Abstract
The hypothalamic components of the hypothalamo–pituitary–adrenal axis (HPA) are corticotropin-releasing hormone (CRH) and vasopressin. To test the hypothesis that HPA regulation changes with age, we compared ether and bacterial lipopolysaccharide (LPS) injection induced stress reactions in adult and 10-day-old Brattleboro rats, which naturally lack vasopressin owing to mutation of the gene (di/di). The LPS stimulus was used also with V1b receptor antagonist pretreatment (SSR149415). In adult di/di or V1b pretreated rats, we observed normal pituitary and adrenocortical secretory responses, while in all 10-day-old rats stress-induced serum corticosterone increases were marked, but adrenocorticotropin (ACTH) increases were significantly smaller. Compared to control pups the adenohypophysis of the 10-day-old di/di rats responded normally to CRH, but their adrenal glands were hyper-responsive to ACTH, while in adults there was greater secretion at both levels with no difference between the genotypes. The serum transcortin level was higher in adults than pups, with the di/di pups having higher transcortin levels than controls. Hence, using the same stressors in adults and pups with both a genetic model and pharmacological pretreatment, we have shown that the role of vasopressin in ACTH regulation is more important during the neonatal period than in adulthood. Blunted hypophysial sensitivity to CRH and similar adrenal gland sensitivity to ACTH in the pups compared to adults suggest that hypothalamic factors could be responsible for the neonatal stress hyporesponsive period.
Introduction
The hypothalamo–pituitary–adrenal axis (HPA) response to stressors is accepted as a vital element for survival and well-being of all vertebrates (McEwen Citation2008). The hormonal stress cascade begins with the secretion of the two neurohormones, corticotropin-releasing hormone (CRH), and arginine vasopressin into the hypothalamo–pituitary portal blood. This is followed by adenohypophysial secretion of adrenocorticotropin (ACTH), which, in turn, stimulates glucocorticoid synthesis and release in the adrenal cortex (Antoni Citation1993). This hormonal cascade is restrained by glucocorticoid negative feedback (Dayanithi and Antoni Citation1989). The responsiveness of the HPA axis to stressors varies both during ontogeny and between individuals, and can be altered by neonatal events (Penke et al. Citation2001).
In the rat, postnatal days 4–14 is the stress hyporesponsive period (SHRP), characterized by a reduced capacity to secrete corticosterone in response to stressful stimuli (Walker and Vrana Citation1993). This hyporesponsiveness seems to be time and stressor specific (Walker et al. Citation1991) and is largely under the control of maternal signals such as feeding, licking, and nursing (Suchecki et al. Citation1993; Grino et al. Citation1994; Levine Citation2002). The main cause of hyporesponsiveness of the HPA axis is considered to be inhibitory maternal factors (Levine Citation2002) and enhanced negative feedback, probably due to elevation in free corticosterone concentration because of low circulating transcortin concentrations (Sapolsky and Meaney Citation1986; Walker et al. Citation1986). A rate-limiting factor could also be the limited availability and/or transport of hypothalamic secretagogues (Suchecki et al. Citation1993).
During the SHRP insulin-induced hypoglycemia increases, ACTH and corticosterone secretion via vasopressin mediation (Muret et al. Citation1992). Our previous results with maternal separation also suggest a prominent role of vasopressin in the regulation of ACTH secretion in 5-, 10- and 20-day-old rats (Zelena et al. Citation2008). So, in the rat, vasopressin may be the main regulator of the HPA axis during the neonatal period (Avishai-Eliner et al. Citation1995; Levine Citation2002) in contrast to adulthood, where CRH is evidently the more important regulator (de Kloet and Oitzl Citation2003).
The spontaneous mutant Brattleboro rat has proven to be a good tool to study the role of vasopressin in physiological processes. These rats carry a single base deletion in the vasopressin gene, and in the homozygous (di/di) rat this recessive mutation results in an inability to process the gene product to vasopressin, while heterozygous (di/+) rats have normal vasopressin function (Schmale et al. Citation1984). We have previously demonstrated that vasopressin is essential for ACTH but not corticosterone secretion induced by maternal separation of rat pups during the SHRP (Zelena et al. Citation2008).
In the present study, we aimed to clarify the age-dependent role of vasopressin in HPA axis regulation by direct comparison of pups with adults and using an ether exposure paradigm (Walker and Dallman Citation1993) and a model of bacterial infection by injection of bacterial lipopolysaccharide (LPS). The results from vasopressin-deficient Brattleboro rats were supported with a study using V1b antagonist (SSR149415) treatment. In vitro experiments were used to clarify possible background mechanisms, specifically altered anterior pituitary and adrenocortical sensitivity to secretagogues. Our hypothesis was that vasopressin is the dominant hypothalamic secretagogue in the HPA axis during the neonatal period while its role is subtle during adulthood. This hypothesis would be supported if a strong inhibition of hormonal stress responses is observed in the vasopressin-deficient Brattleboro rats as well as after V1b antagonist pretreatment in pups only.
Materials and methods
Animals
Adult male Brattleboro rats (200–250 g; local colony, originating from Harlan Laboratories, Indianapolis, IN, USA) were kept individually for 1 week in a controlled environment (temperature: 23 ± 1°C; relative humidity: 50–70%), fed on commercial rat chow (Charles River Laboratories, Hungary) and had free access to tap water. The day/night schedule was 12 h light/12 h dark, with lights on at 07:00 h. All experiments were done between 10:00 and 14:00 h. The genotype of adult Brattleboro rats was assessed before the experiment on the basis of their greatly increased daily water consumption, which compensates for their profound diabetes insipidus. The breeding colony was kept as described earlier (Zelena et al. Citation2009b).
Pregnant female Brattleboro rats were kept individually under standard laboratory conditions. Day 0 was assigned when the litter was found at the regular 09:00 h inspection. Litter size was between 6 and 10 pups, and was not modified. The genotype of the Brattleboro pups was assessed after the experiment by finding absence of vasopressin in the pituitary by radioimmunoassay (RIA). If not otherwise stated, heterozygous rats were compared to their homozygous vasopressin-deficient littermates as they were born and grew up in the same families (from matings of di/+ females with di/di males; Bohus and de Wied Citation1998; Zelena et al. Citation2003b). For Experiment 3, homozygous “wild-type” (+/+) Brattleboro rats were used (Zelena et al. Citation2009b). Experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were reviewed and approved by the Animal Welfare Committee of the Institute of Experimental Medicine.
