan et al. Citation1998) that the size of the DPC micelle increased by 25% (by 0.3 nm in diameter to 2.3 nm) when the Arg-rich sequence was present, in excellent agreement with the expected increase in size from one peptide molecule (Cantor and Schimmel Citation1980). Hence, it was concluded that a well-formed one-to-one complex between the two was formed.
The micelles are, however, problematic, and, as discussed earlier, it has been demonstrated for both peptides (Andersson and Mäler Citation2002, Chou et al. Citation2002) and integral membrane proteins (Jiang et al. Citation2003a, Long et al. Citation2007) that the structure may be distorted by the micelles. Several peptide and protein structures in the presence of fast-tumbling bicelles have been reported (Whiles et al. Citation2001, Andersson and Mäler Citation2002, Chou et al. Citation2002, Bárány-Wallje et al. Citation2004, Poget et al. Citation2007, Poget and Girvin Citation2007), and it has been shown that peptides may adopt different conformation in the more disc-like bicelles as compared to in detergent micelles. In the study of a water-insoluble peptide fragment from HIV-1 Env gp41 protein, it was demonstrated that the helical structure of the peptide was significantly bent in the presence of DHPC micelles, while it was not in the presence of DMPC/DHPC bicelles (Chou et al. Citation2002). In a similar study of the gastrointestinal peptide motilin it was observed that the peptide adopted a significantly different structure in the presence of bicelles (Andersson and Mäler Citation2002) compared to SDS micelles (Jarvet et al. Citation1997). Lau et al. studied integrin β3, and demonstrated that the structures were not the same in bicelles and micelles (Lau et al. Citation2008, Citation2009).
Cell-penetrating peptides
Membrane-interacting peptides, such as antimicrobial peptides and cell-penetrating peptides, which share the common feature of typically being rich in positively charged amino acid residues, are well represented among the peptides for which structures have been determined. Here, a few examples will be given from the somewhat diverse class comprising the cell-penetrating peptides. Cell-penetrating peptides are, from a structural perspective, a class of short, often cationic peptides that have the ability to form various secondary structures under different conditions. Often the choice of membrane mimetic media has been observed to influence the secondary structure content of the peptides (Lindberg et al. Citation2001, Citation2003, Czajlik et al. Citation2002, Magzoub et al. Citation2002, Citation2003, Bárány-Wallje et al. Citation2004). In general these peptides are unstructured in water or buffer solution, but attain secondary structure in the presence of a membrane, or a membrane mimetic. Most CPPs have the possibility to adopt amphiphilic α-helical structure under certain membrane conditions (often including somewhat negatively charged bilayer surfaces and not too high peptide/lipid ratio). Some CPPs adopt this structure as a consequence of electrostatic interactions between the positively charged amino acid residues and a negatively charged bilayer surface, while for others it is found that hydrophobic interactions dominate.
NMR has been successfully used to elucidate the detailed structure of several CPPs in their membrane-bound form, using a variety of membrane models such as detergents, bicelles and simple organic solvents (Lindberg et al. Citation2001, Citation2003, Czajlik et al. Citation2002, Bárány-Wallje et al. Citation2004, Biverståhl et al. Citation2004, Deshayes et al. Citation2004, Citation2005, Ruzza et al. Citation2004, Herbig et al. Citation2005). A wide range of CPPs have been investigated, including the well-studied examples penetratin and transportan, as well as peptides derived from the N-terminal fragments of the prion and doppel proteins. Structures of three different CPPs are shown in . These sequences represent three different classes of CPPs: first, penetratin, a cationic peptide with very few hydrophobic residues (Derossi et al. Citation1994, Citation1996, Citation1998), transportan, which is both cationic and hydrophobic (Pooga et al. Citation1998, Citation2001, Lindgren et al. Citation2000a), while the third peptide derived from the prion protein represents a more hydrophobic sequence (Lundberg et al. Citation2002, Nunziante et al. Citation2003, Sunyach et al. Citation2003, Walmsley et al. Citation2003, Biverståhl et al. Citation2004, Papadopoulos et al. Citation2006). From these structural studies it is evident that all adopt helical structure, but after a closer look these structures differs in many significant ways.
Figure 5. NMR solution structures of three cell-penetrating peptides derived in different membrane mimetic media. The structure of penetratin (a) was in q = 0.5 DMPC/DMPG/DHPC phospholipid bicelles (30% PG), of transportan (b) in q = 0.33 DMPC/DHPC, and of the N-terminal fragment (1–30) of the bovine Prion protein (c) in DHPC. The Figures were generated with PyMol (W.L. DeLano, The PyMol Molecular graphics system [2002], http://www.pymol.org). The coordinates were taken from the PDB (www.rcsb.org) (penetratin: 1OMQ, transportan: 1SMZ and bovine Prion protein-derived peptide: 1SKH). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 5. NMR solution structures of three cell-penetrating peptides derived in different membrane mimetic media. The structure of penetratin (a) was in q = 0.5 DMPC/DMPG/DHPC phospholipid bicelles (30% PG), of transportan (b) in q = 0.33 DMPC/DHPC, and of the N-terminal fragment (1–30) of the bovine Prion protein (c) in DHPC. The Figures were generated with PyMol (W.L. DeLano, The PyMol Molecular graphics system [2002], http://www.pymol.org). The coordinates were taken from the PDB (www.rcsb.org) (penetratin: 1OMQ, transportan: 1SMZ and bovine Prion protein-derived peptide: 1SKH). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/b4d32c1c-4211-4266-b910-94620f23f706/imbc_a_683456_f0005_b.jpg)
Penetratin, which is a cationic peptide derived from the third helix of the Antennapedia homeodomain (Derossi et al. Citation1994) shows an amphipathic character in the structure in both SDS and in phospholipid bicelles (Lindberg et al. Citation2003). In TFE mixtures, the helix is somewhat irregular and evidence of 310 helix character has been reported (Czajlik et al. Citation2002). The structure has been reported to vary with bilayer surface charge, with a change from random to α-helix to β-sheet as the surface charge increases (Magzoub et al. Citation2002, Citation2003). It has also been demonstrated that the structure of the cell-penetrating peptide penetratin is substantially different in SDS, DHPC, different bicelles or LUVs (Magzoub et al. Citation2002). Furthermore, the position of penetratin varies in different membrane mimetic media, and it is more parallel to the surface of the bicelle, as compared to in micelles, while a similar orientation perpendicular to the bilayer normal has been seen in POPC/POPG LUVs (Magzoub et al. Citation2003). Hence it is crucial to use a realistic membrane model for studying the molecular details in peptide and protein location in a bilayer.
Transportan, on the other hand, which is a synthetic peptide composed of mastoparan (a wasp venom peptide) linked together with the N-terminal amino acid residues of the neuropeptide galanin (Pooga et al. Citation1998), has a little more complex structure. The C-terminal half forms an amphipathic structure, while the N-terminus is more unstructured (Bárány-Wallje et al. Citation2004). The structure of the mastoparan part of the peptide is very similar to that of mastoparan alone (Higashijima et al. Citation1988, Wakamatsu et al. Citation1992, Seigneuret and Lévy Citation1995, Vold et al. Citation1997). This structure does not differ significantly between bicelles composed of zwitterionic DMPC or bicelles with a fraction of the negatively charged DMPG incorporated in the bilayer (Bárány-Wallje et al. Citation2006). It has also been reported that the structure is similar in LUVs composed of varying amount of POPC/POPG lipids, again indicating that the structure does not depend on charge interactions (Magzoub et al. Citation2003).
Finally, the N-terminal sequence of the bovine prion protein (including a signal sequence) is different altogether from the other two. Here, the charged residues are all located at the N- and C-terminus of the sequence, leaving a hydrophobic patch in the central part that adopts a rigid helix in both bicelles and DHPC micelles (Biverståhl et al. Citation2004). For this peptide, the structure is, however, observed to differ in membrane mimetic media composed of POPC LUVs, compared to in POPC/POPG LUVs (Magzoub et al. Citation2005).
The latter two peptides have severe effects on membrane and model bilayer integrity, which cannot easily be correlated to structure, and to learn more about their effects, studies on their dynamic behaviour and membrane location are useful.
Dynamics
Overview
NMR provides unique possibilities to investigate the dynamics of molecules on a wide time-scale. Direct analyses of chemical shifts and line-shapes can provide information about slow exchange processes (Jeener et al. Citation1979, Bodenhausen et al. Citation1984, Wagner et al. Citation1985), while faster exchange processes, such as conformational exchange or binding processes, leading to chemical shift averaging can be studied through transverse relaxation methods, including relaxation dispersion (Loria et al. Citation1999, Palmer et al. Citation2001, Wang et al. Citation2001, Palmer Citation2004). Fast dynamics, i.e., molecular tumbling and local flexibility can be investigated by conventional spin relaxation techniques (Lipari and Szabo Citation1982a, Skelton et al. Citation1993, Kowalewski and Mäler Citation2006). Furthermore, NMR offers the possibility for studying peptide-membrane interaction both from the perspective of the peptide or protein, as well as from the lipid. Both pieces of information are necessary to provide a complete picture of how peptides and lipids interact.
For determining backbone peptide dynamics, measurements of the backbone 15N relaxation parameters, R1, R2 and NOE are usually interpreted using the model-free approach (Lipari and Szabo Citation1982a, Citation1982b). This analysis results in dynamic parameters including a generalized order parameter and a local correlation time that describe the internal motion for each 15N-1H bond vector, together with an overall correlation time for the peptide or protein (assuming that the overall motion is isotropic). This method has been used successfully for the last 25 years or so to describe protein motion on the ps–ns time-scale. Many biologically relevant processes occur on slower time-scales, typically conformational exchanges that result in averaging of chemical shifts. These motions can be studied from measurements of transverse relaxation dispersion, either by measurements of R2 using CPMG pulse sequences (Loria et al. Citation1999, Wang et al. Citation2001) or by measurements of R1r with different spin lock field strengths (Szyperski et al. Citation1993, Akke and Palmer Citation1996, Zinn-Justin et al. Citation1997). Both methods provide information about the exchange dynamics (on the ms–ms time scale) species and are in a sense unique in that they can also measure the properties of scarcely populated states, e.g., detect the presence of a small population of an unfolded state of a protein, or invisible protein states (Hansen et al. Citation2008).
