Abstract
Mitochondrial carriers transport inorganic ions, nucleotides, amino acids, keto acids and cofactors across the mitochondrial inner membrane. Structurally they consist of three domains, each containing two transmembrane α-helices linked by a short α-helix and loop. The substrate binds to three major contact points in the central cavity. The class of substrate (e.g., adenine nucleotides) is determined by contact point II on transmembrane α-helix H4 and the type of substrate within the class (e.g., ADP, coenzyme A) by contact point I in H2, whereas contact point III on H6 is most usually a positively charged residue, irrespective of the type or class. Two salt bridge networks, consisting of conserved and symmetric residues, are located on the matrix and cytoplasmic side of the cavity. These residues are part of the gates that are involved in opening and closing of the carrier during the transport cycle, exposing the central substrate binding site to either side of the membrane in an alternating way. Here we revisit the plethora of mutagenesis data that have been collected over the last two decades to see if the residues in the proposed binding site and salt bridge networks are indeed important for function. The analysis shows that the major contact points of the substrate binding site are indeed crucial for function and in defining the specificity. The matrix salt bridge network is more critical for function than the cytoplasmic salt bridge network in agreement with its central position, but neither is likely to be involved in substrate recognition directly.
Introduction
Many transport steps across the mitochondrial inner membrane are required for the generation of metabolic energy from the oxidation of sugars and fats, synthesis of haem and iron sulphur clusters, production of heat, macromolecular synthesis and breakdown, and the synthesis, degradation and interconversion of amino acids. Members of the mitochondrial carrier family (MCF) are involved in the majority of these transport steps (Palmieri Citation2004, Citation2012), but the transport of pyruvate is carried out by the MPC family (Herzig et al. Citation2012). Some MCF members have also been found in membranes of other organelles, such as peroxisomes and chloroplasts (Palmieri et al. Citation2011). On the protein level, mitochondrial carriers are characterized by having three homologous repeats, consisting of about a hundred amino acid residues each (Saraste and Walker Citation1982). The structural fold of the bovine ADP/ATP carrier, the only member of the family for which an atomic structure is available, consists of a barrel of six transmembrane α-helices (H1-6) with three short α-helices in the matrix loops (h12, h34, h56), which are arranged in a three-fold pseudo-symmetrical manner (Pebay-Peyroula et al. Citation2003). At the carboxy-terminal ends of all odd-numbered α-helices H1, H3 and H5 a highly conserved signature motif PX[DE]XX[KR] is found (Nelson et al. Citation1998, Pebay-Peyroula et al. Citation2003). The prolines of the signature motifs are present at sharp kinks in the helices, whereas the charged residues form three salt bridges, which close the cavity to the matrix side. The structure provided the basic fold of all mitochondrial carriers but did not reveal the location of the substrate binding site nor the putative movements upon substrate binding, as the structure is representing an inhibited state, i.e., with carboxy-atractyloside bound.
Computational approaches to identify the substrate binding site
Mitochondrial carriers have a similar structure but can handle a large variety of substrates ranging from single protons (uncoupling proteins) to large cofactors, such as NAD+ and coenzyme A (Palmieri Citation2004, Citation2012). The substrate binding site of mitochondrial carriers was located by three different computational methods. The first used comparative structural models of carriers with known substrate specificity combined with chemical and distance constraints to identify conserved substrate binding sites that were capable of discriminating between (oxo)carboxylates, amino acids and nucleotides (Kunji and Robinson Citation2006, Robinson and Kunji Citation2006). It was discovered that there are three main contact points involved in substrate binding, which were indicated by roman numerals I, II and III. The contact points may involve one or several residues, positioned approximately in the middle of the membrane, on the cavity-exposed face of the even-numbered α-helices. For the bovine mitochondrial ADP/ATP carrier, the contact points were proposed to be R79 on H2 (contact point I) and R279 on H6 (contact point III) for binding of the phosphate groups and G182, I183 and Y186 on H4 for binding of the adenine moiety (contact point II) (Kunji and Robinson Citation2006, Robinson and Kunji Citation2006). The interactions with the contact points allow the coupling of substrate binding to a symmetrical mechanism.
The second approach used molecular dynamics simulations to identify residues of the bovine mitochondrial ADP/ATP carrier that are involved in the trajectory and binding of ADP in the cavity. ADP bound with a minimum of the free energy to the binding site defined above, revealing an aromatic stacking arrangement of the adenine moiety with Y186 and an ionic interaction of the β-phosphate of ADP with an arginine, which comes through the centre of the matrix salt bridge network (Dehez et al. Citation2008, Wang and Tajkhorshid Citation2008). From a sequence perspective, it was clear that the most important interactions of the carriers with the substrate were electrostatic in nature (Kunji and Robinson Citation2006), but this notion has been refined by calculating electrostatic potentials (Wang and Tajkhorshid Citation2008) and by looking at the effects of chlorine ions on binding (Krammer et al. Citation2009).
