Abstract
Clinically, adults who have experienced stresses in childhood present with episodes of serious symptoms of irritable bowel syndrome that are associated with acute stress, but the mechanism is not well understood. This study aimed to investigate the colonic sensory/motor responses to acute water avoidance stress (WAS) in male adult rats subjected to neonatal maternal separation (NMS), and the underlying mechanism of sensory/motor responses. Effects of the combined acute and early life stress on visceral sensation, colonic motility, and the tissue and luminal content of serotonin (5-hydroxytryptamine, 5-HT) in the proximal and distal colon were evaluated using the abdominal withdrawal reflex test, faecal pellet output measurement and capillary electrophoresis analysis, respectively. Results showed that WAS significantly increased not only visceral sensitivity but also colonic motility in NMS rats compared to the normal rats. These alterations were accompanied by significant increase in 5-HT content in the proximal but not the distal colonic tissues; these alterations were also associated with increased density of enterochromaffin (EC) cells in the proximal segment. In contrast, the faecal content of 5-HT increased similarly in both segments. Consecutive administration of parachlorophenylalanine to NMS rats was more potent at 500 mg kg− 1 day− 1 than at 150 mg kg− 1 day− 1 in suppressing colonic sensory/motor responses to WAS, corresponding to the greater reduction of the tissue and faecal content of 5-HT and of EC cell density in the colon. These data indicate that combined early life stress and acute stress effectively induce visceral hyperalgesia and motility disorder through 5-HT pathways in the colon of rats, and the proximal and distal colon have different responses towards the combined stressors.
Introduction
Irritable bowel syndrome (IBS) is a common condition characterised by abdominal pain/discomfort connected with disturbed defecation without structural abnormalities in the gut (Horwitz and Fisher Citation2001). Although there has been progress in the last 20 years in studying the mechanisms of IBS, the pivotal mechanisms responsible for the abdominal pain or visceral sensation in IBS are still unknown (Camilleri Citation2010). Clinical studies have shown that adverse physiological or psychological experiences in early life are associated with the development of IBS symptoms (Lowman et al. Citation1987; Chitkara et al. Citation2008), and acute stresses in adulthood are associated with episodes of serious symptoms in IBS patients (Drossman et al. Citation1988). Therefore, childhood traumatic experiences followed by later exposure to acute stress in adulthood may play key roles in the development as well as in the modulation and maintenance of IBS throughout the patient's life. Despite the widespread occurrence of this condition, treatment is not entirely satisfactory, perhaps largely because the underlying mechanism remains unclear.
Previous studies have shown that early traumatic experiences, including neonatal maternal deprivation, neonatal colonic inflammatory stimuli and neonatal colorectal distension (CRD), result in visceral hyperalgesia, increased gastrointestinal mucosa permeability and bacterial translocation in adulthood in rats (Barreau et al. Citation2007), but these models rarely demonstrate motility dysfunction such as diarrhoea or constipation. It seems that although these models can mimic some aspects of IBS, especially visceral hyperalgesia, they cannot reproduce the motility dysfunction at the same time. Experimental studies also demonstrate that acute exposure to a variety of psychological, physical and immune stressors stimulate faecal output and induce long lasting visceral hyperalgesia (Taché et al. Citation2004; Bradesi et al. Citation2005). Moreover, some studies have shown that acute stimuli can accelerate colonic transit (Coutinho et al. Citation2002; Tian et al. Citation2006) and facilitate visceral hypersensitivity (Coutinho et al. Citation2002; Schwetz et al. Citation2005; Ren et al. Citation2007) in neonatally stressed rats. Evidently, early life stressors may not directly induce motility disorder, but may predispose rats to manifest colonic motility dysfunction in response to acute stress later in life. Although the model of acute stress can successfully mimic both visceral hyperalgesia and colonic motility dysfunction, it cannot reflect the most common situation in IBS patients, in which early life trauma plus adult acute stress play cooperative roles. Therefore, the combination of early life stress with subsequent acute stress in adulthood may mimic better the cause(s) of visceral hyperalgesia and motility disorder, the cardinal features of IBS.
Understanding of the underlying mechanism(s) of the functional alterations in the gut induced by different stressors is rather poor. Serotonin (5-hydroxytryptamine, 5-HT) in the gastrointestinal tract is generally believed to be one of the most important mediators and regulators of bowel sensation and motility (Gershon Citation1999; Crowell et al. Citation2004). Accumulating data show that enterochromaffin (EC) cells, which store the majority of 5-HT in the body (Kim and Camilleri Citation2000), release 5-HT not only into the portal circulation or basolateral border of the mucosa, but also into the lumen of the gastrointestinal tract (Fujimiya et al. Citation1997; Tsukamoto et al. Citation2007). Previous research has shown that luminal release of 5-HT is involved in alterations in gastrointestinal function induced by acute stimuli in normal rats. For example, increased lumen pressure (Fujimiya et al. Citation1997) or restraint stress (Nakade et al. Citation2007) has been reported to stimulate luminal content of 5-HT and increase colonic motility in normal rats. In addition, luminal administration of 5-HT into the proximal colon significantly accelerates colonic transit and increases faecal pellet output in normal rats (Fukumoto et al. Citation2003). However, the combined effects of the acute stress with neonatal maternal separation (NMS) on luminal content of 5-HT have not been investigated. Interestingly, the effects of NMS on the 5-HT content in colonic tissue have been investigated, but with inconsistent results. The colonic tissue content of 5-HT in NMS rats was increased in one study (Tian et al. Citation2006), but not in another (Ren et al. Citation2007). Although both studies found that acute stimuli increased 5-HT in the colonic tissue under chronic stress conditions, differences between the proximal and distal colon were not analysed. Actually, the proximal and the distal colon differ greatly in many respects, including innervation (Altschuler et al. Citation1993), 5-HT content (Tsukamoto et al. Citation2007) and EC cell density (Oshima et al. Citation1999). It is possible that the two colonic segments respond differently to stress, such that non-discriminating assessment of the whole colon may overlook critical regional differences.