Experiments
Exp. 1A: Ether exposure in pups
Ten-day-old male pups of the Brattleboro colony were randomly divided into three groups (Walker and Dallman Citation1993). Control, unstressed, male pups were decapitated, without anesthetic, immediately after removal from the mother. Other pups were stressed by placing for 3 min into a 50 ml centrifuge tube that contained ether-soaked cotton wool. Single stressed pups were decapitated 10 min after the beginning of the ether inhalation session. Doubly stressed pups were kept separately from the mother, and 60 min after the onset of the first ether exposure a second ether session was performed, then the pups were decapitated 70 min after the onset of the first stressor. After decapitation, trunk blood (into 1 ml Eppendorf tubes) and pituitaries (into Eppendorf tubes containing 100 μl 0.1 M HCl) were collected. Genotype of the pups was assessed after the experiment on the basis of the vasopressin content of the pituitary gland, and assigned to groups using these measurements (n = 13–19).
Exp. 1B: Ether exposure in adults
Each genotype was randomly divided into three groups. Controls were decapitated rapidly without anesthetic. Stressed rats were put in a glass jar filled with ether vapor for 1 min, then they were kept anesthetized for another 2 min with an ether-soaked nose cone and returned to their home-cage. Singly stressed rats were decapitated 10 min after the beginning of the stress-procedure (Zelena et al. Citation1999). For doubly stressed rats, the single stressor was repeated 60 min later and the rats were decapitated 70 min after the onset of the first stressor. After decapitation, trunk blood was collected on ice in 10 ml polythene centrifuge-tubes, and after centrifugation the serum was stored at − 20°C (n = 12–21).
Exp. 2: LPS treatment
Groups of 10-day-old pups (n = 13–15) or adults (n = 6–7) were injected intraperitoneally with LPS (100 μg/l ml/kg in saline, SIGMA, O55:B5) or 0.9% saline. The pups were marked with ink and returned to their mothers. Two hours later the rats were decapitated and trunk blood was collected on ice for hormone measurement and the pituitaries of pups were collected in 100 μl 0.1 N HCl for assessment of the genotype by vasopressin measurements.
Exp. 3: V1b antagonist and response to LPS
Groups of Brattleboro +/+ pups (n = 13–16; Zelena et al. Citation2009b) at the age of 10 days or adults (n = 8–9) were injected intraperitoneally with a V1b receptor antagonist (SSR149415; 10 mg/1 ml/kg; generous gift of sanofi-sythelabo) or vehicle (0.9% saline with a few drops of Tween 80; Serradeil-Le Gal et al. Citation2002, Citation2005). The dosage of the antagonist was chosen to be at the border of ineffectiveness in adults (Ramos et al. Citation2006). The pups were marked with ink. Fifteen minutes later, LPS (100 μg/1 ml/kg) or vehicle was injected intraperitoneally and the pups were returned to their mothers. Two hours after the second injection the rats were decapitated and trunk blood was collected for hormone measurement.
Exp. 4A
Serum from trunk blood and endocrine glands were collected from 10-day-old and adult rats after decapitation under resting condition between 08:00 and 10:00 h. The glands were used for in vitro testing of CRH and ACTH sensitivities. The anterior pituitary lobes were collected into Dulbecco's modified medium (Sigma-Aldrich, Budapest, Hungary) with 0.25% bovine serum albumin (Fraction V, Calbiochem, La Jolla; DMEM) for CRH sensitivity measurement (n = 7–13); posterior pituitary lobes from pups only were placed into 100 μl 0.1 M HCl for genotyping by vasopressin measurement; and adrenal glands were placed into DMEM for measurement of in vitro ACTH sensitivity (n = 8–11). Serum corticosterone binding capacity (corticosterone binding globulin, CBG) was also measured in samples from these rats (n = 7–8).
Exp. 4B
Separate sets of rats were used for measurement of organ weights, serum osmolality and pituitary vasopressin and oxytocin content.
Corticosterone binding capacity
Transcortin (CBG) binding capacity was determined using the modified Sephadex-LH-20 based method of Shanks and Meaney (Citation1994). To remove endogenous corticosteroids, 50 μl serum aliquots were incubated with 1 ml dextran charcoal for 30 min on ice (500 mg dextran T-70 plus 5 g Norit A in 100 ml TEGM buffer (30 mM TRIS.HCl, 1 mM Na-ETDA, 10 mM Na-molybdate, 10% (v/v) glycerine, pH 7.4)) then centrifuged (300g, 30 min). For determination of the total binding capacity 200 μl supernatant were incubated overnight at 4°C with 200 μl 3H-corticosterone (2.78 TBq/mmol, Amersham, Birmingham, UK; 3 nM in TEGM). For determination of the nonspecific binding capacity 200 μl supernatant was incubated overnight at 4°C with 200 μl 3H- plus cold corticosterone (3 nM 3H- plus 16 μM cold corticosterone in TEGM). Sephadex LH-20 was swollen in TEGM buffer (20% v/v) overnight at 4°C. Microcolumns were built in 1 ml pipette tips from 1250 μl suspension, then washed with 500 μl TEGM and 100 μl TEGMD (20 ml TEGM+3 mg DTT (DL-Dithiothreitol, Sigma-Aldrich, St. Louise, MO, USA); activation step), and then 100 μl incubated supernatant was loaded on the column. The column was eluted with 100 μl TEGMD and after 25 min unbound radioactivity was eluted with 500 μl TEGMD. Both specific and nonspecific binding capacity were measured with three parallel microcolumns for each sample (n = 7–8/group). Eluates were collected from the step of sample loading, volume was made up to 1 ml, then 50 μl aliquots were transferred into liquid scintillation vials and measured in a counter (Wallace 1409 Liquid Scintillation Counter, GMI, Inc., Ramsey, MN, USA). The radioactivity data for specific and nonspecific binding measurements are presented as nM ± SEM in serum.
Osmolality measurement
The serum osmolality was measured by vapor pressure osmometry (The Advanced Micro Osmometer Model 3MO, Verner Futschek Medizintechnik).