The third approach exploited the principle that mitochondrial carriers have a high degree of three-fold pseudo-symmetry in contrast to the transported substrates that are asymmetric in structure and chemistry (Robinson et al. Citation2008). Therefore, the residues involved in substrate binding must deviate from each other to couple the binding of the asymmetric substrate to a symmetric transport mechanism. Conserved asymmetric residues were found to cluster consistently at a single site that overlapped with the common substrate binding site in all studied mitochondrial carriers (Kunji and Robinson Citation2006, Robinson and Kunji Citation2006, Robinson et al. Citation2008). In addition, conserved negatively charged asymmetric residues were observed in the substrate binding sites of carriers that transport substrates into mitochondria by coupling the transport step to the import of protons, indicating a potential link between proton and substrate binding (Kunji and Robinson Citation2010).
Mutagenesis studies
Many mutagenesis studies on mitochondrial carriers had been carried out prior to any structural information being available. Here we revisit the effects of these mutations to see if they are consistent with the proposed substrate binding site. In these studies the activity of the mutant proteins have been assessed by transport experiments in reconstituted liposomes (Fiermonte et al. Citation1993, Palmieri et al. Citation1996a) or by mutant complementation assays in deletion strains of yeast (Nelson et al. Citation1993). So far, the bovine 2-oxoglutarate carrier OGC is the only mitochondrial carrier in which every residue has been mutated to cysteine and to other amino acids when the Cys-replacement leads to loss of transport (Stipani et al. Citation2001, Cappello et al. Citation2006, Citation2007, Miniero et al. Citation2011). These systematic studies have carved out the conserved functional elements of mitochondrial carriers. The OGC studies demonstrate that the vast majority of the critical residues have their side chains protruding into the cavity of the carrier, whereas only a few critical residues are found on the surface exposed to the mitochondrial membrane, in the cytoplasmic loops and in the matrix helices (Miniero et al. Citation2011). Extensive mutagenesis studies in other carriers have been carried out on cavity residues of the yeast phosphate carrier Mir1 (Wohlrab and Briggs Citation1994, Phelps et al. Citation1996, Briggs et al. Citation1999, Wohlrab et al. Citation2002) and the yeast citrate carrier Ctp1 (Kaplan et al. Citation2000, Ma et al. Citation2004, Citation2007), whereas only selected residues have been investigated in the yeast ADP/ATP carrier Aac2 (Nelson et al. Citation1993, Citation1998, Heidkämper et al. Citation1996, David et al. Citation2008), the human folate carrier (Lawrence et al. Citation2011), the human and rat carnitine/acyl-carnitine carrier (Indiveri et al. Citation2002, De Lucas et al. Citation2008, Tonazzi et al. Citation2009, Giangregorio et al. Citation2010, Tonazzi et al. Citation2012), and the human and rat uncoupling protein (Echtay et al. Citation1997, Citation2001, Modrianský et al. Citation1997). Since the latter mutagenesis studies are incomplete, we have chosen to focus on the cavity residues of OGC, Mir1 and Ctp1 to evaluate if the computational approaches agree with the results of mutagenesis studies. Effects of mutations on the function of the ADP/ATP carrier have been described elsewhere (Nury et al. Citation2006).
Analysis of mutations of residues in the substrate binding site
The pseudo-symmetry and conservation analysis of OGC (), Mir1 () and Ctp1 () demonstrate that the conserved and asymmetric residues in the cavity have a clear tendency of clustering in the proposed substrate binding site at a central location in the membrane, whereas the symmetric ones tend to cluster in and around the cytoplasmic and matrix gate (Robinson et al. Citation2008).