This study hypothesised that early life stress predisposes rats to overreact to acute stress in adulthood, expressed as colonic sensory/motor dysfunction through 5-HT pathways in the colon, and that the proximal and distal colon respond differently to these stressors. To test this hypothesis, the indices of visceral sensitivity and colonic motor function and changes in colonic tissue and colonic luminal content of 5-HT in response to acute water avoidance stress (WAS) in normal and NMS rats were evaluated. In particular, 5-HT concentration and EC cell density in the proximal and distal colon were analysed separately. In addition, the effects of 5-HT depletion with parachlorophenylalanine (PCPA) on colonic sensory and motor responses to combined stressors were investigated. The results support our hypothesis that a combined early life stressor and acute adulthood stressor effectively induce visceral hyperalgesia and motility disorder; the proximal and distal colon respond differently towards the combined stressors, and serotonin pathways mediate these changes in the rat colon.
Methods
Animals and neonatal maternal separation
Primiparous timed-pregnant Sprague-Dawley female rats were obtained from the Laboratory Animal Services Centre, The Chinese University of Hong Kong, on gestational day 15. Rats were maintained on a 12:12 h light-dark cycle (lights on from 06:00 h until 18:00 h) with free access to food and water. They were housed individually in standard polypropylene cages (Eurostandard Type III H, from Tecniplast, Milan, Italy) each sized 42.5 (H) × 26.6 (W) × 18.5 (D) cm containing 2.5 cm of wood chip bedding material to a depth of 2.5 cm. In the animal house, the temperature was kept at 20 ± 2°C and the relative humidity at 55–60%. The handling of rats and all experimental procedures were approved by the Animal Care and Use Committee at Hong Kong Baptist University, in accordance with the Animal Ordinance (Control of Experiments), Hong Kong, China.
The NMS rat model was produced as previously reported (Coutinho et al. Citation2002). Briefly, rat pups were separated from their mothers from postnatal days 2 to 14 for 3 h daily. During the each 3-h separation, rats were kept in a separate cage lined with bedding material under a lamp to keep them warm. The normal handled pups (NH) were left undisturbed in their home cages with the dams. All rat pups were weaned at postnatal day 22 and housed 5 rats per cage. Only male rats 8 weeks old and weighing approximately 290 g (mean ± S.E.M.: 290 ± 21 g in NH group, n = 21, and 285 ± 17 g in NMS group, n = 41) were used in the present study.
Study design
The first series of experiments aimed to investigate the combined effect of NMS and WAS on visceral pain threshold and faecal pellet output, and the PCPA effect on visceral pain threshold and faecal pellets. Group 1, non-handled rats (NH group, n = 5), and Group 2, NMS rats (NMS group, n = 5), received abdominal withdrawal reflex (AWR) tests for visceral pain threshold assessment in order to analyse the effect of NMS on visceral sensory responses. Group 3 with NH rats (n = 5) and Group 4 with NMS rats (n = 5) received WAS treatment for faecal pellet assessment. Group 5 with NMS rats (n = 5) were intraperitoneally (i.p.) given 5-HT synthesis inhibitor PCPA at the dose of 500 mg kg− 1 day− 1 before WAS treatment, whereas Group 6 with NMS rats (n = 4) were administered (i.p.) with PCPA at the dose of 150 mg kg− 1 day− 1 before WAS treatment. For Groups 3–6, AWR tests were conducted 24 h after WAS treatment, thus the results from these groups represent (1) the effects of NMS plus WAS on visceral sensory and motility responses and (2) the effects of PCPA. In order to maximally exclude interference by faeces in rats during AWR tests, all rats were fasted for 24 h before the tests.
The second series of experiments aimed to investigate 5-HT involvement in colonic motor responses to WAS treatment. Unlike in the first series of experiments, all rats were not fasted, and faeces and tissue samples were collected for quantitative analysis. Group 1 with NH rats (n = 6) and Group 2 with NMS rats (n = 8) did not receive WAS treatment. Group 3 with NH rats (n = 5), Group 4 with NMS rats (n = 5) and Group 5 with NMS rats (n = 5) were administered (i.p.) PCPA at the dose of 500 mg kg− 1 day− 1, and Group 6 with NMS rats (n = 4) were administered (i.p.) PCPA at the dose of 150 mg kg− 1 day− 1 and received WAS treatment. Faecal pellets from these rats were collected with a metabolism cage to give a baseline value, and rats were euthanised (with an overdose of pentobarbital sodium, 120 mg/kg, i.p.) 24 h after the WAS test. For all experimental rats, samples of faeces were collected for 5-HT assessment, and tissues of the proximal and distal colon were collected for 5-HT content assessment and EC cell study.
Parachlorophenylalanine treatment
Preparation of PCPA suspension
PCPA was suspended (Sigma Chemical Co., St Louis, MO, USA) in alkaline 0.9% saline solution (pH 10.0–10.5), then two drops of PEG-400 and one drop of Tween-80 was added. Afterwards, HCl (5 mol l− 1) was added drop by drop with continued gentle stirring until the solution changed from a clear solution to a homogenous opalescent suspension (pH 7.6).
PCPA administration
The rats were administered (i.p.) PCPA at the high (500 mg kg− 1 day− 1) or low (150 mg kg− 1 day− 1) dosage for three consecutive days. PCPA was given in a volume of 4 ml kg− 1 between 09:00 and 11:00 h during the peak of 5-HT levels (Bercovici et al. Citation2006). Seventy-two hours after the last injection of PCPA, WAS treatment was applied.
Water avoidance stress
WAS test was conducted with the procedures slightly modified from a previous report (Bonaz and Taché Citation1994). Briefly, a rat was placed for 1 h on a plastic block (10 × 8 × 8 cm) affixed to the centre of the floor of a tank during the day time between 09:00 and 11:00 h. The tank was filled with clean water at room temperature (25°C) reaching to about 1 cm from the top of the block. The total number of faecal pellets during WAS were recorded.