Spin relaxation has previously been used successfully to investigate effects of peptides and protein fragments on lipid dynamics. The DMPC dynamics in bicelles (Andersson and Mäler Citation2005, Andersson et al. Citation2007, Lind et al. Citation2008) have been examined, and it was shown that the lipid dynamics in the bicelle agree well with the dynamics in unilamellar vesicles obtained from EPR methods (Freed Citation2000, Lou et al. Citation2001) as well as from NMR spin relaxation (Brown et al. Citation1983, Citation2000, Lepore et al. Citation1992, Ellena et al. Citation1993).
Integral membrane protein dynamics have been determined for a few proteins (Chill and Naider Citation2011). For OmpA relaxation studies indicated that the loops were flexible on the ps–ns time-scale, while most of the barrel was rigid (Liang et al. Citation2010), with a gradual decrease in order parameters from the middle of the barrel towards the ends. For the voltage-sensor domain from KvAP, two studies have reported on backbone 15N relaxation (Butterwick and MacKinnon Citation2010, Shenkarev et al Citation2010b). Both studies indicated a rigid domain, with flexible S1–S2 and S2–S3 loops on the ns–ps. Both studies also indicated the presence of motion on the slower ms–ms time-scale, as evidenced from increased line-widths or R1 × R2 values. An earlier study focused on the backbone dynamics of the KcsA potassium channel, i.e., the pore domain (Chill et al. Citation2006). The relaxation data for this protein solubilized in SDS indicated a rigid ion channel, and in particular, no large-scale motion in the selectivity filter. These studies clearly demonstrate the feasibility of studying backbone dynamics also in integral membrane proteins. Exchange dynamics have also been measured for membrane proteins, and for the bacterial outer membrane protein PagP it was found that the enzyme alters between two dynamically different states (Hwang et al. Citation2004). The exchange was studied using a combination of Nzz exchange spectroscopy and CPMG relaxation dispersion to determine the kinetics and thermodynamics of the inter-conversion between a relaxed and tense state, and the different dynamical behaviour of these two states. For phospholamban, both the ps–ns dynamics (Metcalfe et al. Citation2004) and exchange dynamics have been determined (Traaseth and Veglia Citation2010). The CPMG-based approach was used to study the ms–ms exchange dynamics between excited, disordered, states and the ordered state (Traaseth and Veglia Citation2010). The results were interpreted using a model for the partial unwinding of the structure. A different strategy to investigate conformational mobility was recently taken using selective 31F-labeling. In study of the β2-adrenergic receptor in complex with different ligands (Liu et al. Citation2012) differences in line-widths and signal intensities, which also report on dynamics, and changes in relative populations of two states, were observed for site-specific 31F-labels in the protein, as a function of ligand. The results were interpreted in the context of signaling pathways, and the impact of different ligands on the conformational dynamics of the receptor.
Peptide dynamics
Fast protein and peptide dynamics are often obtained from spin relaxation data for backbone 15N-1H pairs in 15N-labeled proteins and peptides. Typically, R1, R2 and heteronuclear NOE relaxation parameters are measured and sometimes interpreted within the framework of the so-called model-free approach (Lipari and Szabo Citation1982a, Citation1982b). This method yields a generalized order parameter (S2) and correlation times for the internal motion and for the overall motion, and in the case of more complicated dynamics, additional parameters describing this (Clore et al. Citation1990). This analysis is a little bit more complicated in a membrane environment, as also the influence of the membrane mimetic has to be considered (Ellena et al. Citation1993, Andersson and Mäler Citation2005). Most of these dynamic studies have been performed in different detergent micelles, but a few in bicelles have also been reported.
Peptide dynamics generally depend on the membrane mimicking solvent, and care should be used to ascertain that the solvent reflects a true lipid environment. Wang et al. (Citation2011) reported relaxation measurement for the human antimicrobial peptide LL-23, and they found small differences in the local motion, as evidenced by 15N NOE factors, of the peptide using three different solvents: SDS, DPC and D8PG. In a study of the antimicrobial peptide maximin H6 from toad, elevated dynamics were found in SDS as compared to in DPC, which was in agreement with a somewhat less defined structure (Kosol and Zangger Citation2010).
CPP dynamics
Many of the CPPs have the capability to alter their secondary structure in different environments, such as different membrane mimetic media. The overall and local dynamics for penetratin was therefore studied and it was concluded that the dynamic behaviour of the peptide also depended on the membrane mimetic (Andersson et al. Citation2004). Penetratin was observed to bind more rigidly to SDS micelles, which resulted in a less flexible peptide, than what was observed in zwitterionic bicelles. Hence, for penetratin, there is a correlation between the structure and dynamic properties in different membrane environment.
The dynamics of the CPP transportan was also investigated in both zwitterionic and partly negatively charged bicelles. As discussed previously, the lipid composition of both LUVs and bicelles did not appear to alter the structure of transportan (Bárány-Wallje et al. Citation2004). However, a rather significant difference in the mobility of transportan in the two bicelles was observed (Bárány-Wallje et al. Citation2006). The overall motion of the peptide was much slower in the presence of negatively charged lipids, and could be explained by the peptide residing rigidly at the surface of the bilayer, while the motion in zwitterionic lipids best was explained by a freely rotating peptide within the head-group region of the bilayer. The surface-charge dependence of the dynamics can be correlated with membrane perturbing effects, and it has been observed that transportan variant induces more leakage in vesicles composed of zwitterionic lipids only than in vesicles with some negatively charged PG lipids (Guterstam et al. Citation2009). Hence mobility may be correlated with the perturbation.
Lipid dynamics
The bilayer is a highly dynamic system with many motional modes. The lipid molecule undergoes later diffusion within the bilayer (Lindblom and Orädd Citation1994, Gaede and Gawrisch Citation2003, Filippov et al. Citation2004, Orädd and Lindblom Citation2004a, Citation2004b), rotates around its own axis, and has many degrees of internal flexibility (Mayer et al. Citation1990, Ellena et al. Citation1993). All of these motions affect the interactions between a lipid molecule and a peptide or protein. To understand the effect that a peptide has on lipid properties, NMR spin relaxation of lipids can reveal changes in lipid mobility (Yeagle Citation2005).
Carbon-13 relaxation has for a long time been used as a measure for lipid dynamics and has mainly been performed on phospholipids in vesicles. Such data carry information about the local flexibility of the lipid molecule, and in 1983 Brown and co-workers showed that collective motions strongly influence the relaxation (Brown et al. Citation1983). Attempts have also been made to correlate relaxation data with lateral diffusion (Lepore et al. Citation1992, Ellena et al. Citation1993). Effects of size and shape of the membrane on relaxation parameters have been studied (Gent and Prestegard Citation1974), as well as influences of motions on different time-scales (Fuson and Prestegard Citation1983). Recently, a compilation of measurements of various relaxation parameters and segmental order parameters for lipids have been published (Leftin and Brown Citation2011), useful for interpreting other data, and for use in simulations of membrane dynamics. Most investigations of bilayer dynamics have been made using phospholipid vesicles, using carbon-13 relaxation parameters. Shintani et al. (Citation2011), on the other hand, interpreted proton-proton NOEs together with MD simulations in terms of lipid dynamics in a wide range of lipid vesicle systems.
Bicelles, provide a convenient way of conducting high-resolution NMR studies of both the bound peptide and of lipid properties (Andersson and Mäler Citation2005). Is has further been demonstrated that changes in lipid dynamics can readily be monitored by analyzing changes in relaxation parameters (Andersson et al. Citation2007). These analyses are based on the theories presented by Wennerström and co-workers already in the 1970s (Wennerström et al. Citation1974, Halle and Wennerström Citation1981), which are very similar to those developed by Lipari and Szabo used for analyzing protein dynamics as described previously (Lipari and Szabo Citation1982a, Pastor et al. Citation1988). The main information gained from relaxation is a generalized order parameter (S2) and a local correlation time (τloc) for each carbon-proton vector in the lipid molecule, provided that estimates of the overall motion of the individual lipid molecules can be obtained. It should be noted that the relaxation parameters themselves also provide relative estimates of the amount of local dynamics without fitting the data to obtain the dynamic parameters. Especially the heteronuclear NOE factor provides a good indicating of the degree of local flexibility and can be used to generate a mobility profile for the lipid molecule ().
Figure 6. Mobility profile for DMPC in bicelles. 13C NOE factors for selected carbons in DMPC in q = 0.5 DMPC/CHAPS bicelles. The Figure was produced from data in (Andersson et al. Citation2007). The carbon atom labeling is indicated in the DMPC structure.
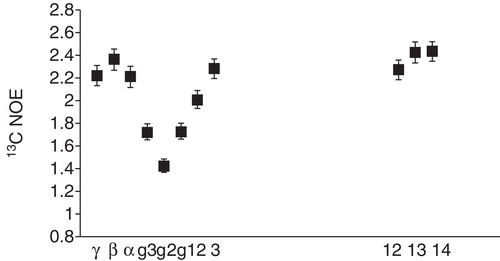
It has been shown that the lipid dynamics in small fast-tumbling bicelles composed of DMPC does not depend on bicelle size, but more so on the choice of detergent (Andersson and Mäler Citation2005). The order parameters for the acyl chains in DMPC are very low, indicating as expected a high degree of mobility, with the ends having almost completely unrestricted motion. On the other hand, the dynamics are more restricted for the glycerol part of the molecule, and part of the head-group (). It has also been shown that altering the acyl chain length from 14 carbon atoms in DMPC to either 12 carbons in DLPC or 16 carbons in DPPC has very limited effect on the acyl chain dynamics in general (Lind et al. Citation2008). In this study, the effect of different model transmembrane peptides on the lipid dynamics was reported. The peptides had the effect of increasing the order parameters for the DLPC lipid, while no effect was observed on the longer lipid chains. This effect was explained by a mismatch between the hydrophobic length of the peptides and the DLPC lipid acyl chain. In a study of the effect of cholesterol and the lytic peptide melittin, it was demonstrated that the incorporation of cholesterol into bicelles had the expected effect of making the lipids more rigid (Andersson et al. Citation2007). At the same time, melittin was also seen to have a profound effect of the dynamics of the whole lipid molecule.