Figure 1. Asymmetry, symmetry and the effects of single cysteine mutations on transport activity of the bovine 2-oxoglutarate carrier OGC. The backbone is shown in yellow and is based on the structure of the bovine AAC1 (Pebay-Peyroula et al. Citation2003). The conservation and average symmetry scores of the OGC subfamily are represented by the size and colour of the Cβ atom, respectively. Large spheres indicate residues that are well-conserved in the subfamily of OGC, whereas small spheres are not. Asymmetric residues are shown in a colour scale from red (highly asymmetric) to white (neutral) (A), whereas symmetric residues are shown in a colour scale from blue (highly symmetric) to white (neutral) (C) (Cappello et al. Citation2006). The asymmetric (B) and symmetric residues (D) were also coloured according to the relative initial transport velocity of the single cysteine mutant proteins compared with the wild-type at the external substrate concentration equal to the km of the wild-type: red, 0–15%; orange 16–50%; green, 51–100%; white, no data. The black encircled residues are the three contact points of the substrate binding site, which in OGC is a symmetrical triplet of arginines. As the substrates malate and oxoglutarate are small, the substrate binding site has only a few asymmetrical adaptations. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
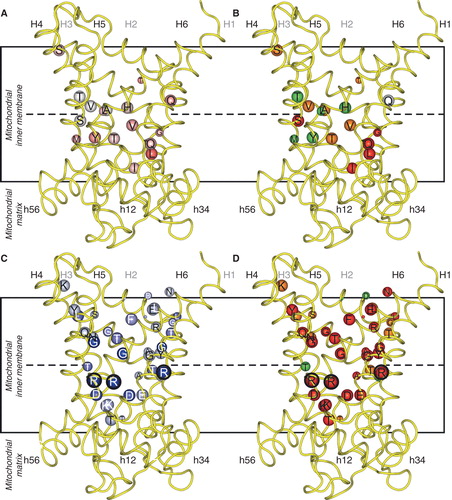
Figure 2. Asymmetry, symmetry and the effects of mutations on transport activity of the yeast phosphate carrier Mir1. Key as in . Where the kinetic parameters were measured, the corresponding initial rates were calculated with the Michaelis-Menten equation at a substrate concentration equal to the km of the wild-type. The black encircled residues are the three contact points of the substrate binding site, which in Mir1 consists of Q86, K179, Q180 and M279, all asymmetric as an adaptation to a small substrate. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
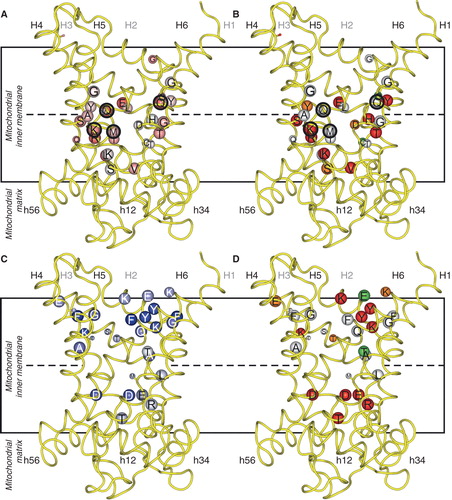
Figure 3. Asymmetry, symmetry and activity of mutants of the yeast citrate carrier Ctp1. Key as in . Where the kinetic parameters were measured, the corresponding initial rates were calculated according to the Michaelis-Menten equation at a substrate concentration equal to the km of the wild-type. The black encircled residues are the contact points of the substrate binding site, which in Ctp1 might consist of the conserved and asymmetric K83 and Q182 together with the symmetric R279 and R181. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
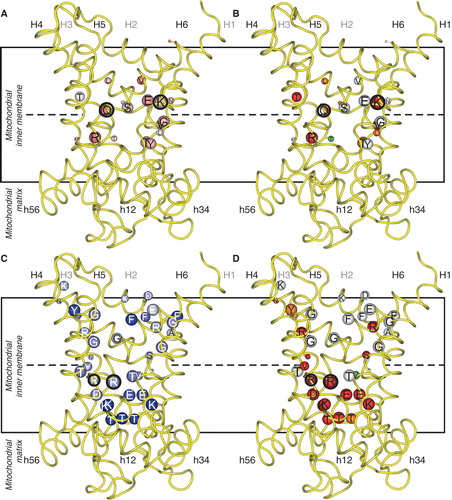
There is a distinct correlation between residues with increasing degree of asymmetry and residues that are critical for transport in OGC (), Mir1 () and Ctp1 (). The analysis also shows that mutations of the contact point residues are detrimental to transport activity. In Mir1 only contact point II is involved, whereas other interactions are carried out by asymmetric residues, because the substrate is small and requires co-transport with a proton (Kunji and Robinson Citation2010). However, the residues of the substrate binding site of OGC and Ctp1 are mostly conserved and symmetrical, as a requirement for the coupling of substrate binding to a symmetric transport mechanism. The carriers that transport carboxylic acids have positively charged residues to bind the negatively charged substrates whereas the surrounding asymmetric residues are likely to define the specificity by modulating the immediate environment. For example 2-oxoglutarate carriers, with the combination of RY[TS][RK], RA and [RK] in the three contact points, transport 2-oxoglutarate, L-malate, malonate, maleate, succinate, and to a small extent D-malate and 2-oxoadipate (Bisaccia et al. Citation1985, Fiermonte et al. Citation1993) whereas dicarboxylate carriers with RY[ST]R, R[AG] and R in the contact points transport L-malate, phosphate, sulphate, thiosulphate, malonate, maleate and succinate (Palmieri et al. Citation1996b, Fiermonte et al. Citation1998). Thus OGC and the dicarboxylate carrier have overlapping, but not identical substrate specificity. Therefore, there must be other determinants that can discriminate between substrates that are similar. Most likely the asymmetric residues close to the contact points take part in this ‘fine-tuning' of the substrate specificity (Palmieri et al. Citation2011).