Abdominal withdrawal reflex test
The AWR test was conducted as previously described (Al-Chaer et al. Citation2000). Briefly, the rats were mildly sedated with ether inhalation before a distension balloon, which was approximately 4 cm in length and made from the finger of a latex glove, lubricated with glycerol, was inserted into the colorectum, with the distal tip 1 cm from the anus. The balloon was secured in place by taping the balloon catheter to the base of the tail. The rat soon recovered from the ether and was then placed in a plexiglass cage (20 × 15 × 15 cm) and allowed to adapt for 30 min. The tube of the balloon was connected via a Y-connector to a sphygmomanometer. CRD was applied in increments of 5 mmHg starting at 10 mmHg until an identifiable contraction of the abdominal wall (score 2 in the study of Al-Chaer et al. Citation2000) was detected by an observer who was blinded to the grouping. Then the balloon was deflated immediately. The visceral pain threshold intensity, which is defined as a pressure that elicits an observable AWR response to CRD, was tested 5 times in each rat at 5-min intervals. The values were averaged for analysis.
Measurement of faecal pellet output
Faecal pellet output was measured as previously described (Bonaz and Taché Citation1994). In brief, rats were housed individually in a metabolism cage (Eurostandard Type III H, from Tecniplast) for 1 week to allow them to become accustomed to their environment. Each rat was fed daily with equal food (pre-weighed) and water. Baseline 24-h faecal pellet output of each rat was monitored for at least three consecutive days before WAS treatment. On the day of the experiment, each rat was exposed to WAS for 1 h; the faecal pellets expelled during the period of WAS were counted. After WAS, each rat was returned to the individual observation cage and fed ad libitum with food (pre-weighed) and water, and faecal pellets were collected over a 24-h period. Colonic transit was expressed as the mean number of faecal pellets per hour.
Tissue preparation
After the measurement of faecal pellet output, the rats were euthanised with an overdose of pentobarbital sodium (120 mg/kg, i.p.); approximately 3 cm of proximal colon (2 cm from the caeco-colonic junction), approximately 3 cm of distal colon (8 cm from the caeco-colonic junction) and the faeces inside were promptly harvested. The distal parts (about 2 cm in length) of the proximal and distal colons and all faeces were immediately frozen at − 80°C for 5-HT detection. The proximal parts (about 1 cm in length) of the proximal and distal colon tissue were fixed in 4% neutral-buffered paraformaldehyde for histological assessment.
Assessment of the tissue and faecal content of 5-HT in the proximal and distal colon
The content of 5-HT was detected following the procedure described in a previous report (Qi et al. Citation2009). Briefly, the frozen tissue or faecal samples were shattered into fine powder in a mortar and weighed. Each sample was processed as previously described, and the sample separation was carried out on a P/ACE MDQ capillary electrophoresis system equipped with a laser-induced fluorescence detector (Beckman Coulter Instrument, Fullerton, CA, USA). The data were collected and processed by Beckman P/ACE 32 Karat Software Version 7.0. The concentration of 5-HT was expressed as ng mg− 1 (wet weight of tissue or faeces). All chemicals used in this experiment were purchased from Sigma-Aldrich (St Louis, MO, USA).
Enterochromaffin cell assay
The colonic tissue samples were fixed in 4% paraformaldehyde for 48 h then paraffin-embedded routinely for staining of EC cells according to the Masson-Fontana silver staining procedure (Barbosa et al. Citation1984) with slight modifications. After deparaffination and rehydration, 4-μm-thick transverse sections were incubated in a dark humidified chamber with 5% ammoniacal silver solution for 4 h at room temperature followed by 2 h incubation at 56°C and returned to room temperature overnight. The appearance of brown to black silver precipitate in cytoplasm of EC cells was taken as a positive reaction. Images of at least five non-overlapping high power fields (magnification: × 200) from each paraffin section were used for analysis of EC cell density. With the aid of the NIH Image J software, the area of colonic mucosa in each image was estimated, and EC cells distributed in the mucosa were counted. EC cell density was expressed as the mean of the number of EC cells per square millimetre (mm2) of colonic mucosa (Wheatcroft et al. Citation2005; Glisií et al. Citation2006).
Statistical analysis
Data are expressed as mean ± S.E.M. Graphs were generated using Sigma Plot v.9 (Systat, Inc., Chicago, USA). Statistical analysis was conducted using SPSS 12 Software. The effects of NMS and WAS on colonic sensation, motility, 5-HT content and EC cell number were done by two-way ANOVA followed by Student's t-tests. The effects of PCPA on colonic sensation, motility, 5-HT content and EC cell number was done by One-way ANOVA followed by post-hoc tests with Bonferroni's correction. In addition, paired Student's t-tests were used when comparing the level of 5-HT between the proximal and distal colon. When Student's t-test was used to make multiple comparisons, p-value was adjusted and difference was considered significant when P < 0.01; other data were considered significantly different when P < 0.05 (two-sided).
Results
Colonic sensory responses in NH and NMS rats
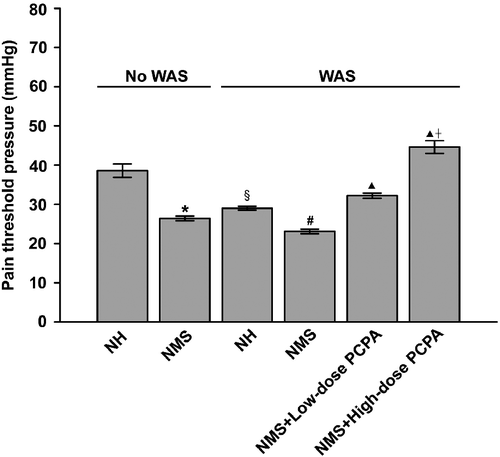
summarises the visceral nociceptive responses of NH and NMS rats without WAS treatment or at 24 h after WAS treatment. Two-way ANOVA showed highly significant effects of NMS (F1, 16: 39.69, P < 0.001), WAS (F1, 16: 20.02, P < 0.001), and the interaction between NMS and WAS (F1, 16: 6.22, P = 0.024). Consistent with our previous report (Chung et al. Citation2007), NMS alone resulted in a significantly lower visceral pain threshold pressure when compared to NH rats (P < 0.001, Student's t-tests). After exposure to WAS, the pain threshold pressure in the NH group was significantly reduced by ∼25% when compared with the NH group without WAS (P < 0.001, Student's t-tests). In NMS rats, the pain threshold was reduced by ∼12%, but there was no statistically significant difference when compared with that of NMS rats without WAS treatment. The threshold pressure in the group of NH rats with WAS remained higher than that in the NMS with WAS group (P < 0.001, Student's t-tests). However, when PCPA was applied, the WAS-aggravated visceral hyperalgesia in NMS rats was substantially suppressed. As shown in , the visceral pain threshold pressures in NMS rats after administration of low and high doses of PCPA were significantly higher than those in the NMS+WAS group (P < 0.001, One-way ANOVA followed by Bonferroni's correction). Furthermore, the pain threshold pressure in the NMS group given high-dose PCPA and WAS was significantly higher than that in the low-dose PCPA group (P < 0.001, One-way ANOVA followed by Bonferroni's correction). These results indicate that the combined effect of WAS and NMS generated significant visceral hyperalgesia, and that 5-HT may be involved in the colonic sensory response to WAS in NH and NMS rats.