The effect of the CPP transportan on lipid dynamics was investigated in DMPC-containing bicelles (Bárány-Wallje et al. Citation2006) and it was concluded that the peptide interacts preferentially with DMPC, and not with the detergent DHPC to the same extent, as dynamic effects were observed mostly for DMPC. It was also concluded that the motion of the entire lipid molecule was mostly affected, indicating that the peptide residues on the surface of the bilayer, affecting the overall rotation of the lipid molecules. These results, in combination with the dynamics of the peptide, indicating a slower motion in a negatively charged bilayer, indicates clearly that the lipid-peptide interaction affects primarily the dynamics of the molecules, and not the structure, which suggests that the dynamics of the lipid-peptide system is a more important parameter.
Peptide location in a bilayer
There are several NMR methods for elucidating the effect of peptides on membrane mobility and order, and hence their location in a bilayer (Seelig Citation1977, Citation1978, Seelig and Seelig Citation1980, Pointer-Keenan et al. Citation2004, Ramamoorthy et al. Citation2006a, Citation2006b, Brender et al. Citation2007). 31P NMR as well as 2H NMR has been used to determine the phase properties of lipid mixtures, including bicelles (Ottiger and Bax Citation1998, Glover et al. Citation2001), and the effect of bioactive peptides on lipid bilayers. Solid-state NMR offers several possibilites to study the location of of peptides, and many of these methods are based on measuring 13C-1H dipolar couplings (Dvinskikh et al. Citation2005, Citation2006) of phospholipids in ordered bilayers or bicelles. More recently bicelles have been used in solid-state NMR investigations of cytochrome P450 and b5 (Dürr et al. Citation2007, Waskell and Ramamoorthy Citation2007) and of Islet amyloid polypeptide (Smith et al. Citation2009). 2H NMR of chain-deuterated lipids has often been used to examine the effect that peptides have on acyl chain ordering and dynamics. In this way information about the location of peptides in a bilayer can be obtained. Several studies of the insertion of model transmembrane peptides into phospholipid bilayers using among other methods 31P and 2H NMR have been reported by Killian and co-workers (de Planque and Killian Citation2003, Killian Citation2003), in which peptide-induced membrane effects, such as hydrophobic mismatch and effect of flanking residues have been investigated (Killian et al. Citation1996, Morein et al. Citation1997, Citation2000, Killian Citation1998, de Planque et al. Citation1998, Citation1999, Citation2001, Citation2002, Citation2003, Killian and von Heijne Citation2000, Weiss et al. Citation2003). These studies have shown that flanking residues have a large impact on the effect of hydrophobic mismatch, where Lys do not induce any ordering in the lipids. The results show that there is a large variety in the response to hydrophobic mismatch and that flanking residues have an important role in this response.
Solution-state NMR, offers several possibilities to monitor the location of peptides and proteins in a membrane mimetic, while these methods are not as direct as the ones used in solid-state NMR. In addition to the dynamic investigations of lipids described earlier, techniques include paramagnetic relaxation enhancement, hydrogen to deuterium exchange and measurements of NOEs between peptides and lipids, among others. Typically several methods are used in combination to give a rather complete picture of the location of a peptide. In early studies by Papavoine et al. (Citation1994, Citation1995), using paramagnetic labels and hydrogen to deuterium exchange in combination with a structural analysis allowed for determining the location of the major coat protein of bacteriophage M13 in DPC micelles. The following presentation of these methods provides an overview of these solution-state techniques for selected applications, including cell-penetrating peptides.
Paramagnetic relaxation enhancement
In the field of solution NMR, the relaxation-based methods described above have been used to characterize the interactions between peptides and lipids. A more simple approach is the use of paramagnetic spin-labels for studying paramagnetic relaxation enhancement (PRE) effects. The presence of paramagnetic species affects NMR properties in many ways. One way is through enhancement of the relaxation rates for nuclei close to the paramagnetic agent. This enhancement depends on the relationship between the amount of molecules in close coordination to the paramagnetic species and those in the bulk, and if they interact weakly or strongly (outer- and inner-sphere PRE, respectively) (Kowalewski and Mäler Citation2006). The theory behind this phenomenon is rather cumbersome, but nevertheless, measurements of enhanced relaxation rates for sites in a protein can at least on a semi-quantitative level be evaluated by measuring the remaining amplitude of cross-peaks in an NMR spectrum.
Spin-labels used in membrane research are typically stable radicals (doxyl or tempo moieties) attached to lipid molecules at varying positions in the lipid (Chattopadhyay and London Citation1987, Abrams and London Citation1993, Damberg et al. Citation2001). Additionally, soluble paramagnetic species, such as Mn2+ ions or various Gd3+ complexes are used (Damberg et al. Citation2001, Pintacuda and Otting Citation2002). By combining data from a set of such spin-labels, a picture of how a peptide or protein is located in the bilayer may be obtained (). As an example we return again to the CPPs. By combining the information from PREs (measured through the remaining amplitude of signals in the NMR spectrum) from POPC labeled with a doxyl group in position 5 and 12 in the acyl chain and from Mn2+ in solution, it was concluded that the location of penetratin was slightly tilted, with the N-terminus embedded inside a DMPC bicelle (Lindberg et al. Citation2003). Interestingly, a non-translocating variant, in which the two Trp residues were mutated to Phe, of penetratin adopted a slightly different orientation, with a more overall shallow penetration. Similar measurements in SDS gave very different results indicating that the peptide interacts with the two membrane mimetics in very different ways, in agreement with the measurements of peptide dynamics discussed earlier.
Figure 7. Cartoon over the location of commonly used spin-labeled lipids and paramagnetic metals (Mn2+) in solution NMR for elucidating peptide position in a DMPC bilayer. The spheres indicate the position the DOXYL label in 5- and 12-DOXYL labeled lipids. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
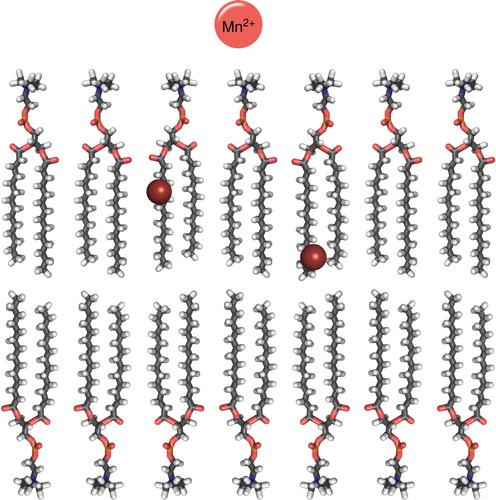
This approach was also used for transportan (Bárány-Wallje et al. Citation2004), where it was observed that the PRE from doxyl-labelled lipids gave a variation in the effect along the sequence with a periodicity of 3–4 residues, indicating a helical structure with an orientation parallel to the surface of the bicelle bilayered part. Contrary to penetratin, in this case the results were similar to what was observed in SDS micelles (Lindberg et al. Citation2001).
The location of the antimicrobial β-hairpin peptide aranicin was studied using similar probes, Mn2+ and 16-doxylstearate (Shenkarev et al. Citation2011). These probes indicated that the peptide, shown in structural structural studies to be a dimeric β-sheet, and located inside the interior of a DPC micelle. In the structural and dynamical study of the antimicrobial peptide maximin H6 (Kosol and Zangger Citation2010), which adopts an α-helical structure in both DPC and SDS, the soluble paramagnetic complex Gd(DTPA-BMA) was used to determine the location of the peptide. The data indicated different orientations in the two solvents, and a location closer to the surface in SDS, which was explained in part by the difference in the detergents (head-group charge).
Backbone HN exchange
In well-folded proteins, it is easy to monitor the rate of exchange between backbone HN protons and solvent. By dissolving the peptide or protein in 2H2O, exchange between the protonated amide groups and 2H in the solvent may take place, which makes the HN signal disappear in, e.g., a 15N-1H HSQC spectrum. Hence, relative peak heights give information about the rate of exchange. Typically, the intrinsic exchange rate for such a proton is very rapid (Englander et al. Citation1972, Molday et al. Citation1972), and one observes either the absence or presence of a peak in the NMR spectrum. This method has been used extensively in protein folding, but also in studies of membrane-bound peptides.
For the CPP transportan, exchange monitored in this way revealed that all amide protons, starting with the most N- and C-terminal had completely exchanged for deuterium within 30 minutes (Bárány-Wallje et al. Citation2006). The same behaviour was observed in both zwitterionic and partly negatively charged bicelles indicating a similar location of the peptide in both media. This is in great contrast to similar measurements for hydrophobic CPPs derived from the prion and doppel proteins, which reside more or less transmembrane in a bilayer (Biverståhl et al. Citation2004, Papadopoulos et al. Citation2006). In those cases, complete exchange for residues in the ‘transmembrane’ part of the peptides was slower than several hours or days.
Wang et al. (Citation2011) studied the amide proton exchange in the human LL-23 antimicrobial peptide. The results indicated that the central part of the helical structure was embedded in the interior of DPC micelles, and they also found that variants of the peptide inserted deeper into the micelle, as evidenced by slower exchange. This could in turn be correlated with their antimicrobial activity, which clearly demonstrates the importance of interaction studies.
Peptide-lipid NOEs
Direct evidence of the location of a peptide in bilayer or micelle can be obtained by observing NOEs (or distances) between certain parts of a lipid molecule and the peptide. This can be done in several ways, and perhaps the most elegant is to use saturation transfer difference spectroscopy, STD (Mayer and Meyer Citation1999, Citation2001). This method is commonly used to study binding between molecules, e.g., between enzymes and substrates or between receptors and ligands. By this method, different resonances in the lipid molecule are irradiated and the effect on signal intensities in the peptide molecule is monitored. Build-up of magnetization, due to cross-relaxation or NOE, on spins close in space to the irradiated lipid spins can thus reveal relative distances between the lipid and the peptide.