Mutations that alter substrate specificity
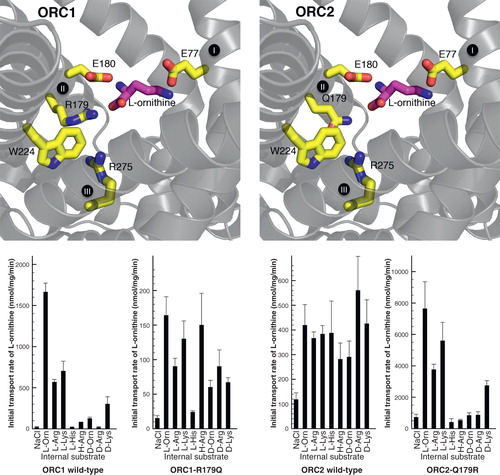
The mutagenesis experiments show that particular residues are important for transport, but this observation does not disclose their role, because they can be directly or indirectly involved in substrate binding, in the structural fold, in the conformational changes during substrate translocation or in the opening and closing of the matrix and cytosoplasmic gates. If a mutation causes an altered substrate specificity then this demonstrates unequivocally that the mutated residue is involved in substrate binding and selection. In our recent paper, the substrate binding site of the two human mitochondrial ornithine carrier isoforms (ORC1 and ORC2) were probed by site directed mutagenesis of the contact point residues (Monné et al. Citation2012). The two transporters differ in specificity and transport rate (Fiermonte et al. Citation2003) and have different residues in contact point II; RE in ORC1 and QE in ORC2. The results demonstrated that this difference is responsible for the difference in substrate specificity between the two isoforms (). When the residues are exchanged in the ORC1-R179Q and ORC2-Q179R mutant proteins the substrate specificity and transport rate are swapped as well. Given that mutations in contact point II also affect the turnover number, substrate binding to this residue is a rate limiting step in the catalytic transport cycle. A mutation in the other residue in contact point II ORC1-E180D also displayed changed substrate specificity, indicating that this position is also important in substrate selection. The most straightforward interpretation is that the Cα carboxylate and amino group of the amino acid substrate bind to R/Q179 and E180 of the ornithine carriers. When bound to this position the only plausible binding site for the terminal amino group is residue E77 in contact point I. This residue is conserved and unique for the ORC subfamily and the results show that it cannot be altered without total loss of function. It was also noted that contact point II might be interacting indirectly with contact point III through cation-π interactions. In this way, substrate binding would engage all three contact points for coupling substrate binding to a symmetrical mechanism.
Figure 4. Swapping specificity of the human ornithine carrier by exchanging a single residue in contact point II of the substrate binding site. L-ornithine (magenta) bound in the substrate binding site of ORC1 and ORC2 is shown with the investigated residues (yellow). Results from transport hetero-exchange experiments of radioactive L-ornithine with the wild-type and mutant ORC proteins reconstituted in proteoliposomes, preloaded internally with the various substrates indicated: L-Orn, L-ornithine; L-Arg, L-arginine; L-Lys, L-lysine; L-His, L-histidine; H-Arg, L-homoarginine; D-Orn, D-ornithine; D-Arg, D-arginine and D-Lys, D-lysine (Monné et al. Citation2012). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
The R294A mutation of the yeast mitochondrial ADP/ATP carrier Aac2 also alters the substrate specificity, demonstrating that contact point III is also involved in substrate binding (Heidkämper et al. Citation1996). This mutant protein transports ADP at an almost unchanged rate compared to the wild-type but ATP transport is reduced to less than 10%, suggesting that R294 is important for binding the γ-phosphate of ATP.
Analysis of mutations of residues involved in the transport mechanism
The systematic mutagenesis of OGC has highlighted the structural elements that are conserved, symmetrical and important for transport (). Apart from those in the vicinity of the substrate binding site (), critical residues are also found in the conserved signature motifs of the odd- and even-numbered helices that form the matrix and cytoplasmic gates, respectively. Similar to what was observed for the asymmetric residues, it seems that residues with increasing degree of symmetry coincide with positions where mutations are affecting the transport more severely in OGC (), Mir1 () and Ctp1 (). Here we review these residues to see whether they are likely to be involved in substrate binding or in critical aspects of the transport mechanism or the structure.
Figure 5. Conserved and symmetrical residues that are critical for function of the mitochondrial oxoglutarate carrier. (A) Residues of the substrate binding site, including the symmetrical and conserved arginine triplet, which are the contact points. (B) Triplets of symmetry-related residues belonging to the GXXXG motif and the PX[DE]XX[RK] motif, which contain a conserved P that is present at the kink and the conserved charge residues of the matrix salt bridge network, which forms in the cytoplasmic state. Underneath are the conserved and symmetrical Q residues, which may interact with the network. (C) Triplets belonging to the aromatic motif [YF]XX[YF] and the cytoplasmic salt bridge network [DE]XX[RK], which are present on the even-numbered α-helices at the cytoplasmic side of the carrier. The charged residues may form a network when the carrier is in the matrix-state. (D) Conserved positively charged [RK] residues, which follow the matrix network and interact with the negatively charged residue of the [ED]G motif. The Y may also be involved in the bonding arrangement, linking the matrix α-helices to the odd-numbered α -helices. The model of OGC, based on the structure of the bovine ADP/ATP carrier, is shown in yellow and the aforementioned residues are shown in red when they are critical to the function, orange if they are important and green when they are not important. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 5. Conserved and symmetrical residues that are critical for function of the mitochondrial oxoglutarate carrier. (A) Residues of the substrate binding site, including the symmetrical and conserved arginine triplet, which are the contact points. (B) Triplets of symmetry-related residues belonging to the GXXXG motif and the PX[DE]XX[RK] motif, which contain a conserved P that is present at the kink and the conserved charge residues of the matrix salt bridge network, which forms in the cytoplasmic state. Underneath are the conserved and symmetrical Q residues, which may interact with the network. (C) Triplets belonging to the aromatic motif [YF]XX[YF] and the cytoplasmic salt bridge network [DE]XX[RK], which are present on the even-numbered α-helices at the cytoplasmic side of the carrier. The charged residues may form a network when the carrier is in the matrix-state. (D) Conserved positively charged [RK] residues, which follow the matrix network and interact with the negatively charged residue of the [ED]G motif. The Y may also be involved in the bonding arrangement, linking the matrix α-helices to the odd-numbered α -helices. The model of OGC, based on the structure of the bovine ADP/ATP carrier, is shown in yellow and the aforementioned residues are shown in red when they are critical to the function, orange if they are important and green when they are not important. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/d6f71395-2cdd-48c5-b12f-1f1ae776af65/imbc_a_737936_f0005_b.jpg)