Figure 1. Effects of WAS and PCPA on colonic nociceptive threshold in normal handled (NH) and NMS rats (n = 5 in all groups except n = 4 in NMS+low-dose PCPA+WAS group). Data are presented as mean ± S.E.M. *P < 0.001 vs. NH group, §P < 0.001 vs. NH group, #P < 0.001 vs. NH+WAS group (t-tests); ▴P < 0.001 vs. NMS+WAS group, †P < 0.001 vs. NMS+low-dose PCPA+WAS group (Bonferroni's correction).
Colonic motor responses in NH and NMS rats
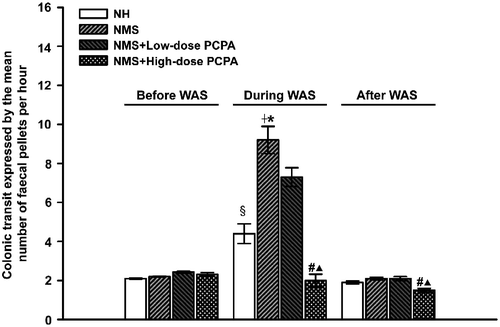
Two-way ANOVA indicated significant effects of WAS (F1, 16: 108.47, P < 0.001) and the interaction of WAS × NMS (F1, 16: 26.88, P < 0.001) on colonic motor function. As shown in , there was no significant difference in faecal pellet number per hour between the NH and NMS groups before WAS treatment. During a 1-h period of exposure to WAS, the faecal pellet number for NH and NMS rats was significantly increased when compared with that before WAS (P < 0.001, Student's t-tests). The faecal pellet number during WAS, for NMS rats was significantly greater than that for NH rats (P < 0.001, Student's t-tests). In addition, the WAS-induced increase of faecal pellet number for NMS rats was markedly attenuated by PCPA at 500 mg kg− 1 day− 1 (P < 0.001, One-way ANOVA followed by Bonferroni's correction). PCPA at 150 mg kg− 1 day− 1 also slightly inhibited colonic motor responses to WAS, but there was no statistically significant difference when compared with that of the NMS group. Furthermore, PCPA at 500 mg kg− 1 day− 1 significantly reduced the faecal pellet number in NMS rats during the 24 h after 1-h WAS treatment, when compared with that in NMS rats (P < 0.001, One-way ANOVA followed by Bonferroni's correction).
Figure 2. Effects of WAS and PCPA on colon motility in normal handled (NH) and NMS rats (n = 5 in all groups except n = 4 in NMS+low-dose PCPA+WAS group). Colon motility was assessed by the mean faecal pellet output per hour before, during and 24 h after WAS. Data are presented as mean ± S.E.M. §P < 0.001 vs. NH group, †P < 0.001 vs. NMS group, *P < 0.001 vs. NH+WAS group (t-tests); #P < 0.001 vs. NMS+WAS group, ▴P < 0.001 vs. NMS+low-dose PCPA+WAS group (Bonferroni's correction).
5-HT contents in the proximal and distal colons
The effects of NMS and/or WAS on the tissue and faecal content of 5-HT in the proximal and distal colon were further analysed. As shown in , 5-HT concentration in the proximal colon tissues was significantly higher than that of the distal colon tissues (P < 0.05, paired Student's t-test). The faecal content of 5-HT in the proximal colon was comparable to that in the distal colon in the different conditions ().
Figure 3. Effects of WAS and PCPA on tissue content of 5-HT in the proximal and distal colon of normal handled (NH, n = 6) and NMS (n = 8) rats. Data presented as mean ± S.E.M. 5-HT concentration in the proximal colon were significantly higher than those in the distal colon in each group (‡P < 0.01, t-test). In the proximal colon, *P < 0.01 vs. NH group (n = 6), #P < 0.01 vs. NH+WAS group (n = 5, t-tests); ▴P < 0.01 vs. NMS+WAS group (n = 5), †P < 0.01 vs. NMS+low-dose PCPA+WAS group (n = 4, Bonferroni's correction). In the distal colon, ▴P < 0.01 vs. NMS+WAS group (n = 5), †P < 0.01 vs. NMS+low-dose PCPA+WAS group (n = 4, Bonferroni's correction).
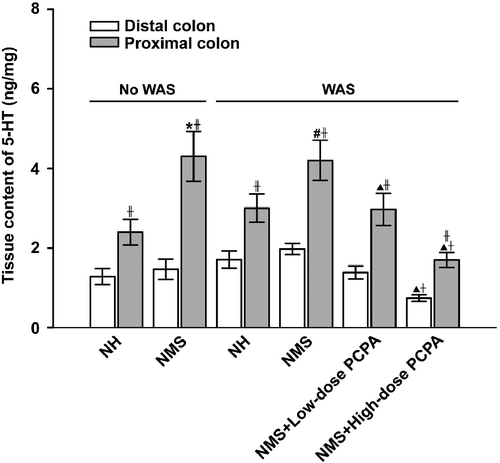
Figure 4. Effects of WAS and PCPA on faecal content of 5-HT in the proximal and distal colon of normal handled (NH, n = 6) and NMS (n = 8) rats. Data are presented as mean ± S.E.M. In the proximal and distal colon, *P < 0.01 vs. NH+WAS group (n = 5, t-tests); #P < 0.01 vs. NMS+WAS group (n = 5, ▴P < 0.01 vs. NMS+low-dose PCPA+WAS group (n = 4, Bonferroni's correction).