In vitro testing: Static incubation
Incubation followed the method of Stachura et al. (Citation1985). Each gland was chopped into small pieces with a sterile scalpel blade and put into 1 ml DMEM. After changing the medium, the pieces were preincubated for 1 h at 37°C under a 95% O2–5% CO2 atmosphere when DMEM was replaced by a fresh solution for another 1 h. After preincubation, the medium was changed above the fragments and collected every 15 min for 6 times, adding 10− 10 M CRH per pituitary (Antoni and Dayanithi Citation1990) or 10− 12 M ACTH per adrenal gland into the second fraction. At the end of the experiment, media were removed, centrifuged at 3000g for 5 min and the supernatant was stored at − 20°C until ACTH or corticosterone measurement. Groups of n = 7–13 were formed after genotyping by phenotype as above.
Hormone measurements
Pituitary samples were placed in a boiling water bath for 5 min, and then homogenized by ultrasound, centrifuged, and vasopressin and oxytocin content were measured from the 100-fold diluted supernatant using specific vasopressin and oxytocin RIAs. The rabbit antibodies were donated by Dr M. Vecsernyés (Szent-Györgyi Medical University, Szeged, Hungary). The cross-reactivity of the vasopressin antibody with oxytocin was < 0.01% and with arginine-vasotocin was 0.03%, while that of the oxytocin antibody with vasopressin was < 0.1% and with arginine-vasotocin was < 0.01%. The limit of detection was 1 pg vasopressin/assay tube or 2 pg oxytocin/assay tube. 125I-labeled tracers were produced by the chloramine-T method. Bound and free fractions were separated by charcoal (Laczi et al. Citation1987; Zelena et al. Citation2006). From serum samples ACTH and corticosterone concentrations were measured by specific RIAs without previous extraction. Both antibodies were developed in our Institute as described elsewhere (Zelena et al. Citation2003a). All the samples from a particular experiment were assayed in the same RIA. The intra-assay variation coefficients of the specific ACTH, corticosterone, vasopressin, and oxytocin RIAs were 7.2, 7.5, 10.7, and 6.6%, respectively.
Statistical analysis
Data were analyzed by three-way ANOVA for factor age, and by two-way ANOVA for factor genotype/treatment and stressor, or in the case of in vitro experiments by repeated measure ANOVA using the Statistica 8.0 program of StatSoft, Inc., Tulsa, OK, USA. Post hoc comparison of the data from different experimental groups was performed by the Newman–Keuls test. For the calculation of the area under the curve (AUC) the following formula was used: 15(t1+t2)/2+15(t2+t3)/2+…15(t5+t6)/2 (15 is the 15 min incubation—x axis—and tn is the hormone value at the n. timepoint—y-axis). Data are presented as mean ± SEM.
Results
Hormone concentrations after stressors
Exp. 1: Ether exposure
Pups
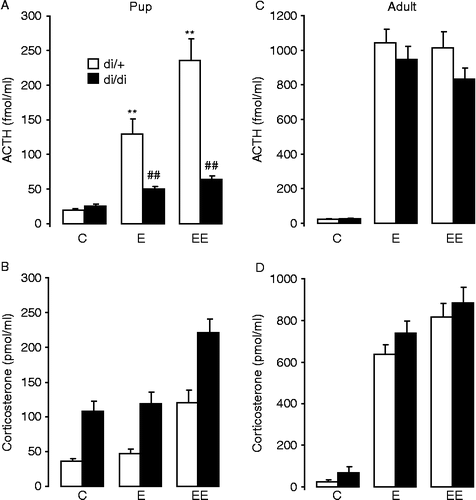
In 10-day-old heterozygous pups the serum ACTH concentrations showed significant effects for ether inhalation, and the second ether exposure induced a significantly greater (10-fold) ACTH increase than the single exposure (fivefold) ; F(2,89) = 30.4; p < 0.001]. In the vasopressin deficient homozygous pups ether inhalation failed to elevate significantly the circulating ACTH concentrations [effect of genotype: F(1,89) = 38.3; p < 0.001 and stressor × genotype interaction: F(2,89) = 14.8; p < 0.001]. In pups, the serum corticosterone concentrations showed significant effects both for ether inhalation and genotype ; ether: F(2,89) = 32.0; p < 0.001; genotype: F(1,89) = 56.1; p < 0.001]. In contrast to ACTH, the serum corticosterone concentrations showed no interaction between stressor and genotype. The resting corticosterone concentrations were already significantly greater in the vasopressin deficient homozygous control group than in the vasopressin-producing heterozygous pups. Single ether exposure failed to elevate circulating corticosterone concentrations while the repetition of the ether-stressor approximately doubled the serum corticosterone concentration in both genotypes.
Figure 1. Serum hormone concentrations in 10-day-old and adult Brattleboro (di/di) rats after single (E; 3 min ether+7 min rest) or repeated (EE; 3 min ether+57 min rest+3 min ether+7 min rest) ether inhalation. In heterozygous controls, both single and repeated ether exposure induced a significant elevation of the ACTH concentrations both in pups (A) and adults (C). The increase after ether exposure was similar in di/di and di/+ adults; however, in di/di pups this elevation was much less than in di/+ pups (A). In pups, only the repeated ether inhalation increased the serum corticosterone concentrations (B), while in adults the single stimulus was also sufficient (D). There was no difference in corticosterone concentrations between the genotypes except for overall greater concentrations in di/di than in di/+ pups. Data are expressed as mean ± SEM. Statistical analysis was done by three-way ANOVA (age, genotype, stress). n = 12–21 per group. **p < 0.01 vs. control; ##p < 0.01 vs. di/+.
Adults
In adult Brattleboro rats, the resting ACTH and serum corticosterone concentrations were comparable to those in the 10-day-old pups but the hormone concentrations after stressor exposure were considerably greater in adults than those in the neonates (about fourfold greater for ACTH and sevenfold for corticosterone) [effect of age for ACTH: F(1,180) = 334.4; p < 0.001 and for corticosterone: F(1,180) = 304.6; p < 0.001]. In adult rats, serum ACTH concentrations showed an approximately 40-fold increase after single or repeated ether inhalation ; F(2,91) = 101.3; p < 0.001] and there was no significant difference between the di/+ and di/di rats under basal or stress conditions. Similarly, serum corticosterone concentrations increased approximately 10-fold after single or repeated ether inhalation ; F(2,91) = 104.3; p < 0.001] and there was no significant difference between the di/+ and di/di rats.