The matrix gate mutations include residues of the signature motif PX[DE]XX[RK] that are highly conserved in all carriers and can therefore not have a discriminatory role in substrate recognition (). Carriers transporting NAD+ (Todisco et al. Citation2006, Palmieri et al. Citation2009), pyrimidine nucleotides (Marobbio et al. Citation2006, Floyd et al. Citation2007), FAD/folate (Tzagoloff et al. Citation1996, Titus and Moran, Citation2000, Bedhomme et al. Citation2005), and coenzyme A, FAD and NAD+ in peroxisomes (Agrimi et al. Citation2012a, Citation2012b, Bernhardt et al. Citation2012) have a conserved W instead of [DE] in the second signature motif; FAD/folate carriers and ATP-Mg2+/phosphate carriers (Fiermonte et al. Citation2004, Traba et al. Citation2008, Citation2009) have a glutamine and [QNAT], respectively, instead of the negatively charged residue of the third signature motif; dicarboxylate carriers (Palmieri et al. Citation1996b, Citation2008, Fiermonte et al. Citation1998) have an asparagine or methionine instead of the positively charged residue of the second motif; the fungal oxaloacetate/sulphate/α-isopropylmalate carrier (Palmieri et al. Citation1999, Marobbio et al. Citation2008) lacks the negatively charged residue and the positively charged residue in the second and third motifs, the former residue being replaced by [FY] and the latter by [LM]; and the phosphate carriers (Runswick et al. Citation1987, Dolce et al. Citation1994, Wohlrab and Briggs Citation1994) have a hydrophobic substitution instead of the positively charged residue in the third motif. These mostly polar modifications would be capable to either cation-π or hydrogen bond interactions, which have an interaction energy that is approximately half of an ionic bond. There is no straightforward correlation to substrate specificity, since the tryptophan modification, for example, is the same in the NAD+, pyrimidine nucleotides, FAD/folate and peroxisomal coenzyme A/FAD/NAD+ transporters (see above), but their substrates are very different in chemistry and biophysical properties. It is possible that the strength of the matrix network is modulated to be lower than the interaction energy of the substrate with the binding site. Just below the matrix salt bridge network are conserved and symmetrical glutamines, which could form a hydrogen bond with residues involved in the matrix salt bridge network, but there is no correlation to substrate specificity (). The positively charged residue that follows the matrix salt bridge network residues interacts with a negatively charged residue of the [DE]G motif and conserved Y, linking the matrix α-helices to the odd-numbered α-helices, but they are outside of the central cavity and occluded (). The residues at the cytoplasmic gate in and around the motif [YF]XX[YF] and [DE]XX[RK] are (), although less conserved, found in a wide range of carriers with different substrates and are therefore also unlikely to form a basis for substrate specificity. There are also many conserved and symmetric glycines, of which some are found in the GXXXG motif below the cytoplasmic gate in the odd-numbered helices that have been shown to be crucial for transport (). There are two separate hypotheses to describe their role in carrier activity: (i) They could form important pivot points for close helix-helix interactions (Melnyk et al. Citation2004, Robinson et al. Citation2008), or (ii) they could form hinges in the helices necessary for the opening and closing of the gates (Palmieri and Pierri Citation2010).
Thus it is unlikely that the critical residues of the matrix and cytoplasmic gates could be involved in substrate binding and it is much more likely that they are involved in the closing and opening of the gates in the transport cycle. Since they are in the translocation pathway, it is also possible that they modulate or facilitate the entry of substrates to the central substrate binding site or the exclusion of others.
Conclusions/perspectives
In this review we have shown that the conclusions drawn from theoretical and experimental approaches to define the substrate binding site in mitochondrial carriers largely agree. In fact, the sequence/structure analysis complements interpretation of the mutant data and vice versa. The approach of combining site-directed mutagenesis and transport assays with a set of substrates has proved to be successful for determining the residues that are directly involved in substrate interactions. Taken together, the mutagenesis studies of well-characterized carriers have validated the importance of specific residues in substrate binding and transport mechanism. Furthermore, the identification of contact point residues in the mitochondrial carrier substrate-binding site has helped and will continue to be useful in explaining differences in half-saturation constants of substrates for specific carriers (Marobbio et al. Citation2008) and in predicting the substrates, or at least the class of substrates, that are transported by yet uncharacterized mitochondrial carriers (Castegna et al. Citation2010, Palmieri et al. Citation2011, Stael et al. Citation2011).
Acknowledgements
The collaboration was funded by the European Community's Seventh Framework Programme FP7/2007-2013 under grant agreement no. HEALTH-F4-2007-201924, EDICT Consortium.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agrimi G, Russo A, Pierri CL, Palmieri F. 2012a. The peroxisomal NAD(+) carrier of Arabidopsis thaliana transports coenzyme A and its derivatives. J Bioenerg Biomembr 44:333–340.
- Agrimi G, Russo A, Scarcia P, Palmieri F. 2012b. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+. Biochem J 443:241–247.