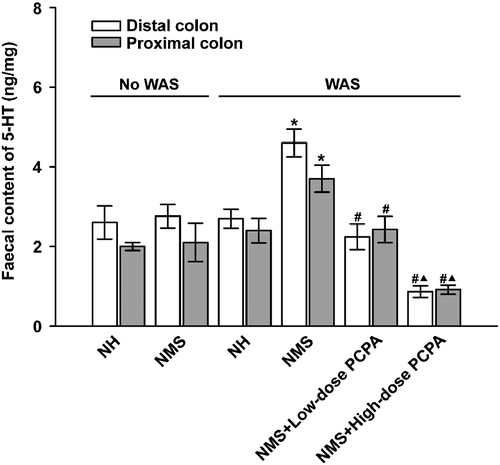
Two-way ANOVA showed that NMS treatment significantly increased the tissue content of 5-HT in the proximal (F1, 18: 16.53, P = 0.01) but not the distal colon, whereas the factors of WAS as well as the interaction of NMS and WAS demonstrate non-significant effects on the tissue 5-HT content. As shown in , the 5-HT content of proximal colon tissue was significantly increased in NMS rats when compared with that of the NH group (P = 0.006, Student's t-tests); by contrast, NMS had no significant effect on the faecal content of 5-HT either in the proximal or in the distal colon. After exposure to WAS, 5-HT concentration in the proximal colonic tissue of NMS rats was still significantly higher than that in NH rats (P = 0.008, Student's t-tests), but there was no significant difference between the NH and NH+WAS groups, or between the NMS and NMS+WAS groups. However, the faecal 5-HT content in the proximal and distal colon of NMS rats was significantly increased after WAS treatment when compared with that of the NMS group before WAS (F1, 18: 5.83, P = 0.025, in proximal colon, F1, 18: F = 5.94, P = 0.024, in distal colon), when compared with that of the NMS group before WAS (P = 0.009 in proximal, and P = 0.005 in distal colon, Student's t-tests).
In addition, 5-HT concentrations were found to be significantly reduced in rats treated with PCPA at the dose of 500 mg kg− 1 day− 1, with ∼59 and ∼57% reductions in the proximal and distal colon, respectively, when compared with NMS rats without PCPA administration (P < 0.001, One-way ANOVA followed by Bonferroni's correction, ). Such reductions caused by PCPA were much greater at the high dose of 500 mg kg− 1 day− 1 than that at the lower dose (150 mg kg− 1 day− 1). Similarly, in the high-dose PCPA group, the faecal content of 5-HT was significantly reduced by ∼74 and ∼79% in the proximal and distal colon, respectively, when compared with that of the NMS group with WAS (P < 0.01 for lower dose PCPA, P < 0.001 for high dose PCPA, One-way ANOVA followed by Bonferroni's correction).
EC cell density in colonic mucosa of NH and NMS rats
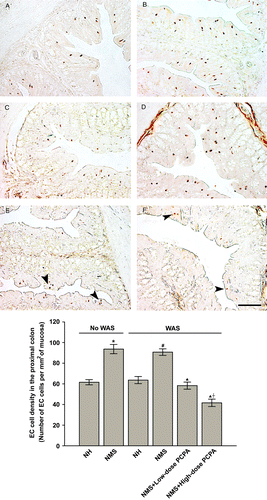
To further investigate the stress-induced change of 5-HT content in the colonic tissues, alterations in EC cell density in the proximal and distal colon were examined. There was a significant effect of NMS treatment on the EC cell density (F1, 22: 14.74, P < 0.001), whereas WAS as well as the interaction of NMS and WAS demonstrated non-significant effects. As shown in , the density of silver-stained EC cells in the NMS group was significantly larger than that in the NH group (p = 0.009, Student t-tests), and the NMS+WAS group also had greater EC cell density than that of the NH+WAS group (P = 0.009, Student's t-tests). There was no statistically significant difference in EC cell density between the NMS and NMS+WAS groups or between the NH and NH+WAS groups. The EC cell density in the distal colon showed no significant difference between the NH and NMS groups (data not shown). In the PCPA-treated NMS groups, EC cell density was significantly reduced when compared with that of the NMS+WAS group (P < 0.01, One-way ANOVA followed by Bonferroni's correction). Furthermore, the high dose of PCPA led to a greater reduction in EC cell density than did the lower dose (P < 0.01, One-way ANOVA followed by Bonferroni's correction).
Figure 5. Representative EC cell staining and statistical analysis of WAS and PCPA on EC cell density in transverse sections of the proximal colon of normal handled (A, NH, n = 6), NMS (B, NMS, n = 8), NH+WAS (C, n = 5), NMS+WAS (D, n = 5), NMS+low-dose PCPA+WAS (E, n = 4), NMS+high-dose PCPA+WAS (F, n = 5) groups. Data are presented as mean ± S.E.M. *P < 0.01 vs. NH group, #P < 0.01 vs. NH+WAS group (t-tests); ▴P < 0.01 vs. NMS+WAS group, †P < 0.01 vs. NMS+low-dose PCPA+WAS group (Bonferroni's correction). Arrow heads indicate EC cells. The scale bar is 100 μm.