Exp. 2: LPS injection
Pups
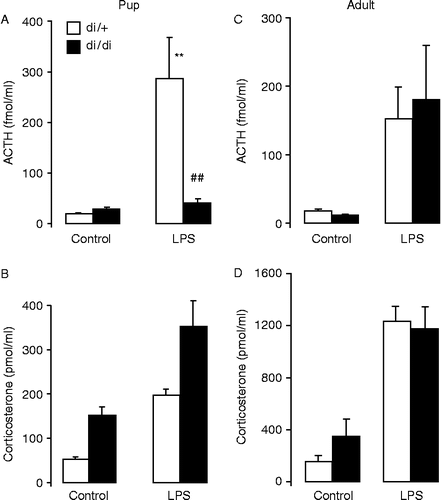
In 10-day-old heterozygous pups the serum ACTH concentrations showed significant effects for LPS injection with an approximately 10-fold increase ; F(1,53) = 9.14; p < 0.01]. In the vasopressin-deficient homozygous pups, LPS injection failed to elevate significantly the circulating ACTH concentrations [effect of genotype: F(1,53) = 6.54; p < 0.01 and stressor × genotype interaction: F(1,53) = 7.99; p < 0.01]. In pups, the serum corticosterone concentrations showed significant effects both for LPS injection (approximately fourfold increase) ; F(1,53) = 27.29; p < 0.001] and genotype [F(1,53) = 13.59; p < 0.001]. In contrast to ACTH, the serum corticosterone concentrations showed no interaction between genotype and stressor. The corticosterone concentrations after saline injection were already significantly greater in the vasopressin-deficient homozygous control group than in the vasopressin-producing heterozygous pups. LPS injection increased the serum corticosterone concentration in both genotypes.
Figure 2. Serum hormone concentrations in 10-day-old and adult Brattleboro rats 2 h after ip LPS (100 μg/ml/kg) or vehicle (control) injection. In di/+ rats, the LPS injection significantly increased ACTH (A,C) and corticosterone (B, D) concentrations at both ages. The vasopressin deficiency prevented the ACTH increase (A) and induced greater overall corticosterone concentrations (B) in pups, but had no effect on the responses to LPS in adults. Data are expressed as mean ± SEM. Statistical analysis was done by three-way ANOVA (age, genotype, stress). n = 6–15 rats per group. **p < 0.01 vs. control; ##p < 0.01 vs. di/+.
Adults
In adult saline-injected control rats, ACTH concentrations were comparable to those in the pups (no effect of age). In the adults, serum ACTH concentrations increased markedly (approximately eightfold) after LPS injection ; F(1,22) = 11.5; p < 0.01] and there was no significant difference between the di/+ and di/di rats under basal or stressed conditions. Similarly, serum corticosterone concentrations increased markedly after LPS injection (approximately eightfold) ; F(1,22) = 57.9; p < 0.001], and was also with no effect of genotype and no interaction between stressor and genotype.
The ACTH concentrations in the adults after LPS were similar to the concentrations in the di/+ LPS-treated pups for both phenotypes (,C). Nevertheless, 2 h after the injection (saline or LPS) the corticosterone concentrations in the adults were substantially greater than those in the equivalent 10-day-old pups (saline injected group: three times greater concentrations, LPS-injected group: six times greater concentrations) [effect of age: F(1,75) = 126.1; p < 0.001].
Exp. 3: LPS injection and V1b receptor antagonist
Pups
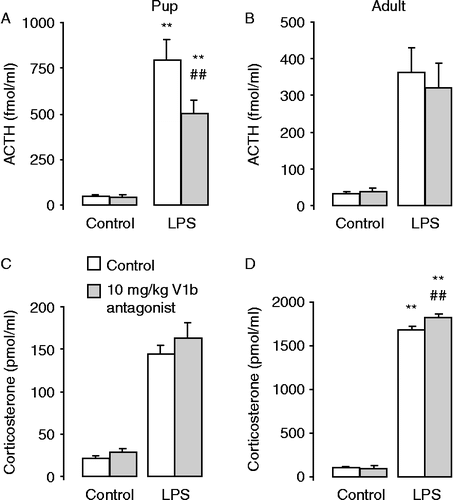
In the 10-day-old +/+ Brattleboro rat pups, the serum ACTH concentrations showed significant effects for LPS injection and V1b antagonist treatment as well as for their interaction ; effect of stressor: F(1,53) = 85.3; p < 0.001; effect of V1b antagonist treatment: F(1,53) = 5.11; p < 0.05 and stressor × V1b antagonist interaction: F(1,53) = 4.80; p < 0.05]. Hence V1b pretreatment significantly reduced the LPS injection induced increase in ACTH concentration. By contrast, the increased corticosterone concentrations after LPS were not affected by V1b pretreatment ; F(1,53) = 121.5; p < 0.001].
Figure 3. Serum hormone concentrations in 10-day-old and adult homozygous vasopressin producing (+/+) Brattleboro rats 15 min after pretreatment with a V1b antagonist (SSR149415; 10 mg/1 ml/kg or saline with few drops of Tween 80) and 2 h after LPS (100 μg/ml/kg) injection. LPS induced significant increases in serum ACTH (A, B) and corticosterone (C, D) concentrations. The V1b antagonist pretreatment diminished the ACTH increase in pups (A) but not in adults (B). Data are expressed as mean ± SEM. Statistical analysis was done by three-way ANOVA (age, V1b treatment, stress). n = 8–16 rats per group. **p < 0.01 vs. control for LPS injection; ##p < 0.01 vs. control for V1b injection.
Adults
In adult +/+ Brattleboro rats 2 h after the saline injection ACTH concentrations were comparable to those in the control pups, while LPS injection induced a smaller ACTH increase in the adults ; effect of age: F(1,82) = 11.66; p < 0.001]. By contrast, the corticosterone concentrations were considerably greater (threefold after saline and approximately 10-fold after LPS injection; ) than in the pups [effect of age: F(1,80) = 3107; p < 0.001]. In adult rats, the increase in serum ACTH concentrations after LPS injection ; F(1,29) = 39.3; p < 0.001] was not altered by V1b receptor antagonist pretreatment; nor was basal ACTH concentration in controls (). Serum corticosterone concentrations were increased markedly after LPS injection ; F(1,27) = 2237; p < 0.001], but the effect of V1b antagonist treatment [F(1,27) = 3.73; p = 0.06] as well as the LPS-injection and V1b antagonist interaction [F(1,27) = 4.08; p = 0.05] were only marginally significant. Nevertheless, the V1b antagonist pretreated group had greater corticosterone concentrations after the LPS stimulus than the LPS alone group, contra-indicating a stimulatory role for vasopressin.