- Bedhomme M, Hoffmann M, McCarthy EA, Gambonnet B, Moran RG, Rébeillé F, 2005. Folate metabolism in plants: An Arabidopsis homolog of the mammalian mitochondrial folate transporter mediates folate import into chloroplasts. J Biol Chem 280:34823–34831.
- Bernhardt K, Wilkinson S, Weber APM, Linka N. 2012. A peroxisomal carrier delivers NAD+ and contributes to optimal fatty acid degradation during storage oil mobilization. Plant J 69:1–13.
- Bisaccia F, Indiveri C, Palmieri F. 1985. Purification of reconstitutively active alpha-oxoglutarate carrier from pig heart mitochondria. Biochim Biophys Acta 810:362–369.
- Briggs C, Mincone L, Wohlrab H. 1999. Replacements of basic and hydroxyl amino acids identify structurally and functionally sensitive regions of the mitochondrial phosphate transport protein. Biochemistry 38:5096–5102.
- Cappello AR, Curcio R, Valeria Miniero D, Stipani I, Robinson AJ, Kunji ERS, 2006. Functional and structural role of amino acid residues in the even-numbered transmembrane alpha-helices of the bovine mitochondrial oxoglutarate carrier. J Mol Biol 363:51–62.
- Cappello AR, Miniero DV, Curcio R, Ludovico A, Daddabbo L, Stipani I, 2007. Functional and structural role of amino acid residues in the odd-numbered transmembrane alpha-helices of the bovine mitochondrial oxoglutarate carrier. J Mol Biol 369:400–412.
- Castegna A, Scarcia P, Agrimi G, Palmieri L, Rottensteiner H, Spera I, 2010. Identification and functional characterization of a novel mitochondrial carrier for citrate and oxoglutarate in S. cerevisiae. J Biol Chem 285:17359–17370.
- David C, Arnou B, Sanchez J-F, Pelosi L, Brandolin G, Lauquin GJM, 2008. Two residues of a conserved aromatic ladder of the mitochondrial ADP/ATP carrier are crucial to nucleotide transport. Biochemistry 47:13223–13231.
- De Lucas JR, Indiveri C, Tonazzi A, Perez P, Giangregorio N, Iacobazzi V, 2008. Functional characterization of residues within the carnitine/acylcarnitine translocase RX2PANAAXF distinct motif. Mol Membr Biol 25:152–163.
- Dehez F, Pebay-Peyroula E, Chipot C. 2008. Binding of ADP in the mitochondrial ADP/ATP carrier is driven by an electrostatic funnel. J Am Chem Soc 130:12725–12733.
- Dolce V, Iacobazzi V, Palmieri F, Walker JE. 1994. The sequences of human and bovine genes of the phosphate carrier from mitochondria contain evidence of alternatively spliced forms. J Biol Chem 269:10451–10460.
- Echtay KS, Bienengraeber M, Klingenberg M. 1997. Mutagenesis of the uncoupling protein of brown adipose tissue. Neutralization Of E190 largely abolishes pH control of nucleotide binding. Biochemistry 36:8253–8260.
- Echtay KS, Bienengraeber M, Klingenberg M. 2001. Role of intrahelical arginine residues in functional properties of uncoupling protein (UCP1). Biochemistry 40:5243–5248.
- Fiermonte G, Dolce V, David L, Santorelli FM, Dionisi-Vici C, Palmieri F, 2003. The mitochondrial ornithine transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution of two human isoforms. J Biol Chem 278:32778–32783.
- Fiermonte G, De Leonardis F, Todisco S, Palmieri L, Lasorsa FM, Palmieri F. 2004. Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution. J Biol Chem 279:30722–30730.
- Fiermonte G, Palmieri L, Dolce V, Lasorsa FM, Palmieri F, Runswick MJ, 1998. The sequence, bacterial expression, and functional reconstitution of the rat mitochondrial dicarboxylate transporter cloned via distant homologs in yeast and Caenorhabditis elegans. J Biol Chem 273:24754–24759.
- Fiermonte G, Walker JE, Palmieri F. 1993. Abundant bacterial expression and reconstitution of an intrinsic membrane-transport protein from bovine mitochondria. Biochem J 294:293–299.
- Floyd S, Favre C, Lasorsa FM, Leahy M, Trigiante G, Stroebel P, 2007. The insulin-like growth Factor-I-mTOR signaling pathway induces the mitochondrial pyrimidine nucleotide carrier to promote cell growth. Mol Biol Cell 18:3545–3555.
- Giangregorio N, Tonazzi A, Console L, Indiveri C, Palmieri F. 2010. Site-directed mutagenesis of charged amino acids of the human mitochondrial carnitine/acylcarnitine carrier: Insight into the molecular mechanism of transport. Biochim Biophys Acta 1797:839–845.
- Heidkämper D, Müller V, Nelson DR, Klingenberg M. 1996. Probing the role of positive residues in the ADP/ATP carrier from yeast. The effect of six arginine mutations on transport and the four ATP versus ADP exchange modes. Biochemistry 35:16144–16152.
- Herzig S, Raemy E, Montessuit S, Veuthey J-L, Zamboni N, Westermann B, 2012. Identification and functional expression of the mitochondrial pyruvate carrier. Science 337:93–96.