Discussion
The purpose of this study was (i) to examine the combined effects of an early life stress and acute stress in adulthood on colonic visceral and motility responses to the acute stressor, and (ii) to elucidate the underlying mechanism. We first hypothesised that the combination of early life stressor and acute adulthood stressor exposure may better mimic the visceral hyperalgesia and motility disorder in IBS patients than does either early life stress or acute stress in adulthood alone. Our results showed that (i) NMS treatment significantly reduced pain threshold distension pressures, (ii) WAS significantly reduced the pain threshold pressure with immediate and delayed effects, (iii) WAS can increase the faecal pellet output during the stress period but was without delayed effects, and (iv) NMS treatment alone did not change the baseline of faecal pellet output, but NMS treatment plus WAS resulted in significant increase of faecal pellet output during the period of acute stress. It is necessary to point out that faecal pellet output is considered to reflect distal rather than proximal, or entire colonic motor function (Million et al. Citation2000). However, if the distal and proximal colonic motor function could be evaluated separately, the results could more precisely reflect the nature of NMS effect on gastrointestinal motility.
These data indicate (i) NMS can produce visceral hyperalgesia but not motility dysfunction in adults, (ii) WAS can produce both immediate and delayed effects on visceral nociception in NMS rats, (iii) WAS has immediate but not delayed effects on colonic motility, and (iv) NMS treatment did predispose the adults to react strongly to WAS, resulting in motility disorder. These data signify that, in rats, the combination of early life stress and acute adult stress mimic the benchmarks of IBS in humans. This model is more consistent with the clinical situation in which adults that have experienced stresses in childhood present with episodes of serious symptoms that are associated with acute stresses (Drossman et al. Citation1988).
How can early life stress couple with acute adulthood stress to affect the sensory and motor response in these rats? Based on the previous understanding about serotonin's important role in bowel sensation and motility (Gershon Citation1999; Crowell et al. Citation2004), we hypothesised that these effects are mediated through 5-HT pathways in the colon of rats. Previous work has reported the effect of an early life stress on colonic 5-HT with inconsistent results. One study showed that colonic 5-HT concentrations in adult rats exposed to neonatal colorectal distension were significantly higher than those in normal rats (Tian et al. Citation2006). In contrast, another study showed that colonic 5-HT content was comparable in NMS and NH rats (Ren et al. Citation2007). We suggest that this inconsistency may be due to (i) the different types of chronic stressors applied (NMS vs. neonatal colorectal distention) and (ii) the different regions of the colon (colonic mucosa vs. whole section) observed in the studies. The present study found that NMS alone significantly increased 5-HT content in the proximal but not the distal colonic tissue. Interestingly, the amount of 5-HT in the proximal colonic tissue was comparable in NMS and NMS-with-WAS groups, and this result may easily lead to a conclusion that WAS has no effect on 5-HT content in the gut. However, our study also showed that the faecal 5-HT content in NMS rats (both distal colon and proximal colon) was significantly higher than that of NMS rats without WAS. Thus, we can conclude that WAS did increase the total 5-HT concentration, although the tissue concentration did not significantly increase.
In order to further study the participation of serotonin pathways in stress-mediated colonic responses in NMS rats, we investigated the effect of 5-HT depletion by systemic application of PCPA on colonic sensory and motor responses in rats exposed to combined early life and adulthood stressors. In the present study, PCPA was applied at two dosages: 150 mg kg− 1 day− 1 for three consecutive days, which can induce nearly complete 5-HT depletion in the central nervous system (Fletcher et al. Citation2001; Slattery et al. Citation2005; Stean et al. Citation2005; Bercovici et al. Citation2006), which stores only ∼2% of the total 5-HT in the body, and 500 mg kg− 1 day− 1 for three consecutive days (Yamamoto et al. Citation1999; Zhu et al. Citation2001; Fukumoto et al. Citation2003). PCPA at 500 mg kg− 1 day− 1 dramatically reduced both tissue and faecal content of 5-HT in the colon, while the lower dose of 150 mg kg− 1 day− 1 had a much weaker effect. Likewise, EC cell density was dose-dependently reduced by PCPA treatment. Changes in the number of EC cells following the application of PCPA is sparsely reported in the literature (Grube et al. Citation1974; Bargsten and Grube Citation1992). Interestingly, in this study colonic EC cell number was found to be markedly decreased after PCPA treatment, which is morphological evidence in support of PCPA-induced colonic 5-HT depletion. Whether PCPA reduced EC cell markers or EC cell number is not clear. To answer this question, further research needs to be carried out.
We have found that consecutive administrations of PCPA vehicle alone to normal rats for three days can elevate, but not lower the levels of 5-HT and EC cell density in colonic tissues (data not shown). Thus, we confirm that PCPA produced all the suppressive effects on 5-HT content and EC cell density we observed. Moreover, as the dose of PCPA increased from 150 to 500 mg kg− 1 day− 1, the stress-enhanced colonic visceral sensitivity and motility decreased correspondingly. Thus, at least for these two dosage levels, the stress-mediated colonic sensory/motor responses were suppressed by PCPA in a dose-dependent manner, accompanied by equivalent degrees of 5-HT depletion and EC cell reduction. These data, together with the aforementioned findings that WAS-induced colonic sensory/motor responses in NMS rats coincided with increases in colonic tissue and luminal 5-HT and EC cell density, provide strong evidence that peripheral serotonin in the gut plays a key role in colonic sensitivity and motility disorders under combined early life and adult stress.
It should be pointed out that central 5-HT is also involved in the generation and modulation of visceral hyperalgesia in functional bowel disease. Previous study (Carstens et al. Citation1983) has shown that central 5-HT participates in descending inhibition of spinal nociceptive transmission. Additionally, depleting central 5-HT with PCPA has been shown to facilitate pain hypersensitivity (Telner et al. Citation1979; Bodnar et al. Citation1984). These data imply that central 5-HT has anti-nociceptive activity. In contrast, peripheral 5-HT in the gut, by activating its receptors located on primary afferent neurons in response to various stimuli, plays pivotal roles in the generation of visceral hyperalgesia (Gershon Citation1999; Crowell et al. Citation2004). It seems that, at least under certain circumstances, central and peripheral 5-HT play opposite roles in visceral pain processing. In the present study, when PCPA was administered at doses that could cause not only the nearly total depletion of central 5-HT as previously reported (Fletcher et al. Citation2001; Slattery et al. Citation2005; Stean et al. Citation2005; Bercovici et al. Citation2006) but also the significant depletion of peripheral 5-HT, colonic visceral hyperalgesia was strongly inhibited. This result clearly implicates the participation of peripheral serotonin, notwithstanding that the action of central 5-HT cannot be completely excluded.