Studies on the mechanism: Experiment 4
Anterior pituitary sensitivity to CRH
Pups
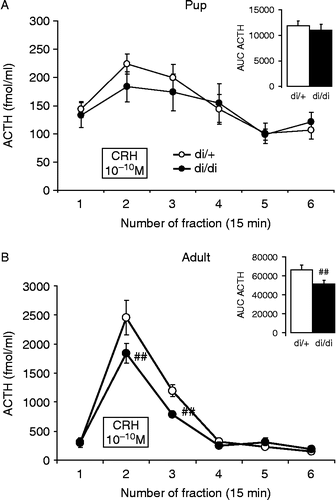
In 10-day-old pups, adding 10 nM CRH to the incubation medium significantly stimulated ACTH release from the anterior lobe of the pituitary ; effect of time: F(5,100) = 7.94; p < 0.001], being increased not only during the presence of the stimulus, but also in the next fraction (+15 min). There was no difference between the two genotypes either in any fraction or in the total amount of secreted hormone, represented by the AUC during the whole observation period , insert].
Figure 4. In vitro CRH sensitivity of anterior pituitary glands of 10-day-old (A) and adult (B) di/+ and di/di Brattleboro rats. In all rats, the 10− 10 M CRH significantly increased ACTH secretion, however this increase was much smaller for anterior pituitaries from pups (A). In pups, there was no difference between the genotypes, while in adults the di/di rats had reduced reactivity. The overall amount secreted is represented by the AUC parameter in the inserts. Data are expressed as mean ± SEM. Statistical analysis was done by repeated measure ANOVA (age, genotype, time) or two-way ANOVA (age, genotype). n = 7–13 ##p < 0.01 vs. di/+.
Adults
The in vitro ACTH release was much higher in adults than in the 10-day-old Brattleboro rat pups, reaching 4–5 times the baseline release while the increase for the pups was less than twofold [effect of age: F(1,33) = 303.8; p < 0.001]. In contrast to pups, in adults, in addition to the significant effect of the CRH stimulus ; effect of time: F(5,65) = 104.5; p < 0.001], there was a significantly smaller increase for anterior pituitaries from di/di rats [effect of genotype: F(1,13) = 4.99; p < 0.05; CRH × genotype interaction: F(5,65) = 3.53; p < 0.01]. This significant difference was present in two fractions, during the 10 nM CRH stimulus and also in the next 15 min fraction. The same significant differences were detected with respect to the total secreted amount of ACTH (AUC) , insert, effect of genotype: F(1,13) = 5.35; p < 0.05].
Adrenal cortex sensitivity to ACTH
Pups
Corticosterone secretion by adrenals of 10-day-old pups was significantly increased in an in vitro system by incubation with 1 pM ACTH ; effect of time: F(5,85) = 71.5; p < 0.001]. This stimulation lasted for 45 min (three fractions) and the greatest stimulation was visable after removing the ACTH from the medium. Although the effect of genotype per se did not reach the level of significance [F(1,17) = 3.69; p = 0.07], the treatment × genotype interaction was significant [F(5,85) = 4.0; p < 0.01], indicating greater reaction by di/di pup adrenals; however, there was only a tendency for greater total corticosterone secretion by di/di pup adrenals , insert, F(1,17) = 4.11; p = 0.06].
Figure 5. In vitro ACTH sensitivity of the adrenal cortex of 10-day-old (A) and adult (B) di/di and di/+ Brattleboro rats. The 10− 12 M ACTH significantly increased corticosterone secretion in all groups. The lesser secretion in A compared with B is accounted for by the smaller pup adrenals. In pups, the di/di rats were more reactive to the stimulus (A) in the two post-stimulation fractions but not in the case of the whole secreted amount represented by AUC in the insert. Data are expressed as mean ± SEM. Statistical analysis was done by repeated measure ANOVA (age, genotype, and time) or two-way ANOVA (age, genotype). n = 8–11 rat samples per group # < 0.05; ##p < 0.01 vs. di/+.
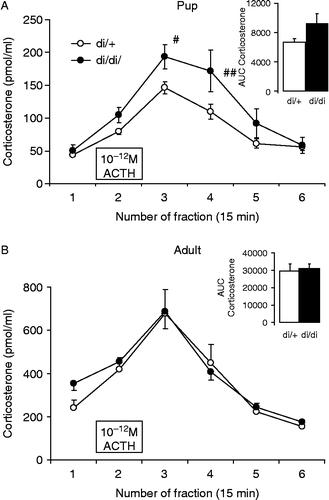
Adults
Baseline in vitro corticosterone secretion was significantly greater from adult adrenals than from pup adrenals, reflecting the differences in adrenal gland weight () [F(1,35) = 74.8; p < 0.001]. Adding 1 pM ACTH to the incubation medium significantly increased ACTH secretion ; F(5,90) = 46.5; p < 0.001], with greatest release in the poststimulus fraction. Neither the genotype nor the treatment × genotype interaction was significant suggesting a similar corticosterone secretion capability in di/+ and di/di adult rat adrenals.
Table I. Comparison of various parameters of 10-day-old and adult vasopressin producing (di/+) and deficient (di/di) rats.
The adrenal gland weight was greater in adults [F(1,33) = 529.6; p < 0.001], and smaller in di/di rats [F(1,33) = 5.41; p < 0.05] without interaction (). The corresponding body weight was similarly increased with age [F(1,33) = 1931; p < 0.001] and reduced by the vasopressin deficiency [F(1,33) = 21.3; p < 0.001]; here also the interaction was significant [F(1,33) = 17.6; p < 0.001].