- Indiveri C, Giangregorio N, Iacobazzi V, Palmieri F. 2002. Site-directed mutagenesis and chemical modification of the six native cysteine residues of the rat mitochondrial carnitine carrier: Implications for the role of cysteine-136. Biochemistry 41:8649–8656.
- Kaplan RS, Mayor JA, Brauer D, Kotaria R, Walters DE, Dean AM. 2000. The yeast mitochondrial citrate transport protein. Probing the secondary structure of transmembrane domain iv and identification of residues that likely comprise a portion of the citrate translocation pathway. J Biol Chem 275:12009–12016.
- Krammer E-M, Ravaud S, Dehez F, Frelet-Barrand A, Pebay-Peyroula E, Chipot C. 2009. High-chloride concentrations abolish the binding of adenine nucleotides in the mitochondrial ADP/ATP carrier family. Biophys J 97:L25–L27.
- Kunji ERS, Robinson AJ. 2006. The conserved substrate binding site of mitochondrial carriers. Biochim Biophys Acta 1757:1237–1248.
- Kunji ERS, Robinson AJ. 2010. Coupling of proton and substrate translocation in the transport cycle of mitochondrial carriers. Curr Opin Struct Biol 20:440–447.
- Lawrence SA, Hackett JC, Moran RG. 2011. Tetrahydrofolate recognition by the mitochondrial folate transporter. J Biol Chem 286:31480–31489.
- Ma C, Kotaria R, Mayor JA, Eriks LR, Dean AM, Walters DE, 2004. The mitochondrial citrate transport protein: Probing the secondary structure of transmembrane domain III, identification of residues that likely comprise a portion of the citrate transport pathway, and development of a model for the putative TMDIII-TMDIII. J Biol Chem 279:1533–1540.
- Ma C, Remani S, Sun J, Kotaria R, Mayor JA, Walters DE, 2007. Identification of the substrate binding sites within the yeast mitochondrial citrate transport protein. J Biol Chem 282:17210–17220.
- Marobbio CMT, Di Noia MA, Palmieri F. 2006. Identification of a mitochondrial transporter for pyrimidine nucleotides in Saccharomyces cerevisiae: Bacterial expression, reconstitution and functional characterization. Biochem J 393:441–446.
- Marobbio CMT, Giannuzzi G, Paradies E, Pierri CL, Palmieri F. 2008. α-Isopropylmalate, a leucine biosynthesis intermediate in yeast, is transported by the mitochondrial oxalacetate carrier. J Biol Chem 283:28445–28553.
- Melnyk RA, Kim S, Curran AR, Engelman DM, Bowie JU, Deber CM. 2004. The affinity of GXXXG motifs in transmembrane helix-helix interactions is modulated by long-range communication. J Biol Chem 279:16591–16597.
- Miniero DV, Cappello AR, Curcio R, Ludovico A, Daddabbo L, Stipani I, 2011. Functional and structural role of amino acid residues in the matrix α-helices, termini and cytosolic loops of the bovine mitochondrial oxoglutarate carrier. Biochim Biophys Acta 1807:302–310.
- Modrianský M, Murdza-Inglis DL, Patel HV, Freeman KB, Garlid KD. 1997. Identification by site-directed mutagenesis of three arginines in uncoupling protein that are essential for nucleotide binding and inhibition. J Biol Chem 272:24759–24762.
- Monné M, Miniero DV, Daddabbo L, Robinson AJ, Kunji ERS, Palmieri F. 2012. Substrate specificity of the two mitochondrial ornithine carriers can be swapped by single mutation in substrate binding site. J Biol Chem 287:7925–7934.
- Nelson DR, Felix CM, Swanson JM. 1998. Highly conserved charge-pair networks in the mitochondrial carrier family. J Mol Biol 277:285–308.
- Nelson DR, Lawson JE, Klingenberg M, Douglas MG. 1993. Site-directed mutagenesis of the yeast mitochondrial ADP/ATP translocator. Six arginines and one lysine are essential. J Mol Biol 230:1159–1170.
- Nury H, Dahout-Gonzalez C, Trézéguet V, Lauquin GJM, Brandolin G, Pebay-Peyroula E. 2006. Relations between structure and function of the mitochondrial ADP/ATP carrier. Annu Rev Biochem 75:713–741.
- Palmieri F. 2004. The mitochondrial transporter family (SLC25): Physiological and pathological implications. Pflügers Arch 447:689–709.
- Palmieri F. 2012. The mitochondrial transporter family (SLC25): Identification, properties and physiopathology. Mol Aspects Med in press http://dx.doi.org/10.1016/j.mam.2012.05.005.
- Palmieri F, Bisaccia F, Capobianco L, Dolce V, Fiermonte G, Iacobazzi V, 1996a. Mitochondrial metabolite transporters. Biochim Biophys Acta 1275:127–132.
- Palmieri F, Pierri CL. 2010. Structure and function of mitochondrial carriers – role of the transmembrane helix P and G residues in the gating and transport mechanism. FEBS Lett 584:1931–1939.
- Palmieri F, Pierri CL, De Grassi A, Nunes-Nesi A, Fernie AR. 2011. Evolution, structure and function of mitochondrial carriers: A review with new insights. Plant J 66:161–181.