It is known that serotonin reuptake transporter (SERT)-dependent 5-HT uptake plays an important role in 5-HT signalling pathways. The reduction in SERT expression can be coupled with an increase in EC cell number (Linden et al. Citation2003). In addition, our previous study has shown that NMS rats have higher SERT expression than NH rats (Bian et al. Citation2010). We suggest that higher SERT expression may contribute to NMS-induced visceral hyperalgesia and motility dysfunction.
Since 5-HT in the gut is mainly synthesised in EC cells, we further explored whether the quantity of EC cells was changed under WAS and/or NMS. The results show that NMS alone significantly increased EC cell density in the proximal colon, when compared with NH groups without WAS treatment. Thus, it is reasonable to conclude that NMS elevates the level of 5-HT in the colonic tissue by increasing the number of EC cells. Additionally, the equivalence in EC cell density between NMS and NMS-with-WAS groups indicates that 1-h WAS treatment produces no significant effect on the quantity of colonic EC cells.
The results from the present study also indicate that the proximal and the distal colon respond differently to stressors. Although previous study has demonstrated that the density of EC cells in the proximal colon is six- to seven-fold higher than that in the distal colon in rats (Oshima et al. Citation1999), the pathophysiological implications of this disparity have not been explored. The present study found that the tissue content of 5-HT in the proximal colon is significantly higher than that in the distal colon in each group, and this is accompanied by greater expression of EC cells in the proximal colon; this is consistent with at least one previous study (Oshima et al. Citation1999). Interestingly, the tissue content of 5-HT significantly increased in the proximal but not in the distal colon in NMS rats, suggesting that NMS has a stronger effect on the proximal colon. To explain this phenomenon, we note differences in innervation in the proximal and the distal colon. Anatomical evidence reveals that the distal colon is innervated by pelvic nerves, whereas the proximal colon is innervated by vagal nerve fibres (Altschuler et al. Citation1993), and that EC cells are in close association with vagal neurons in the colon (Leslie and Reynolds Citation1993). Moreover, in vivo electrophysiological studies indicate that corticotropin-releasing factor (CRF) released following acute stress is able to stimulate vagal efferents (Lenz et al. Citation1988) and that stimulating vagal nerves activates EC cells in the colon (Zinner et al. Citation1982). Based on these findings, it will be of great interest to explore in future work whether it is through vagal and CRF pathways that the combination of early life and adult stresses selectively influence the proximal colon.
In contrast to the differences in the tissue content of 5-HT between the proximal and distal colon, the faecal content of 5-HT in the proximal colon was similar to that in the distal colon both in NH and NMS rats. One possible explanation is that, in addition to 5-HT released from the distal colon, 5-HT released from the proximal segment can be transferred to the distal colon with faeces, resulting in comparable contents of 5-HT in the faeces in the two segments (Tsukamoto et al. Citation2007). In this study, NMS alone did not affect the faecal content of 5-HT in either the proximal or the distal colon; however, after exposure to acute stress, the faecal content of 5-HT was significantly increased. This suggests that WAS largely accounts for the high level of faecal 5-HT in rats subjected to NMS and WAS. Furthermore, WAS strongly increased faecal 5-HT in NMS rats with comparable potency in the proximal and distal colon. This result is particularly intriguing because the relatively constant concentration of 5-HT in the faeces throughout the colon of stressed NMS rats implies that it could be a non-invasive biomarker for stress-aggravated IBS.
In summary, our data demonstrate that the combined early life stress and an acute stressor in adulthood effectively induce visceral hyperalgesia and motility disorder; the proximal and distal colon has different responses towards these combined stressors, and the changes are mediated through serotonin pathways in the colon of rats.
Acknowledgements
The present work was supported by the Hong Kong Baptist University Research Grant No. (FRG/08-09//I-53).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Al-Chaer ED, Kawasaki M, Pasricha PJ. 2000. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 119:1276–1285.
- Altschuler SM, Escardo J, Lynn RB, Miselis RR. 1993. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 104:502–509.
- Barbosa AJ, Castro LP, Margarida A, Nogueira MF. 1984. A simple and economical modification of the Masson-Fontana method for staining melanin granules and enterochromaffin cells. Stain Technol. 59:193–196.
- Bargsten G, Grube D. 1992. Serotonin storage and chromogranins: An experimental study in rat gastric endocrine cells. J Histochem Cytochem. 40:1147–1155.
- Barreau F, Ferrier L, Fioramonti J, Bueno L. 2007. New insights in the etiology and pathophysiology of IBS: Contribution of neonatal stress models. Pediatr Res. 62:240–245.
- Bercovici E, Cortez MA, Wang X, Snead OC3rd. 2006. Serotonin depletion attenuates AY-9944-mediated atypical absence seizures. Epilepsia. 47:240–246.
- Bian ZX, Zhang M, Han QB, Xu HX, Sung JJ. 2010. Analgesic effects of JCM-16021 on neonatal maternal separation-induced visceral pain in rats. World J Gastroenterol. 16:837–845.
- Bodnar RJ, Kordower JH, Reches A, Wallace MM, Fahn S. 1984. Reductions in pain thresholds and morphine analgesia following intracerebroventricular parachlorophenylalanine. Pharmacol Biochem Behav. 21:79–84.
- Bonaz B, Taché Y. 1994. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of faecal output: Role of corticotropin-releasing factor. Brain Res. 641:21–28.
- Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. 2005. Repeated exposure to water avoidance stress in rats: A new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 289:G42–G53.
- Camilleri M. 2010. New receptor targets for medical therapy in irritable bowel syndrome. Aliment Pharmacol Ther. 31:35–46.