Serum transcortin concentrations
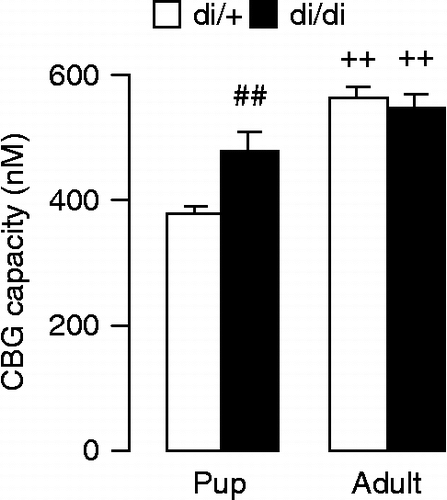
CBG in the serum of 10-day-old pups was significantly greater in the absence of vasopressin ; F(1,12) = 9.06; p = 0.01]. The adult rats had higher CBG [F(1,26) = 36.0; p < 0.001], but in adulthood there was no difference between di/+ and di/di rats [genotype × age interaction: F(1,26) = 7.32; p = 0.01].
Figure 6. CBG binding capacity in the serum of 10-day-old and adult Brattleboro rats. In pups, the vasopressin deficiency resulted in a significant increase in CBG binding capacity. In adults, CBG binding capacity was greater than in pups, with no difference between the genotypes. Data are expressed as mean ± SEM. Statistical analysis was done by two-way ANOVA (age, genotype). n = 7–8 rats per group. ##p < 0.01 vs. di/+;++p < 0.01 vs. pups.
Serum osmolality was significantly greater in di/di rats [F(1,33) = 298.5; p < 0.001], which effect showed age dependence [genotype × age interaction: F(1,33) = 47.8; p < 0.001] being enhanced in pups ().
Pituitary neuropeptide content
The vasopressin content of the pituitary was used to assess the genotype of the pups, and was significantly lower in di/di than di/+ rats ; F(1,33) = 99.8; p < 0.001]. As the size of the hypophysis increased with age (approximately 10-fold, data not shown) the adults had approximately sevenfold more vasopressin in their hypophysis.
The oxytocin content of the pituitary was much lower than its vasopressin content except in the adult di/di rats, where the vasopressin/oxytocin ratio changed (). In di/+ rats, the increased oxytocin content in adults [F(1,29) = 70.6; p < 0.001] was more pronounced (increased approximately 30-fold) than the increase in pituitary weight.
Discussion
The data support the hypothesis that during the neonatal period vasopressin is a more important regulator of ACTH secretion than in adulthood as both the genetically vasopressin-deficient and the V1b receptor antagonist pretreated rats showed diminished ACTH increases to stressors only in pups. The data further support the existence of an ACTH-independent corticosterone regulation of corticosterone secretion, and indicate that enhanced adrenal gland sensitivity to ACTH and higher transcortin levels in di/di pups might also contribute to the observed higher circulating corticosterone concentrations.
Age dependence
In the repeated ether exposure experiment, the previously reported blunted characteristics of the SHRP on stressor induced ACTH and corticosterone secretion was corroborated in the present study (Sapolsky and Meaney Citation1986; Levine Citation2002). Moreover, all other corticosterone characteristics of the 10-day-old pups (after LPS injections or in vitro studies) were similar to what was described in the Wistar strain (Walker and Dallman Citation1993). By contrast, 2 h after LPS injections, the neonatal rats revealed an even higher increase in circulating ACTH level than their adult counterparts (either di/+ rats in Experiment 2 or +/+ rats in Experiment 3). Together with the finding that the in vitro CRH sensitivity of anterior pituitaries from 10-day-old pups was significantly blunted we assume that during the neonatal period there should be other factors that are important in the maintenance of the ACTH secretion. While the in vitro adrenal gland sensitivity to ACTH seemed to be similar in pups and adults (approximately threefold increase in corticosterone release in response to the same dose of ACTH in both cases) we can infer that hypothalamic factors are responsible for the SHRP. Enhanced feedback due to low transcortin levels could also contribute to the SHRP.
Both in vasopressin-deficient Brattleboro pups and in 10-day-old rats that were given V1b antagonist treatment, the decreased ACTH reactivity after different stressors indicates a prominent role of vasopressin in the regulation of the hypophyseal level of the HPA axis. By contrast, adult vasopressin-deficient homozygous Brattleboro rats and their controls showed similar ACTH and corticosterone responses to single or repeated ether exposure or LPS injection, indicating a minor role of vasopressin in HPA axis regulation in adult rats also during these two stressful stimuli (Zelena et al. Citation2009a). Although in Brattleboro rats a compensatory mechanism for the absence of vasopressin may be present (e.g. CRH (Mlynarik et al. Citation2007), and oxytocin (Zelena et al. Citation2009b) (), the present data with the V1b antagonist (SSR149415, at a moderate dose, which was effective in pups only) highlight the different regulatory role of vasopressin at different ages.
ACTH responses to stressors
Our present findings with repeated ether exposure and bacterial LPS injection is similar to the strongly inhibited ACTH secretory response to maternal separation in the homozygous, but not in the heterozygous 10-day-old pups, observed in our previous study (Zelena et al. Citation2008). No differences were observed in the adults in a similar experimental design. Further support for the important role of vasopressin in stimulating ACTH release in the neonatal rat comes from the experiment using a V1b antagonist, which inhibited LPS induced ACTH release in the 10-day-old pups but not in the adults. As in vitro CRH induced a significant stimulation of ACTH secretion from the hypophysis even in pups, it cannot be concluded that the regulatory role of vasopressin is exclusively during the neonatal period (Baram and Lerner Citation1991; Dent et al. Citation2000). Rather there is a shift from a more pronounced regulatory role of vasopressin during the neonatal period to CRH dominance in adults, despite the increasing amount of synthesized vasopressin ().
Theoretically, vasopressin deficiency or V1b antagonism might interfere with stressor-induced ACTH release at several points in the cascade of events. One possibility is that in the neonatal rat, vasopressin is a dominant hypothalamic ACTH secretagogue and its absence or inhibition results in diminished ACTH release. Previously, vasopressin of nucleus paraventricularis hypothalami origin was suggested to be the dominant ACTH secretagogue in the neonate rat (Muret et al. Citation1992; Levine Citation2002), so the lack of the ligand on the pituitary V1b receptors may result in reduced ACTH secretion.