- Palmieri F, Rieder B, Ventrella A, Blanco E, Do PT, Nunes-Nesi A, 2009. Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins. J Biol Chem 284:31249–31259.
- Palmieri L, Palmieri F, Runswick MJ, Walker JE. 1996b. Identification by bacterial expression and functional reconstitution of the yeast genomic sequence encoding the mitochondrial dicarboxylate carrier protein. FEBS Lett 399:299–302.
- Palmieri L, Picault N, Arrigoni R, Besin E, Palmieri F, Hodges M. 2008. Molecular identification of three Arabidopsis thaliana mitochondrial dicarboxylate carrier isoforms: Organ distribution, bacterial expression, reconstitution into liposomes and functional characterization. Biochem J 410:621–629.
- Palmieri L, Vozza A, Agrimi G, De Marco V, Runswick MJ, Palmieri F, 1999. Identification of the yeast mitochondrial transporter for oxaloacetate and sulfate. J Biol Chem 274:22184–22190.
- Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJ-M, Brandolin G. 2003. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426:39–44.
- Phelps A, Briggs C, Mincone L, Wohlrab H. 1996. Mitochondrial phosphate transport protein. Replacements of glutamic, aspartic, and histidine residues affect transport and protein conformation and point to a coupled proton transport path. Biochemistry 35:10757–10762.
- Robinson AJ, Kunji ERS. 2006. Mitochondrial carriers in the cytoplasmic state have a common substrate binding site. Proc Natl Acad Sci USA 103:2617–2622.
- Robinson AJ, Overy C, Kunji ERS. 2008. The mechanism of transport by mitochondrial carriers based on analysis of symmetry. Proc Natl Acad Sci USA 105:17766–17771.
- Runswick MJ, Powell SJ, Nyren P, Walker JE. 1987. Sequence of the bovine mitochondrial phosphate carrier protein: Structural relationship to ADP/ATP translocase and the brown fat mitochondria uncoupling protein. EMBO J 6:1367–1373.
- Saraste M, Walker JE. 1982. Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett 144:250–254.
- Stael S, Rocha AG, Robinson AJ, Kmiecik P, Vothknecht UC, Teige M. 2011. Arabidopsis calcium-binding mitochondrial carrier proteins as potential facilitators of mitochondrial ATP-import and plastid SAM-import. FEBS Lett 585:3935–3940.
- Stipani V, Cappello AR, Daddabbo L, Natuzzi D, Miniero DV, Stipani I, 2001. The mitochondrial oxoglutarate carrier: Cysteine-scanning mutagenesis of transmembrane domain IV and sensitivity of Cys mutants to sulfhydryl reagents. Biochemistry 40:15805–15810.
- Titus SA, Moran RG. 2000. Retrovirally mediated complementation of the glyB phenotype. Cloning of a human gene encoding the carrier for entry of folates into mitochondria. J Biol Chem 275:36811–36807.
- Todisco S, Agrimi G, Castegna A, Palmieri F. 2006. Identification of the mitochondrial NAD+ transporter in Saccharomyces cerevisiae. J Biol Chem 281:1524–1531.
- Tonazzi A, Console L, Giangregorio N, Indiveri C, Palmieri F. 2012. Identification by site-directed mutagenesis of a hydrophobic binding site of the mitochondrial carnitine/acylcarnitine carrier involved in the interaction with acyl groups. Biochim Biophys Acta 1817:697–704.
- Tonazzi A, Giangregorio N, Indiveri C, Palmieri F. 2009. Site-directed mutagenesis of the His residues of the rat mitochondrial carnitine/acylcarnitine carrier: Implications for the role of His-29 in the transport pathway. Biochim Biophys Acta 1787:1009–1015.
- Traba J, Froschauer EM, Wiesenberger G, Satrústegui J, Del Arco A. 2008. Yeast mitochondria import ATP through the calcium-dependent ATP-Mg/Pi carrier Sal1p, and are ATP consumers during aerobic growth in glucose. Mol Microbiol 69:570–585.
- Traba J, Satrústegui J, del Arco A. 2009. Characterization of SCaMC-3-like/slc25a41, a novel calcium-independent mitochondrial ATP-Mg/Pi carrier. Biochem J 418:125–133.
- Tzagoloff A, Jang J, Glerum DM, Wu M. 1996. FLX1 codes for a carrier protein involved in maintaining a proper balance of flavin nucleotides in yeast mitochondria. J Bioenerg Biomembr 271:7392–7397.
- Wang Y, Tajkhorshid E. 2008. Electrostatic funneling of substrate in mitochondrial inner membrane carriers. Proc Natl Acad Sci USA 105:9598–9603.
- Wohlrab H, Annese V, Haefele A. 2002. Single replacement constructs of all hydroxyl, basic, and acidic amino acids identify new function and structure-sensitive regions of the mitochondrial phosphate transport protein. Biochemistry 41:3254–3261.
- Wohlrab H, Briggs C. 1994. Yeast mitochondrial phosphate transport protein expressed in Escherichia coli. Site-directed mutations at threonine-43 and at a similar location in the second tandem repeat (isoleucine-141). Biochemistry 33:9371–9375.