- Carstens E, MacKinnon JD, Guinan MJ. 1983. Serotonin involvement in descending inhibition of spinal nociceptive transmission produced by stimulation of medial diencephalon and basal forebrain. J Neurosci. 3:2112–2120.
- Chitkara DK, Van Tilburg MAL, Blois-Martin N, Whitehead WE. 2008. Early life risk factors that contribute to irritable bowel syndrome in adults: A systematic review. Am J Gastroenterol. 103:765–774.
- Chung EK, Zhang X, Li Z, Zhang H, Xu H, Bian Z. 2007. Neonatal maternal separation enhances central sensitivity to noxious colorectal distention in rat. Brain Res. 1153:68–77.
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. 2002. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 282:G307–G316.
- Crowell MD, Shetzline MA, Moses PL, Mawe GM, Talley NJ. 2004. Enterochromaffin cells and 5-HT signaling in the pathophysiology of disorders of gastrointestinal function. Curr Opin Investig Drugs. 5:55–60.
- Drossman DA, McKee DC, Sandler RS, Mitchell CM, Cramer EM, Lowman BC, Burger AL. 1988. Psychosocial factors in the irritable bowel syndrome. A multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology. 95:701–708.
- Fletcher PJ, Selhi ZF, Azampanah A, Sills TL. 2001. Reduced brain serotonin activity disrupts prepulse inhibition of the acoustic startle reflex: Effects of 5,7-dihydroxytryptamine and p-chlorophenylalanine. Neuropsychopharmacology. 24:399–409.
- Fujimiya M, Okumiya K, Kuwahara A. 1997. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol. 108:105–113.
- Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. 2003. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. 284:R1269–R1276.
- Gershon MD. 1999. Review article: Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 13:15–30.
- Glisić R, Koko V, Todorović V, Drndarević N, Cvijić G. 2006. Serotonin-producing enterochromaffin (EC) cells of gastrointestinal mucosa in dexamethasone-treated rats. Regul Pept. 136:30–39.
- Grube D, Aum¨ller G, Forssmann WG. 1974. EC-cells following inhibition of tryptophan-5-hydroxylase due to PCPA. Verh Anat Ges. 68:645–651.
- Horwitz BJ, Fisher RS. 2001. The irritable bowel syndrome. N Engl J Med. 344:1846–1850.
- Kim DY, Camilleri M. 2000. Serotonin: A mediator of the brain-gut connection. Am J Gastroenterol. 95:2698–2709.
- Lenz HJ, Raedler A, Greten H, Vale WW, Rivier JE. 1988. Stress-induced gastrointestinal secretory and motor responses in rats are mediated by endogenous corticotropin-releasing factor. Gastroenterology. 95:1510–1517.
- Leslie RA, Reynolds DJM. 1993. Neurotransmitters and receptors in the emetic pathway. In: Andrews PLR, Sanger GJ. editors. Emesis in anti-cancer therapy: Mechanisms and treatment. London: Chapman & Hall Medical. p 92–112.
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. 2003. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 285:G207–G216.
- Lowman BC, Drossman DA, Cramer EM, McKee DC. 1987. Recollection of childhood events in adults with irritable bowel syndrome. J Clin Gastroenterol. 9:324–330.
- Million M, Wang L, Martinez V, Tache Y. 2000. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res. 877:345–353.
- Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. 2007. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 292:G1037–G1044.
- Oshima S, Fujimura M, Fukimiya M. 1999. Changes in number of serotonin containing cells and serotonin levels in the intestinal mucosa of rats with colitis induced by dextral sodium sulfate. Histochem Cell Biol. 112:257–263.
- Qi SD, Tian SL, Xu HX, Joseph JYS, Bian ZX. 2009. Quantification of luminally released serotonin in rat proximal colon by capillary electrophoresis with laser-induced fluorescence detection. Anal Bioanal Chem. 393:2059–2066.
- Ren TH, Wu J, Yew D, Ziea E, Lao L, Leung WK, Berman B, Hu PJ, Sung JJY. 2007. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 292:G849–G856.
- Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Taché Y, Plotsky PM, Mayer EA. 2005. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long Evans rats. Am J Physiol Gastrointest Liver Physiol. 289:G704–G712.
- Slattery DA, Desrayaud S, Cryan JF. 2005. GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J Pharmacol Exp Ther. 312:290–296.
- Stean TO, Atkins AR, Heidbreder CA, Quinn LP, Trail BK, Upton N. 2005. Postsynaptic 5-HT1B receptors modulate electroshock-induced generalised seizures in rats. Br J Pharmacol. 144:628–635.
- Taché Y, Martinez V, Wang L, Million M. 2004. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: Implications for irritable bowel syndrome. Br J Pharmacol. 141:1321–1330.
- Telner J, Lepore F, Guillemot JP. 1979. Effects of serotonin content on pain sensitivity in the rat. Pharmacol Biochem Behav. 10:657–661.
- Tian XY, Bian ZX, Hu XG, Zhang XJ, Liu L, Zhang HQ. 2006. Electro-acupuncture attenuates stress-induced defecation in rats with chronic visceral hypersensitivity via serotonergic pathway. Brain Res. 1088:101–108.
- Tsukamoto K, Ariga H, Mantyh C, Pappas TN, Yanagi H, Yamamura T, Takahashi T. 2007. Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats. Am J Physiol Regul Integr Comp Physiol. 293:R64–R69.
- Wheatcroft J, Wakelin D, Smith A, Mahoney CR, Mawe G, Spiller R. 2005. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 17:863–870.
- Yamamoto I, Kuwahara A, Fujimura M, Kadowaki M, Fujimiya M. 1999. Involvement of 5-HT3 and 5-HT4 receptors in the motor activity of isolated vascularly perfused rat duodenum. Neurogastroenterol Motil. 11:457–465.
- Zhu JX, Zhu XY, Owyang C, Li Y. 2001. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 530:431–442.
- Zinner MJ, Jaffe BM, DeMagistris L, Dahlstrom A, Ahlman H. 1982. Effect of cervical and thoracic vagal stimulation on luminal serotonin release and regional blood flow in cats. Gastroenterology. 82:1403–1408.