Alternatively, cross-peaks between lipids (or detergents) and the peptide in a NOESY experiment provide information about the proximity of the two. It is, however, often difficult to accurately determine the intensities of these peaks, which precludes determination of a precise location of the peptide. Nevertheless, observation of such cross-peaks provides evidence of a peptide-lipid interaction.
Turning again to the field of CPPs, the location of penetratin (or pAntp) in q = 1 DMPC/DMPG/DHPC bicelles was studied by Zhang and Smith (Citation2005) and they used a combination of solution-state and solid-state NMR techniques. They identified several cross-peaks between penetratin and lipid molecules, and in particular between Trp residues, crucial for translocation of the peptide, and lipid acyl chain protons in NOESY experiments. The results differed markedly for the non-translocating variant, in which the two Trp residues were mutated to Phe. Their findings indicate, in agreement with other studies, that penetratin inserts into the bilayer, and they proposed a mechanism for the translocation of the peptide based on electroporation.
Conclusions
NMR is a method by which many different molecular properties connected to molecular interactions can be studied. Hence, details in lipid-protein or peptide interactions can readily be investigated. Standard methods for determining solution structures can be used provided that the size of the protein/peptide in complex with detergents or lipids is not too large. Detergents are thus still mainly used to derive high quality structures of membrane proteins. In most cases this works, and the properties and activity of the protein or peptide can be checked against more realistic membrane mimetic media such as bicelles or nanodiscs. In certain cases, however, differences between structures determined in detergents compared to in more realistic lipid mixtures are observed.
The most interesting features of peptide-lipid interactions are perhaps those associated with the position of a peptide in a bilayer, and how dynamic both the peptide and lipid surrounding is. Combined with effects that the peptide has on lipid dynamics and order, this information is essential for the understanding of the action of peptides on membranes. NMR is somewhat unique as it can provide information about both peptide and lipid properties in the same sample. For such studies to be valuable, realistic membrane models are crucial. Detergents typically cover the hydrophobic parts of peptides and proteins, which do not give a realistic picture of the position.
In summary, the action of peptides on bilayer properties is typically governed by the possibility to undergo structural conversions, which implies a high degree of dynamics, in combination with affecting the lipid arrangements around the peptide. Hence, the emphasis on peptide-lipid interaction should be not so much on determining one static structure but on dynamics, and the influence of lipids, and conversely, the influence of peptides on lipids.
Acknowledgements
The author thanks Sofia Unnerståle, Weihua Ye and Johannes Björnerås for discussions, and Jesper Lind and Hans Adolfsson for help with the Figures. This work was supported by grants from the Swedish Research Council (grant nr 621-2011-5964), Carl Trygger Foundation, and Magnus Bergvall Foundation.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Abrams FS, London E. 1993. Extension of the parallax analysis of membrane penetration depth to the polar region of model membranes: use of fluorescence quenching by a spin-label attached to the phospholipid polar headgroup. Biochemistry 32:10826–10831.
- Akke M, Palmer AG. 1996. Monitoring macromolecular motions on microsecond to millisecond time scales by R(1)r-R(1) constant relaxation time NMR spectroscopy. J Am Chem Soc 118:911–912.
- Andersson A, Mäler L. 2002. NMR solution structure and dynamics of motilin in isotropic phospholipid bicellar solution. J Biomol NMR 24:103–112.
- Andersson A, Almqvist J, Hagn F, Mäler L. 2004. Diffusion and dynamics of penetratin in different membrane mimicking media. BBA 1661:18–25.
- Andersson A, Mäler L. 2005. Magnetic resonance investigations of lipid motion in isotropic bicelles. Langmuir 21:7702–7709.
- Andersson A, Biverståhl H, Nordin J, Danielsson J, Lindahl E, Mäler L. 2007. The membrane-induced structure of melittin is correlated with the fluidity of the lipids. Biochim Biophys Acta 1768:115–121.
- Aniansson E, Wall S, Almgren M, Hoffmann H, Kielmann I, Ulbricht W, 1976. Theory of the kinetics of micellar equilibria and quantitative interpretation of chemical relaxation studies of micellar solutions of ionic surfactants. J Phys Chem 80:905–922.
- Arora A, Tamm LK. 2001. Biophysical approaches to membrane protein structure determination. Curr Opin Struct Biol 11:540–547.
- Arora A, Abildgaard F, Bushweller JH, Tamm LK. 2001. Structure of outer membrane protein A transmembrane domain by NMR spectroscopy. Nat Struct Biol 8:334–338.
- Baas BJ, Denisov IG, Sligar SG. 2004. Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. Arch Biochem Biophys 430:218–228.
- Bárány-Wallje E, Andersson A, Gräslund A, Mäler L. 2004. NMR solution structure and position of transportan in neutral phospholipid bicelles. FEBS Lett 567:265–269.
- Bárány-Wallje E, Andersson A, Gräslund A, Mäler L. 2006. Dynamics of transportan in bicelles is surface charge dependent. J Biomol NMR 35:137–147.
- Batenburg AM, van Esch JH, de Kruijff B. 1988. Melittin-induced changes of the macroscopic structure of phosphatidylethanolamines. Biochemistry 27:2324–2331.
- Bayburt TH, Grinkova YV, Sligar SG. 2002. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Letters 2:853–856.
- Bayburt TH, Sligar SG. 2002. Single-molecule height measurements on microsomal cytochrome P450 in nanometer-scale phospholipid bilayer disks. Proc Natl Acad Sci USA 99:6725.
- Bayburt TH, Sligar SG. 2003. Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein Sci 12:2476–2481.
- Biverståhl H, Andersson A, Gräslund A, Mäler L. 2004. NMR solution structure and membrane interaction studies of the N-terminal sequence (1–30) of the bovine prion protein. Biochemistry 43:14940–14947.
- Bodenhausen G, Wagner G, Rance M, Sørensen O, Wüthrich K, Ernst R. 1984. Longitudinal two-spin order in 2D exchange spectroscopy (NOESY). J Magn Reson 59:542–550.
- Borch J, Hamann T. 2009. The nanodisc: a novel tool for membrane protein studies. Biol Chem 390:805–814.
- Brender JR, Dürr UHN, Heyl D, Budarapu MB, Ramamoorthy A. 2007. Membrane fragmentation by an amyloidogenic fragment of human islet amyloid polypeptide detected by solid-state NMR spectroscopy of membrane nanotubes. Biochim Biophys Acta 1768:2026–2029.
- Brown MF, Ribeiro AA, Williams GD. 1983. New view of lipid bilayer dynamics from 2H and 13C NMR relaxation time measurements. Proc Natl Acad Sci USA 80:4325–4329.
- Brown MF, Thurmond RL, Dodd SW, Otten D, Beyer K. 2000. Elastic deformation of membrane bilayers probed by deuterium NMR relaxation. Biochemistry 39:1833.
- Butterwick JA, MacKinnon R. 2010. Solution structure and phospholipid interactions of the isolated voltage-sensor domain from KvAP. J Mol Biol 403:591–606.
- Callaghan P, Komlosh M, Nydén M. 1998. High magnetic field gradient PGSE NMR inthe presence of a large polarizing field. J Magn Reson 133:177–182.
- Cantor CR, Schimmel PR. 1980. Biophysical chemistry. San Francisco: WH Freeman.
- Cavanagh J, Fairbrother WJ, Palmer AG, Rance M, Skelton NJ. 2007. Protein NMR spectroscopy. Burlington, MA: Academic Press.
- Chattopadhyay A, Harikumar K. 1996. Dependence of critical micelle concentration of a zwitterionic detergent on ionic strength: Implications in receptor solubilization. FEBS Lett 391:199–202.
- Chattopadhyay A, London E. 1987. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry 26:39–45.
- Chill JH, Louis JM, Baber JL, Bax A. 2006. Measurement of 15N relaxation in detergent-solubilized tetrameric KcsA potassium channel. J Biomol NMR 36:123–136.
- Chill JH, Naider F. 2011. A solution NMR view of protein dynamics in the biological membrane. Curr Opin Struct Biol 21:627–633.
- Chou JJ, Kaufman JD, Stahl SJ, Wingfield PT, Bax A. 2002. Micelle-induced curvature in a water-insoluble HIV-1 env peptide revealed by NMR dipolar coupling measurement in stretched polyacrylamide gel. J Am Chem Soc 124:2450–2451.
- Chou JJ, Baber JL, Bax A. 2004. Characterization of phospholipid mixed micelles by translational diffusion. J Biomol NMR 29:299–308.
- Chromy BA, Arroyo E, Blanchette CD, Bench G, Benner H, Cappuccio JA, 2007. Different apolipoproteins impact nanolipoprotein particle formation. J Am Chem Soc 129:14348–14354.
- Clore GM, Szabo A, Bax A, Kay LE, Driscoll PC, Gronenborn AM. 1990. Deviations from the simple two-parameter model-free approach to the interpretation of nitrogen-15 nuclear magnetic relaxation of proteins. J Am Chem Soc 112:4989–4991.
- Clore GM, Gronenborn AM. 1994. Multidimensional heteronuclear nuclear magnetic resonance of proteins. Meth Enzymol 239:349–363.
- Czajlik A, Meskó E, Penke B, Perczel A. 2002. Investigation of penetratin peptides part 1. The environment dependent conformational properties of penetratin and two of its derivatives. J Peptide Sci 8:151–171.
- Damberg P, Jarvet J, Gräslund A. 2001. Micellar systems as solvents in peptide and protein structure determination. Meth Enzymol 339:271–285.
- De Angelis AAD, Jones D, Grant C, Park S, Mesleh M, Opella S. 2005. NMR experiments on aligned samples of membrane proteins. Meth Enzymol 394:350–382.