Another possibility is a decreased neuronal stimulation reaching the hypothalamus when vasopressin is lacking or its receptors are under pharmacological inhibition. Central V1b receptors were detected in the amygdala and hippocampus of the rat (Young et al. Citation2006) and could serve as putative mediators of either the maternal signals in the maintenance of the SHRP (Schmidt et al. Citation2004) or the stressor-induced stimulatory influences reaching the hypothalamic CRH/vasopressin neurons. However, in vitro CRH sensitivity of the anterior pituitary indicates an age-dependent change at the hypophysial level (pup anterior pituitaries reacted to CRH with an approximately 40–50% increase in ACTH secretion, while in adulthood the reaction to the same stimulus was 600–800%).
Increased glucocorticoid feedback inhibition in the homozygous 10-day-old pups is unlikely to account for the lack of stressor-induced ACTH release. Although resting corticosterone concentrations are higher in the vasopressin-deficient pups their serum has greater CBG activity, which might limit the amount of free corticosterone reaching the feedback sites. Although hemoconcentration reflected by increased osmolality in the di/di rats could be, at least partially, responsible for the enhanced CBG concentrations, this does not alter the above suggestion regarding the free corticosterone fraction. Moreover, findings from V1b antagonist treatment also contraindicate a role for altered feedback.
Corticosterone responses to stressors
An intriguing observation was the maintained corticosterone stress response to repeated ether exposure or LPS injection without increased ACTH secretion in the 10-day-old di/di pups. This increased serum corticosterone following stressors parallels the earlier observation of a marked corticosterone response to maternal separation without a parallel ACTH response in 10-day-old homozygous Brattleboro pups (Zelena et al. Citation2008). Several possibilities might explain this phenomenon. We already excluded the possibility of an earlier ACTH increase, and enhanced sensitivity of the adrenal gland did not seem to be a significant explanation (Zelena et al. Citation2008). The enhanced CBG capacity of di/di pups could also contribute to their higher total corticosterone concentrations, but is unable to explain the stressor-induced elevations. Moreover, our observation is not an exception but is supported by many other studies (Bornstein et al. Citation2008).
One possibility is that in the neonatal rat pups the stressor-induced corticosterone secretion has a strong neurally mediated adrenal component. It is well-known that a number of neural mediators such as catecholamines, acetylcholine, and vasoactive intestinal polypeptide occur in nerve fibers in the adrenal cortex and are capable of stimulating corticosterone synthesis and release (Ehrhart-Bornstein et al. Citation1995).
As LPS is an immune stimulus we could consider cytokine-induced direct adrenal gland stimulation (Engstrom et al. Citation2008), but it does not seem to be likely in the case of ether exposure or maternal separation. Theoretically it is possible, that with different stressors different additional pathways are activated, but it is unlikely that these factors always contribute to corticosterone secretion of the same magnitude.
Another possibility is that the lack of vasopressin in the hypophysial portal blood during the SHRP alters the proteolytic procession of pro-opiomelanocortin (POMC) preprohormone in the anterior pituitary gland, thus corticosteroidogenic POMC products, other than ACTH immunoreactive peptides, might be secreted by the pituitary corticotropes. Such peptides may alter the responsiveness of the adrenal cortex to neuronal or humoral influences. This suggestion implies that homozygous and heterozygous pups secrete different ACTH fragments also under resting conditions, and in adults this effect of vasopressin deficiency should disappear. Moreover, V1b antagonist treatment should have a similar influence on the proteolytic process. It was reported that some ACTH fragments have a weak corticosterone secretion stimulating action (e.g. 70 times weaker than the effect of ACTH1-39; Feuilloley et al. Citation1990). So altered proteolytic processing is quite unlikely to be responsible for the ACTH-corticosterone dissociation in neonates.
In summary, the present result suggests that vasopressin is an important contributing factor in the normal function of the HPA axis during the SHRP. The findings suggest a yet unknown mechanism of regulation of circulating glucocorticoid concentrations in the absence of serum ACTH increases. Blunted anterior pituitary sensitivity to CRH and similar adrenal gland sensitivity to ACTH in the pups indicate that hypothalamic factors could be responsible for the stress hyporesponsivity during the neonatal period. The role of vasopressin in HPA axis regulation appears to diminish with age, which may be due to a shift to a more important role of CRH.
Acknowledgements
Supported by grants F 48783, IN 67249 and NN 71629 from OTKA, Hungary and a grant 059/2006 from ETT, Hungary.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Antoni FA. 1993. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 14:76–122.
- Antoni FA, Dayanithi G. 1990. Secretion of ACTH by perifused isolated rat anterior pituitary cells: Pulses of secretagogue enhance the secretory response and modify the effect of atriopeptin. J Endocrinol. 125:365–373.
- Avishai-Eliner S, Yi SJ, Newth CJ, Baram TZ. 1995. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic–pituitary–adrenal components in the infant rat. Neurosci Lett. 192:49–52.
- Baram TZ, Lerner SP. 1991. Ontogeny of corticotropin releasing hormone gene expression in rat hypothalamus-comparison with somatostatin. Int J Dev Neurosci. 9:473–478.
- Bohus B, de Wied D. 1998. The vasopressin-deficient Brattleboro rats: A natural knockout model used in the search for CNS effects of vasopressin. Prog Brain Res. 119:555–573.
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. 2008. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 19:175–180.
- Dayanithi G, Antoni FA. 1989. Rapid as well as delayed inhibitory effects of glucocorticoid hormones on pituitary adrenocorticotropic hormone release are mediated by type II glucocorticoid receptors and require newly synthesized messenger ribonucleic acid as well as protein. Endocrinology. 125:308–313.
- de Kloet ER, Oitzl MS. 2003. Who cares for a stressed brain? The mother, the kid or both?. Neurobiol Aging. 24 Suppl 1: S61–5 discussion S67–8.
- Dent GW, Okimoto DK, Smith MA, Levine S. 2000. Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology. 71:333–342.
- Ehrhart-Bornstein M, Bornstein SR, Gonzalez-Hernandez J, Holst JJ, Waterman MR, Scherbaum WA. 1995. Sympathoadrenal regulation of adrenocortical steroidogenesis. Endocrinol Res. 21:13–24.
- Engstrom L, Rosen K, Angel A, Fyrberg A, Mackerlova L, Konsman JP, Engblom D, Blomqvist A. 2008. Systemic immune challenge activates an intrinsically regulated local inflammatory circuit in the adrenal gland. Endocrinology. 149:1436–1450.
- Feuilloley M, Stolz MB, Delarue C, Fauchere JL, Vaudry H. 1990. Structure–activity relationships of monomeric and dimeric synthetic ACTH fragments in perifused frog adrenal slices. J Steroid Biochem. 35:583–592.
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. 1994. Blockade of alpha 2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology. 135:2549–2557.
- Laczi F, Vecsernyes M, Kovacs GL, Szabo G, Janaky T, Telegdy G, Laslo FA. 1987. Effects of beta-endorphin2-9 on arginine-8-vasopressin and oxytocin levels in hypothalamic and limbic brain regions. Brain Res. 403:155–157.
- Levine S. 2002. Regulation of the hypothalamic–pituitary–adrenal axis in the neonatal rat: The role of maternal behavior. Neurotox Res. 4:557–564.
- McEwen BS. 2008. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 583:174–185.
- Mlynarik M, Zelena D, Bagdy G, Makara GB, Jezova D. 2007. Signs of attenuated depression-like behavior in vasopressin-deficient Brattleboro rats. Horm Behav. 51:395–405.
- Muret L, Priou A, Oliver C, Grino M. 1992. Stimulation of adrenocorticotropin secretion by insulin-induced hypoglycemia in the developing rat involves arginine vasopressin but not corticotropin-releasing factor. Endocrinology. 130:2725–2732.
- Penke Z, Felszeghy K, Fernette B, Sage D, Nyakas C, Burlet A. 2001. Postnatal maternal deprivation produces long-lasting modifications of the stress response, feeding and stress-related behaviour in the rat. Eur J Neurosci. 14:747–755.
- Ramos AT, Troncone LR, Tufik S. 2006. Suppression of adrenocorticotrophic hormone secretion by simultaneous antagonism of vasopressin 1b and CRH-1 receptors on three different stress models. Neuroendocrinology. 84:309–316.
- Sapolsky RM, Meaney MJ. 1986. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 396:64–76.
- Schmale H, Ivell R, Breindl M, Darmer D, Richter D. 1984. The mutant vasopressin gene from diabetes insipidus (Brattleboro) rats is transcribed but the message is not efficiently translated. EMBO J. 3:3289–3293.
- Schmidt M, Enthoven L, van Woezik JH, Levine S, de Kloet ER, Oitzl MS. 2004. The dynamics of the hypothalamic–pituitary–adrenal axis during maternal deprivation. J Neuroendocrinol. 16:52–57.
- Serradeil-Le Gal C, Wagnon J, Valette G, Garcial G, Pasca M, Maffrand JP, Le Fur G. 2002. Nonpeptide vasopressin receptor antagonists: Development of selective and orally active V1a, V2 and V1b receptor ligands. Prog Brain Res. 139:197–210.
- Serradeil-Le Gal C, Wagnon JIII, Tonnerre B, Roux R, Garcia G, Griebel G, Aulombard A. 2005. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS Drug Rev. 11:53–68.
- Shanks N, Meaney MJ. 1994. Hypothalamic–pituitary–adrenal activation following endotoxin administration in the developing rat: A CRH-mediated effect. J Neuroendocrinol. 6:375–383.
- Stachura ME, Tyler JM, Farmer PK. 1985. Human pancreatic growth hormone-releasing factor-44 differentially stimulates release of stored and newly synthesized rat growth hormone in vitro. Endocrinology. 116:698–706.
- Suchecki D, Rosenfeld P, Levine S. 1993. Maternal regulation of the hypothalamic–pituitary–adrenal axis in the infant rat: The roles of feeding and stroking. Brain Res Dev Brain Res. 75:185–192.
- Walker CD, Dallman MF. 1993. Neonatal facilitation of stress-induced adrenocorticotropin secretion by prior stress: Evidence for increased central drive to the pituitary. Endocrinology. 132:1101–1107.
- Walker CD, Perrin M, Vale W, Rivier C. 1986. Ontogeny of the stress response in the rat: Role of the pituitary and the hypothalamus. Endocrinology. 118:1445–1451.
- Walker CD, Scribner KA, Cascio CS, Dallman MF. 1991. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 128:1385–1395.
- Walker SJ, Vrana KE. 1993. Pituitary corticotroph function during the stress hyporesponsive period in neonatal rats. Neuroendocrinology. 57:1003–1010.
- Young WS, Li J, Wersinger SR, Palkovits M. 2006. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 143:1031–1039.
- Zelena D, Domokos A, Barna I, Mergl Z, Haller J, Makara GB. 2008. Control of the hypothalamo–pituitary–adrenal axis in the neonatal period: Adrenocorticotropin and corticosterone stress responses dissociate in vasopressin-deficient brattleboro rats. Endocrinology. 149:2576–2583.
- Zelena D, Kiem DT, Barna I, Makara GB. 1999. Alpha 2-adrenoreceptor subtypes regulate ACTH and beta-endorphin secretions during stress in the rat. Psychoneuroendocrinology. 24:333–343.
- Zelena D, Mergl Z, Foldes A, Kovacs KJ, Toth Z, Makara GB. Role of hypothalamic inputs in maintaining pituitary–adrenal responsiveness in repeated restraint. Am J Physiol Endocrinol Metab. 2003a; 285:E1110–E1117.
- Zelena D, Mergl Z, Makara GB. Maternal genotype influences stress reactivity of vasopressin-deficient brattleboro rats. J Neuroendocrinol. 2003b; 15:1105–1110.
- Zelena D, Filaretova L, Mergl Z, Barna I, Toth ZE, Makara GB. 2006. Hypothalamic paraventricular nucleus, but not vasopressin, participates in chronic hyperactivity of the HPA axis in diabetic rats. Am J Physiol Endocrinol Metab. 290:E243–E250.
- Zelena D, Domokos A, Jain SK, Jankord R, Filaretova L. The stimuli-specific role of vasopressin in the hypothalamus–pituitary–adrenal axis response to stress. J Endocrinol. 2009a; 202:263–278.
- Zelena D, Langnaese K, Domokos A, Pinter O, Landgraf R, Makara GB, Engelmann M. Vasopressin administration into the paraventricular nucleus normalizes plasma oxytocin and corticosterone levels in Brattleboro rats. Endocrinology. 2009b; 150:2791–2798.