- De Angelis AA, Opella SJ. 2007. Bicelle samples for solid-state NMR of membrane proteins. Nature Protocols 2:2332–2338.
- De Angelis AA, Howell SC, Nevzorov AA, Opella SJ. 2005. Structure determination of a membrane protein with two trans-membrane helices in aligned phospholipid bicelles by solid-state NMR spectroscopy. J Am Chem Soc 128:12256–12267.
- de Planque MRR, Greathouse DV, Koeppe RE II, Schäfer H, Marsh D, Killian JA. 1998. Influence of lipid/peptide hydrophobic mismatch on the thickness of diacylphosphatidylcholine bilayers. A 2H NMR and ESR study using designed transmembrane α-helical peptides and gramicidin A. Biochemistry 37:9333–9345.
- de Planque MRR, Kruijtzer JAW, Liskamp RMJ, Marsh D, Greathouse DV, Koeppe RE, 1999. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane α-helical peptides. J Biol Chem 274:20839–20846.
- de Planque MRR, Goormaghtigh E, Greathouse DV, Koeppe RE II, Kruijtzer JAW, Liskamp RMJ, 2001. Sensitivity of single membrane-spanning α-helical peptides to hydrophobic mismatch with a lipid bilayer: Effects on backbone structure, orientation, and extent of membrane incorporation. Biochemistry 40:5000–5010.
- de Planque MRR, Boots JWP, Rijkers DTS, Liskamp RMJ, Greathouse DV, Killian JA. 2002. The effects of hydrophobic mismatch between phosphatidylcholine bilayers and transmembrane α-helical peptides depend on the nature of interfacially exposed aromatic and charged residues. Biochemistry 41:8396–8404.
- de Planque MRR, Bonev BB, Demmers JAA, Greathouse DV, Koeppe RE II, Separovic F, 2003. Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry 42:5341–5348.
- de Planque MRR, Killian JA. 2003. Protein-lipid interactions studied with designed transmembrane peptides: role of hydrophobic matching and interfacial anchoring. Mol Membr Biol 20:271–284.
- Dempsey CE. 1990. The actions of melittin on membranes. Biochim Biophys Acta 1031:143–161.
- Denisov I, Grinkova Y, Lazarides A, Sligar S. 2004. Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J Am Chem Soc 126:3477–3487.
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. 1994. The third helix of the antennapedia homeodomain translocates through biological membranes. J Biol Chem 269:10444–10450.
- Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. 1996. Cell internalization of the third helix of the antennapedia homeodomain is receptor-independent. J Biol Chem 271:18188–18193.
- Derossi D, Chassing G, Prochiantz A. 1998. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol 8:84–87.
- Deshayes S, Plénat T, Aldrian-Herrada G, Divita G, Le Grimellec C, Heitz F. 2004. Primary amphipathic cell-penetrating peptides: structural requirements and interactions with model membranes. Biochemistry 43:7698–7706.
- Deshayes S, Morris M, Divita G, Heitz F. 2005. Cell-penetrating peptides: Tools for intracellular delivery of therapeutics. Cell Mol Life Sci 62:1839–1849.
- Dufourc EJ, Smith ICP, Dufourcq J. 1986. Molecular details of melittin-induced lysis of phospholipid membranes as revealed by deuterium and phosphorus NMR. Biochemistry 25:6448–6455.
- Dufourcq J, Faucon JF, Fourche G, Dasseux JL, Le Maire M, Gulik-Krzywicki T. 1986. Morphological changes of phosphatidylcholine bilayers induced by melittin: Vesicularization, fusion, discoidal particles. Biochim Biophys Acta 859:33–48.
- Dürr UHN, Yamamoto K, Im SC, Waskell L, Ramamoorthy A. 2007. Solid-state NMR reveals structural and dynamical properties of a membrane-anchored electron-carrier protein, cytochrome b 5. J Am Chem Soc 129:6670–6671.
- Dvinskikh S, Dürr U, Yamamoto K, Ramamoorthy A. 2006. A high resolution solid state NMR approach for the structural studies of bicelles. J Am Chem Soc 128:6326.
- Dvinskikh SV, Castro V, Sandström D. 2005. Probing segmental order in lipid bilayers at variable hydration levels by amplitude-and phase-modulated cross-polarization NMR. Phys Chem Chem Phys 7:3255–3257.
- Ellena JF, Lepore LS, Cafiso DS. 1993. Estimating lipid lateral diffusion in phospholipid vesicles from carbon-13 spin-spin relaxation. J Phys Chem 97:2952–2957.
- Englander S, Downer N, Teitelbaum H. 1972. Hydrogen exchange. Annu Rev Biochem 41:903–924.
- Evans FD, Wennerström H. 1999. The colloidal domain. New York: Wiley. 1999.
- Fernández C, Hilty C, Bonjour S, Adeishvili K, Pervushin K, Wüthrich K. 2001. Solution NMR studies of the integral membrane proteins OmpX and OmpA from Escherichia coli. FEBS Lett 504:173–178.
- Fernández C, Hilty C, Wider G, Guntert P, Wüthrich K. 2004. NMR structure of the integral membrane protein OmpX. J Mol Biol 336:1211–1221.
- Filippov A, Orädd G, Lindblom G. 2004. Lipid lateral diffusion in ordered and disordered phases in raft mixtures. Biophys J 86:891–896.
- Freed J. 2000. New technologies in electron spin resonance. Annu Rev Phys Chem 51:655–689.
- Funasaki N, Hada S, Neya S. 1991. Odd-even alternation in the aggregation number dependence of stepwise aggregation constants. J Phys Chem 95:1846–1850.
- Fuson M, Prestegard J. 1983. Dynamics of an interfacial methylene in dimyristoylphosphatidylcholine vesicles using carbon-13 spin relaxation. Biochemistry 22:1311–1316.
- Gabriel NE, Roberts MF. 1984. Spontaneous formation of stable unilamellar vesicles. Biochemistry 23:4011–4015.
- Gaede HC, Gawrisch K. 2003. Lateral diffusion rates of lipid, water, and a hydrophobic drug in a multilamellar liposome. Biophys J 85:1734–1740.
- Gaemers S, Bax A. 2001. Morphology of three lyotropic liquid crystalline biological NMR media studied by translational diffusion anisotropy. J Am Chem Soc 123:12343–12352.
- Gautier A, Mott HR, Bostock MJ, Kirkpatrick JP, Nietlispach D. 2010. Structure determination of the seven-helix transmembrane receptor sensory rhodopsin II by solution NMR spectroscopy. Nature Struct Mol Biol 17:768–774.
- Gent M, Prestegard J. 1974. Comparison of 13C spin-lattice relaxation times in phospholipid vesicles and multilayers. Biochem Biophys Res Commun 58:549–555.
- Glover KJ, Whiles JA, Wu G, Yu N, Deems R, Struppe JO, 2001. Structural evaluation of phospholipid bicelles for solution-state studies of membrane-associated biomolecules. Biophys J 81:2163–2171.
- Glück JM, Wittlich M, Feuerstein S, Hoffmann S, Willbold D, Koenig BW. 2009. Integral membrane proteins in nanodiscs can be studied by solution NMR spectroscopy. J Am Chem Soc 131:12060–12061.
- Gräslund A, Mäler L. 2011. Testing membrane interactions of CPPs. Methods Mol Biol 683:33–40.
- Guterstam P, Madani F, Hirose H, Takeuchi T, Futaki S, EL Andaloussi S, 2009. Elucidating cell-penetrating peptide mechanisms of action for membrane interaction, cellular uptake, and translocation utilizing the hydrophobic counter-anion pyrenebutyrate. Biochim Biophys Acta 1788:2509–2517.
- Habermann E. 1972. Bee and wasp venoms. Science 177:314.
- Halle B, Wennerström H. 1981. Interpretation of magnetic resonance data from water nuclei in heterogeneous systems. J Chem Phys 75:1928–1943.
- Hansen DF, Vallurupalli P, Kay LE. 2008. Using relaxation dispersion NMR spectroscopy to determine structures of excited, invisible protein states. J Biomol NMR 41:113–120.
- Hauser H. 2000. Short-chain phospholipids as detergents. Biochim Biophys Acta 1508:164–181.
- Herbig ME, Weller K, Krauss U, Beck-Sickinger AG, Merkle HP, Zerbe O. 2005. Membrane surface-associated helices promote lipid interactions and cellular uptake of human calcitonin-derived cell penetrating peptides. Biophys J 89:4056–4066.
- Higashijima T, Uzu S, Nakajima T, Ross EM. 1988. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem 263:6491.
- Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. 2008. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321:1206.
- Hope M, Bally M, Mayer L, Janoff A, Cullis P. 1986. Generation of multilamellar and unilamellar phospholipid vesicles. Chem Phys Lipids 40:89–107.
- Hristova K, Wimley WC. 2011. A look at arginine in membranes. J Membr Biol 239:49–56.
- Hwang PM, Bishp RE, Kay LE. 2004. The integral membrane enzyme PagP alternates between two dynamically distinct states. Proc Natl Acad Sci USA 101:9618–9623.
- Itri R, Amaral L. 1991. Distance distribution function of sodium dodecyl sulfate micelles by x-ray scattering. J Phys Chem 95:423–427.
- Jarvet J, Zdunek J, Damberg P, Gräslund A. 1997. Three-dimensional structure and position of porcine motilin in sodium dodecyl sulfate micelles determined by 1H NMR. Biochemistry 36:8153–8163.
- Jeener J, Meier BH, Bachmann P, Ernst RR. 1979. Investigation of exchange processes by two-dimensional NMR spectroscopy. J Chem Phys 71:4546.
- Jhun BH, Berenski CJ, Craik JD, Paterson ARP, Cass CE, Jung CY. 1991. Glucose and nucleoside transporters of human erythrocytes: effects of detergents on immunoadsorption of a membrane protein to its monoclonal antibody. Biochim Biophys Acta 1061:149–155.
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, 2003a. X-ray structure of a voltage-dependent K+ channel. Nature 423:33–41.
- Jiang Y, Ruta V, Chen J, Lee A, MacKinnon R. 2003b. The principle of gating charge movement in a voltage-dependent K+ channel. Nature 423:42–48.
- Jonas A. 1986. Reconstitution of high-density lipoproteins. Meth Enzymol 128:553–582.
- Kainosho M, Torizawa T, Iwashita Y, Terauchi T, Mei Ono A, Güntert P. 2006. Optimal isotope labelling for NMR protein structure determinations. Nature 440:52–57.
- Kang CB, Li Q. 2011. Solution NMR study of integral membrane proteins. Curr Opin Chem Biol 15:560–569.
- Katsaras J, Harroun TA, Pencer J, Nieh MP. 2005. “Bicellar” lipid mixtures as used in biochemical and biophysical studies. Naturwissenschaften 92:355–366.
- Killian JA, Salemink I, de Planque MRR, Lindblom G, Koeppe RE II, Greathouse DV. 1996. Induction of nonbilayer structures in diacylphosphatidylcholine model membranes by transmembrane α-helical peptides: Importance of hydrophobic mismatch and proposed role of tryptophans. Biochemistry 35:1037–1045.
- Killian JA. 1998. Hydrophobic mismatch between proteins and lipids in membranes. Biochim Biophys Acta 1376:401–415.
- Killian JA, von Heijne G. 2000. How proteins adapt to a membrane-water interface. Trends Biochem Sci 25:429–434.
- Killian JA. 2003. Synthetic peptides as models for intrinsic membrane proteins. FEBS Lett 555:134–138.
- Kim HJ, Howell SC, Van Horn WD, Jeon YH, Sanders CR. 2009. Recent advances in the application of solution NMR spectroscopy to multi-span integral membrane proteins. Prog Nucl Magn Reson Spectrosc 55:335.
- Kobayashi S, Takeshima K, Park CB, Kim SC, Matsuzaki K. 2000. Interactions of the novel antimicrobial peptide buforin 2 with lipid bilayers: Proline as a translocation promoting factor. Biochemistry 39:8648–8654.
- Korstanje LJ, van Faassen EE, Levine YK. 1989. Reorientational dynamics in lipid vesicles and liposomes studied with ESR: Effects of hydration, curvature and unsaturation. Biochim Biophys Acta 982:196–204.
- Kosol S, Zangger K. 2010. Dynamics and orientation of a cationic antimicrobial peptide in two membrane-mimetic systems. J Struct Biol 170:172–179.
- Kowalewski J, Mäler L. 2006. Nuclear spin relaxation in liquids: Theory, experiments, and applications. Boca Raton, FL: Taylor & Francis.
- Langel Ü. 2002. Cell-penetrating peptides. Processes and applications. Boca Raton, FL: CRC Press.
- Lau TL, Kim C, Ginsberg MH, Ulmer TS. 2009. The structure of the integrin αIIbβ3 transmembrane complex explains integrin transmembrane signalling. EMBO J 28:1351–1361.
- Lau TL, Partridge AW, Ginsberg MH, Ulmer TS. 2008. Structure of the integrin β3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry 47:4008–4016.
- Lauterwein J, Bosch C, Brown LR, Wuthrich K. 1979. Physicochemical studies of the protein-lipid interactions in melittin-containing micelles. Biochim Biophys Acta 556:244–264.
- Leftin A, Brown MF. 2011. An NMR database for simulations of membrane dynamics. Biochim Biophys Acta 1808:818–839.
- le Maire M, Champeil P, Møller JV. 2000. Interaction of membrane proteins and lipids with solubilizing detergents. Biochimica et Biophysica Acta (BBA) – Biomembranes 1508:86–111.
- Lepore LS, Ellena JF, Cafiso DS. 1992. Comparison of the lipid acyl chain dynamics between small and large unilamellar vesicles. Biophys J 61:767–775.
- Liang B, Arora A, Tamm LK. 2010. Fast-time scale dynamics of outer membrane protein A by extended model-free analysis of relaxation data. Biochim Biophys Acta 1798:68–76.
- Lin TL, Chen SH, Gabriel NE, Roberts MF. 1986. The use of small-angle neutron scattering to determine the structure and interaction of dihexanoylphosphatidylcholine micelles. J Am Chem Soc 108:3499–3507.
- Lin TL, Chen SH, Gabriel NE, Roberts MF. 1987. Small-angle neutron scattering techniques applied to the study of polydisperse rodlike diheptanoylphosphatidylcholine micelles. J Phys Chem 91:406–413.
- Lin TL, Liu CC, Roberts MF, Chen SH. 1991. Structure of mixed short-chain lecithin/long-chain lecithin aggregates studied by small-angle neutron scattering. J Phys Chem 95:6020–6027.
- Lind J, Rämö T, Klement MLR, Bárány-Wallje E, Epand RM, Epand RF, 2007. High cationic charge and bilayer interface-binding helices in a regulatory lipid glycosyltransferase. Biochemistry 46:5664–5677.
- Lind J, Nordin J, Mäler L. 2008. Lipid dynamics in fast-tumbling bicelles with varying bilayer thickness: Effect of model transmembrane peptides. Biochim Biophys Acta 1778:2526–2534.
- Lindberg M, Jarvet J, Langel U, Gräslund A. 2001. Secondary structure and position of the cell-penetrating peptide transportan in SDS micelles as determined by NMR. Biochemistry 40:3141–3149.
- Lindberg M, Biverståhl H, Gräslund A, Mäler L. 2003. Structure and positioning comparison of two variants of penetratin in two different membrane mimicking systems by NMR. FEBS Journal 270:3055–3063.
- Lindblom G, Orädd G. 1994. NMR studies of translational diffusion in lyotropic liquid crystals and lipid membranes. Prog Nuc Mag Reson Specstrosc 26:483–516.
- Lindgren M, Gallet X, Soomets U, Hällbrink M, Bråkenhielm E, Pooga M, 2000a. Translocation properties of novel cell penetrating transportan and penetratin analogues. Bioconjug Chem 11:619–626.
- Lindgren M, Hällbrink M, Prochiantz A, Langel Ü. 2000b. Cell-penetrating peptides. Trends Pharmacol Sci 21:99–103.
- Lipari G, Szabo A. 1982a. Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J Am Chem Soc 104:4546–4559.
- Lipari G, Szabo A. 1982b. Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 2. Analysis of experimental results. J Am Chem Soc 104:4559–4570.
- Liu JJ, Horst R, Katritch V, Stevens RC, Wüthrich K. 2012. Biased signaling pathways in b2-adrenergic receptor characterized by 19F-NMR. Science 335:1106–110.
- Long SB, Tao X, Campbell EB, MacKinnon R. 2007. Atomic structure of a voltage-dependent K channel in a lipid membrane-like environment. Nature 450:376–382.
- Loria JP, Rance M, Palmer AG III. 1999. A relaxation-compensated carr-purcell-meiboom-gill sequence for characterizing chemical exchange by NMR spectroscopy. J Am Chem Soc 121:2331–2332.
- Lou Y, Ge M, Freed JH. 2001. A multifrequency ESR study of the complex dynamics of membranes. J Phys Chem B 105:11053–11056.
- Lu Z, Van Horn WD, Chen J, Mathew S, Zent R, Sanders CR. 2012. Bicelles at low concentrations. Mol. Pharmaceutics 9:752–761.
- Luchette PA, Vetman TN, Prosser RS, Hancock REW, Nieh MP, Glinka CJ, 2001. Morphology of fast-tumbling bicelles: A small angle neutron scattering and NMR study. Biochim Biophys Acta 1513:83–94.
- Lundberg P, Magzoub M, Lindberg M, Hällbrink M, Jarvet J, Eriksson L, 2002. Cell membrane translocation of the N-terminal (1–28) part of the prion protein. Biochem Biophys Res Commun 299:85–90.
- Lyukmanova EN, Shenkarev ZO, Paramonov AS, Sobol AG, Ovchinnikova TV, Chupin VV, 2008. Lipid-protein nanoscale bilayers: A versatile medium for NMR investigations of membrane proteins and membrane-active peptides. J Am Chem Soc 130:2140–2141.
- Madani F, Lindberg S, Langel U, Futaki S, Gräslund A. 2011. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011:414729.
- Magzoub M, Eriksson LE, Gräslund A. 2002. Conformational states of the cell-penetrating peptide penetratin when interacting with phospholipid vesicles: Effects of surface charge and peptide concentration. Biochim Biophys Acta 1563:53–63.
- Magzoub M, Eriksson LE, Gräslund A. 2003. Comparison of the interaction, positioning, structure induction and membrane perturbation of cell-penetrating peptides and non-translocating variants with phospholipid vesicles. Biophys Chem 103:271–288.
- Magzoub M, Gräslund A. 2004. Cell-penetrating peptides: From inception to application. Q Rev Biophys 37:147–195.
- Magzoub M, Oglecka K, Pramanik A, Eriksson LE, Graslund A. 2005. Membrane perturbation effects of peptides derived from the N-termini of unprocessed prion proteins. Biochim Biophys Acta 1716:126–136.
- Mäler L, Gräslund A. 2009. Artificial membrane models for the study of macromolecular delivery. Methods Mol Biol 480:129–139.
- Mäler L, Gräslund A. 2011. NMR studies of three-dimensional structure and positioning of CPPs in membrane model systems. Methods Mol Biol 683:57–67.
- Marcotte I, Auger M. 2005. Bicelles as model membranes for solid-and solution-state NMR studies of membrane peptides and proteins. Concepts Magn Reson 24:17–37.
- Matsuzaki K, Murase O, Fujii N, Miyajima K. 1995. Translocation of a channel-forming antimicrobial peptide, magainin 2, across lipid bilayers by forming a pore. Biochemistry 34:6521–6526.
- Mayer C, Grobner G, Muller K, Weisz K, Kothe G. 1990. Orientation-dependent deuteron spin-lattice relaxation times in bilayer membranes: Characterization of the overall lipid motion. Chem Phys Lett 165:155–161.
- Mayer L, Hope M, Cullis P. 1986. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta 858:161–168.
- Mayer M, Meyer B. 1999. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angewandte Chemie 38:1784–1788.
- Mayer M, Meyer J. 2001. Group epitope mapping by saturation transfer difference NMR to identify segments of a ligand in direct contact with a protein receptor. J Am Chem Soc 123:6108–6117.
- Metcalfe EE, Zamoon J, Thomas DD, Veglia G. 2004. 1H/15N Heteronuclear NMR spectroscopy shows four dynamic domains for phospholamban reconstituted in dodecylphosphocholine micelles. Biophys J 87:1205–1214.
- Molday R, Englander S, Kallen R. 1972. Primary structure effects on peptide group hydrogen exchange. Biochemistry 11:150–158.
- Morein S, Koeppe RE II, Lindblom G, de Kruijff B, Antoinette Killian J. 2000. The effect of peptide/lipid hydrophobic mismatch on the phase behavior of model membranes mimicking the lipid composition in Escherichia coli membranes. Biophys J 78:2475–2485.
- Morein S, Strandberg E, Killian J, Persson S, Arvidson G, Koeppe R, 1997. Influence of membrane-spanning alpha-helical peptides on the phase behavior of the dioleoylphosphatidylcholine/water system. Biophys J 73:3078–3088.
- Nakano M, Fukuda M, Kudo T, Miyazaki M, Wada Y, Matsuzaki N, 2009. Static and dynamic properties of phospholipid bilayer nanodiscs. J Am Chem Soc 131:8308–8312.
- Nath A, Atkins WM, Sligar SG. 2007. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry 46:2059–2069.
- Nieh M-P, Glinka CJ, Krueger S, Prosser RS, Katsaras J. 2001. SANS study of the structural phases of magnetically alignable lanthanide-doped phospholipid mixtures. Langmuir 17:2629–2638.
- Nunziante M, Gilch S, Schätzl HM. 2003. Essential role of the prion protein N terminus in subcellular trafficking and half-life of cellular prion protein. J Biol Chem 278:3726.
- Öhman A, Lycksell P, Juréus A, Langel Ü, Bartfai T, Gräslund A. 1998. NMR study of the conformation and localization of porcine galanin in SDS micelles. Comparsion with an inactive analog and a galanin receptor antagonist. Biochemistry 37:9169–9178.
- Orädd G, Lindblom G. 2004a. Nmr studies of lipid lateral diffusion in the DMPC/gramicidin D/water system: Peptide aggregation and obstruction effects. Biophys J 87:980–987.
- Orädd G, Lindblom G. 2004b. Lateral diffusion studied by pulsed field gradient NMR on oriented lipid membranes. Magn Reson Chem 42:123–131.
- Ottiger M, Bax A. 1998. Characterization of magnetically oriented phospholipid micelles for measurement of dipolar couplings in macromolecules. J Biomol NMR 12:361–372.
- Oxenoid K, Sönnichsen FD, Sanders CR. 2002. Topology and secondary structure of the N-terminal domain of diacylglycerol kinase. Biochemistry 41:12876–12882.
- Oxenoid K, Kim HJ, Jacob J, Sönnichsen FD, Sanders CR. 2004. NMR assignments for a helical 40 kDa membrane protein. J Am Chem Soc 126:5048–5049.
- Palmer AG. 2004. NMR characterization of the dynamics of biomacromolecules. Chem Rev 104:3623–3640.
- Palmer AG, Kroenke CD, Loria JP. 2001. Nuclear magnetic resonance methods for quantifying microsecond-to-millisecond motions in biological macromolecules. Meth Enzymol 339:204–238.
- Papadopoulos E, Oglecka K, Mäler L, Jarvet J, Wright PE, Dyson HJ, 2006. NMR solution structure of the peptide fragment 1–30, derived from unprocessed mouse doppel protein, in DHPC micelles. Biochemistry 45:159–166.
- Papavoine CHM, Christiaans BEC, Folmer RHA, Konings RNH, Hilbers CW. 1998. Solution structure of the M13 major coat protein in detergent micelles: A basis for a model of phage assembly involving specific residues. J Mol Biol 282:401–419.
- Papavoine CHM, Remerowski ML, Horstink LM, Konings RNH, Hilbers CW, van de Ven FJM. 1997. Backbone dynamics of the major coat protein of bacteriophage M13 in detergent micelles by 15N nuclear magnetic resonance relaxation measurements using the model-free approach and reduced spectral density mapping. Biochemistry 36:4015–4026.
- Papavoine CHM, Aelen J, Konings RNH, Hilbers CW, Ven FJM. 1995. NMR studies of the major coat protein of bacteriophage M13. Eur J Biochem 232:490–500.
- Papavoine C, Konings R, Hilbers C, van der Van F. 1994. Location of M13 coat protein in sodium dodecyl micelles as determined by NMR. Biochemistry 33:12990–12997.
- Park SH, Berkamp S, Cook GA, Chan MK, Viadiu H, Opella SJ. 2011. Nanodiscs vs. macrodiscs for NMR of membrane proteins. Biochemistry 50:8983–8985.
- Pastor RW, Venable RM, Karplus M, Szabo A. 1988. A simulation based model of NMR T relaxation in lipid bilayer vesicles. J Chem Phys 89:1128.
- Patching SG. 2011. NMR structures of polytopic integral membrane proteins. Mol Membr Biol 28:370–397.
- Pervushin K, Riek R, Wider G, Wütrich K. 1997. Attenuated T2 relaxation by mutual cancellation of dipole-dipole couplings and chemical shift anisotropy inidcates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci USA 94:12366–12371.
- Pintacuda G, Otting G. 2002. Identification of protein surfaces by NMR measurements with a paramagnetic gd (III) chelate. J Am Chem Soc 124:372–373.
- Poget SF, Cahill SM, Girvin ME. 2007. Isotropic bicelles stabilize the functional form of a small multidrug-resistance pump for NMR structural studies. J Am Chem Soc 129:2432–2433.
- Poget SF, Girvin ME. 2007. Solution NMR of membrane proteins in bilayer mimics: Small is beautiful, but sometimes bigger is better. Biochimica et Biophysica Acta (BBA) – Biomembranes 1768:3098–3106.
- Pointer-Keenan CD, Lee DK, Hallok K, Tan A, Zand R, Ramamoorthy A. 2004. Investigation of the interaction of myelin basic protein with phospholipid bilayers using solid-state NMR spectroscopy. Chem Phys Lipids 132:47–54.
- Pooga M, Hällbrink M, Zorko M, Langel Ü. 1998. Cell penetration by transportan. FASEB J 12:67.
- Pooga M, Kut C, Kihlmark M, Hällbrink M, Fernaeus S, Raid R, 2001. Cellular translocation of proteins by transportan. FASEB J 15:1451–1453.
- Popot JL. 2010. Amphipols, nanodiscs, and fluorinated surfactants: Three nonconventional approaches to studying membrane proteins in aqueous solutions. Annu Rev Biochem 79:737–775.
- Prestegard J. 1990. Magnetically orientable phospholipid bilayers containing small amounts of a bile salt analogue, CHAPSO. Biophys J 58:447–460.
- Prosser RS, Hwang JS, Vold RR. 1998. Magnetically aligned bicelles with a positive ordering: A new model membrane system. Biophys J 74:2405–2418.
- Prosser RS, Evanics F, Kitevski JL, Al-Abdul-Wahid MS. 2006. Current applications of bicelles in NMR studies of membrane-associated amphiphiles and proteins. Biochemistry 45:8453–8465.
- Ram P, Prestegard J. 1988. Magnetic field induced ordering of bile salt/phospholipid micelles: New media for NMR structural investigations. Biochim Biophys Acta 940:289–294.
- Ramamoorthy A, Thennarasu S, Lee DK, Tan A, Maloy L. 2006a. Solid-state NMR investigation of the membrane-disrupting mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from magainin 2 and melittin. Biophys J 91:206–216.
- Ramamoorthy A, Thennarasu S, Tan A, Lee DK, Clayberger C, Krensky AM. 2006b. Cell selectivity correlates with membrane-specific interactions: A case study on the antimicrobial peptide G15 derived from granulysin. Biochim Biophys Acta 1758:154–163.
- Raschle T, Hiller S, Yu TY, Rice AJ, Walz T, Wagner G. 2009. Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. J Am Chem Soc 131:17777–17779.
- Raschle T, Hiller S, Etzkorn M, Wagner G. 2010. Nonmicellar systems for solution NMR spectroscopy of membrane proteins. Curr Opin Struct Biol 20:471–479.
- Reckel S, Gottstein D, Stehle J, Löhr F, Verhoefen M-K, Takeda M, 2011. Solution NMR structure of proteorhodopsin. Angew Chem Int Ed 50:11942–11946.
- Ritchie T, Grinkova Y, Bayburt T, Denisov I, Zolnerciks J, Atkins W, 2009. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Meth Enzymol 464:211–231.
- Rosen MJ, Kunjappu JT. 2012. Surfactants and interfacial phenomena. Hoboken: Wiley.
- Rowe BA, Neal SL. 2003. Fluorescence probe study of bicelle structure as a function of temperature: Developing a practical bicelle structure model. Langmuir 19:2039–2048.
- Ruzza P, Calderan A, Guiotto A, Osler A, Borin G. 2004. Tat cell-penetrating peptide has the characteristics of a poly (proline) II helix in aqueous solution and in SDS micelles. J Pept Sci 10:423–426.
- Sanders CR, Schwonek JP. 1992. Characterization of magnetically orientable bilayers in mixtures of dihexanoylphosphatidylcholine and dimyristoylphosphatidylcholine by solid-state NMR. Biochemistry 31:8898–8905.
- Sanders CR II, Hare BJ, Howard KP, Prestegard JH. 1994. Magnetically-oriented phospholipid micelles as a tool for the study of membrane-associated molecules. Prog Nucl Magn Reson Spectrosc 26:421–444.
- Sanders CR, Landis GC. 1995. Reconstitution of membrane proteins into lipid-rich bilayered mixed micelles for NMR studies. Biochemistry 34:4030–4040.
- Sanders CR, Prosser RS. 1998. Bicelles: A model membrane system for all seasons? Structure 6:1227–1234.
- Sanders CR, Oxenoid K. 2000. Customizing model membranes and samples for NMR spectroscopic studies of complex membrane proteins1. Biochim Biophys Acta 1508:129–145.
- Sanders CR, Sonnichsen F. 2006. Solution NMR of membrane proteins: Practice and challenges. Magn Reson Chem 44:S24–S40.
- Sargent D, Schwyzer R. 1986. Membrane lipid phase as catalyst for peptide-receptor interactions. Proc Natl Acad Sci USA 83:5774.
- Schnell JR, Chou JJ. 2008. Structure and mechanism of the M2 proton channel of influenza A virus. Nature 451:591–595.
- Seelig J. 1978. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta 515:105–140.
- Seelig J, Seelig A. 1980. Lipid conformation in model membranes and biological membranes. Q Rev Biophys 13:19–61.
- Seelig J. 1977. Deuterium magnetic resonance: Theory and application to lipid membranes. Q Rev Biophys 10:353–418.
- Seigneuret M, Lévy D. 1995. A high-resolution 1 H NMR approach for structure determination of membrane peptides and proteins in non-deuterated detergent: Application to mastoparan X solubilized in n-octylglucoside. J Biomol NMR 5:345–352.
- Sharma M, Yi M, Dong H, Qin H, Peterson E, Busath DD, 2010. Insight into the mechanism of the influenza A proton channel from a structure in a lipid bilayer. Science 330:509.
- Shenkarev ZO, Lyukmanova EN, Paramonov AS, Shingarova LN, Chupin VV, Kirpichnikov MP, 2010a. Lipid-protein nanodiscs as reference medium in detergent screening for high-resolution NMR studies of integral membrane proteins. J Am Chem Soc 132:5628–5629.
- Shenkarev ZO, Paramonov AS, Lyukmanova EN, Shingarova LN, Yakimov SA, Dubinnyi MA, 2010b. NMR structural and dynamical investigation of the isolated voltage-sensing domain of the potassium channel KvAP: Implications for voltage gating. J Am Chem Soc 132:5630–5637.
- Shenkarev ZO, Balandin SV, Trunov KI, Paramonov AS, Sukhanov SV, Barsukov LI, 2011. Molecular mechanism of action of b-hairpin antimicrobial peptide arenicin: Oligomeric structure in dodecylphosphocholine micelles and pore formation in planar lipid bilayers. Biochemistry 50:6255–6265.
- Shintani M, Yoshida K, Sakuraba S, Nakahara M, Matubayasi N. 2011. NMR-NOE and MD simulation study on phospholipid membranes: Dependence on membrane dimater and multiple time scale dynamics. J Phys Chem B 115:9106–9115.
- Singer SJ, Nicolson GL. 1972. Fluid mosaic model of the structure of cell membranes. Science 175:720–731.
- Skelton NJ, Palmer AG III, Akke M, Kördel J, Rance M, Chazin WJ. 1993. Practical aspects of two-dimentional proton-detected 15N spin relaxation measurements. J Magn Reson B 102:253–264.
- Smith PES, Brender JR, Ramamoorthy A. 2009. Induction of negative curvature as a mechanism of cell toxicity by amyloidogenic peptides: The case of islet amyloid polypeptide. J Am Chem Soc 131:4470–4478.
- Stafford RE, Fanni T, Dennis EA. 1989. Interfacial properties and critical micelle concentration of lysophospholipids. Biochemistry 28:5113–5120.
- Stejskal EO, Tanner JE. 1965. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J Chem Phys 42:288.
- Struppe J, Whiles JA, Vold RR. 2000. Acidic phospholipid bicelles: A versatile model membrane system. Biophys J 78:281–289.
- Sunyach C, Jen A, Deng J, Fitzgerald KT, Frobert Y, Grassi J, 2003. The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J 22:3591–3601.
- Szyperski T, Lüginbuhl P, Otting G, Güntert P, Wüthrich K. 1993. Protein dynamics studied by rotating frame N-15 spin relaxation times. J Biomol NMR 3:151–164.
- Takeshima K, Chikushi A, Lee KK, Yonehara S, Matsuzaki K. 2003. Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J Biol Chem 278:1310–1315.
- Tanford C, Reynolds JA. 1976. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta 457:133–170.
- Tjandra N, Bax A. 1997. Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science 278:1111–1114.
- Toke O, Bánóczi Z, Király P, Heinzmann R, Bürck J, Ulrich AS, 2011. A kinked antimicrobial peptide from Bombina maxima. I. Three-dimensional structure determined by NMR in membrane-mimicking environments. Eur J Biophys 40:447–462.
- Tolman J, Flanagan J, Kennedy MA, Prestegard J. 1995. Nuclear magnetic dipole interactions in field-oriented proteins: Information for structure determination in solution. Proc Natl Acad Sci USA 92:9279.
- Traaseth NJ, Veglia G. 2010. Probing excited states and activation energy for the integral membrane protein phospholamban by NMR CPMG relaxation dispersion experiments. Biochim Biophys Acta 1798:77–81.
- Triba MN, Devaux PF, Warschawski DE. 2006. Effects of lipid chain length and unsaturation on bicelles stability. A phosphorus NMR study. Biophys J 91:1357–1367.
- Triba MN, Warschawski DE, Devaux PF. 2005. Reinvestigation by phosphorus NMR of lipid distribution in bicelles. Biophys J 88:1887–1901.
- Unnerståle S, Lind J, Papadopoulos E, Mäler L. 2009. Solution structure of the HsapBK K -channel voltage-sensor paddle sequence. Biochemistry 48:5813–5821.
- van Dam L, Karlsson G, Edwards K. 2004. Direct observation and characterization of DMPC/DHPC aggregates under conditions relevant for biological solution NMR. Biochim Biophys Acta 1664:241–256.
- Van Horn WD, Kim HJ, Ellis CD, Hadziselimovic A, Sulistijo ES, Karra MD, 2009. Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase. Science 324:1726.
- Vinogradova O, Sönnichsen F, Sanders CR. 1998. On choosing a detergent for solution NMR studies of membrane proteins. J Biomol NMR 11:381–386.
- Vold RR, Prosser RS, Deese AJ. 1997. Isotropic solutions of phospholipid bicelles: A new membrane mimetic for high-resolution NMR studies of polypeptides. J Biomol NMR 9:329–335.
- Von Meerwall E, Kamat M. 1989. Effect of residual field gradients on pulsed-gradient NMR diffusion measurements. J Magn Reson 83:309–323.
- Wagner G, Bodenhausen G, Mueller N, Rance M, Soerensen OW, Ernst RR, 1985. Exchange of two-spin order in nuclear magnetic resonance: Separation of exchange and cross-relaxation processes. J Am Chem Soc 107:6440–6446.
- Wakamatsu K, Okada A, Miyazawa T, Ohya M, Higashijima T. 1992. Membrane-bound conformation of mastoparan-X, a G-protein-activating peptide. Biochemistry 31:5654–5660.
- Walmsley AR, Zeng F, Hooper NM. 2003. The N-terminal region of the prion protein ectodomain contains a lipid raft targeting determinant. J Biol Chem 278:37241–37248.
- Wang C, Grey MJ, Palmer AG. 2001. CPMG sequences with enhanced sensitivity to chemical exchange. J Biomol NMR 21:361–366.
- Wang G. 2008. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J Biol Chem 283:32637–32643.
- Wang G, Elliott M, Cogen AL, Ezell EL, Gallo RL, Hancock REW. 2011. Structure, dynamics and antimicrobial and immune modulatory activities of human LL-23 and its single-residue variants mutated on the basis of homologous primate cathelicidins. Biochemistry 51:653–664.
- Warschawski DE, Arnold AA, Beaugrand M, Gravel A, Chartrand É, Marcotte I. 2011. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochim Biophys Acta 1808:1957–1974.
- Waskell L, Ramamoorthy A. 2007. The cytochromes P450 and b5 and their reductases – promising targets for structural studies by advanced solid-state NMR spectroscopy. Biochimica et Biophysica Acta (BBA) – Biomembranes 1768:3235–3259.
- Weiss TM, van der Wel PCA, Killian JA, Koeppe RE II, Huang HW. 2003. Hydrophobic mismatch between helices and lipid bilayers. Biophys J 84:379–385.
- Wennerström H, Lindblom G, Lindman B. 1974. Theoretical aspects on the NMR of quadrupolar ionic nuclei in micellar solutions and amphiphilic liquid crystals. Chem Scr 6:97–103.
- Whiles JA, Glover KJ, Vold RR, Komives EA. 2002. Methods for studying transmembrane peptides in bicelles: Consequences of hydrophobic mismatch and peptide sequence. J Magn Reson 158:149–156.
- Whiles JA, Brasseur R, Glover KJ, Melacini G, Komives EA, Vold RR. 2001. Orientation and effects of mastoparan X on phospholipid bicelles. Biophys J 80:280–293.
- Wimley WC, Hristova K. 2011. Antimicrobial peptides: Successes, challenges and unanswered questions. J Membr Biol 239:27–34.
- Wüthrich K. 1986. NMR of proteins and nucleic acids. New York: Wiley.
- Yeagle P. 2005. The structure of biological membranes. Boca Raton, FL: CRC Press.
- Zasloff M. 1987. Magainins, a class of antimicrobial peptides from xenopus skin: Isolation, characterization of two active forms, partial cDNA sequence of a precursor. Proc Natl Acad Sci USA 84:5549–5453.
- Zinn-Justin S, Berthault P, Guenneugues M, Desvaux H. 1997. Off-resonance r.f. fields in heteronuclear NMR: Application to the study of slow motions. J Biomol NMR 10:363–372.
- Zhang W, Smith SO. 2005. Mechanism of penetration of antp (43–58) into membrane bilayers. Biochemistry 44:10110–10118.
