Abstract
Interleukin-18 (IL-18) has recently been considered a promising marker of stress responses. In this study, to evaluate IL-18 as a noninvasive stress marker in pigs, we investigated the expression of IL-18 in porcine salivary glands and its presence in saliva, and its dynamics during acute immobilization stress in pigs. IL-18 mRNA was detected robustly in the pig salivary glands by RT-PCR. Immunohistochemical staining of IL-18 protein expression revealed that the expression patterns differed among the three types of salivary glands (parotid, submandibular, and sublingual gland). IL-18 was also detected in pig saliva by ELISA, and a diurnal rhythm with a peak in the afternoon was observed. The IL-18 concentration in saliva was significantly increased during a 60-min acute immobilization stress in thirteen 5-month-old pigs. These results are the first evidence of a stress-related change of IL-18 in pig saliva. Salivary IL-18 may thus become a useful noninvasive marker for the evaluation of acute stress in pigs.
Introduction
There has been increasing interest in animal welfare with regard to the handling, housing, and feeding of domestic animals, especially in Europe, and the spread of intensive agriculture and changes in environmental factors may be enhancing the physiological stress of farmed animals (Caporale et al. Citation2005). Such stress negatively affects immune responses and resistance to diseases (Spencer and Howell Citation1989; Calcagni and Elenkov Citation2006; Mitchell et al. Citation2008), quality of meat (Southern et al. Citation2006), and reproduction (Einarsson et al. Citation2008) in a variety of animals. Recently, animal welfare guidelines for the handling, housing, and feeding of pigs, laying chickens, broiler chickens, and dairy cattle have been introduced in Japan (http://jlta.lin.gr.jp/chikusan/aw/aw.html).
Most of the past research on stress markers in pigs has been carried out using blood samples, such as the measurement of levels of serum cortisol, acute phase proteins, or cytokines (Becker et al. Citation1985; Carstensen et al. Citation2005; Breinekova et al. Citation2007), and leukocyte populations and functions such as natural killer cytotoxicity (Niekamp et al. Citation2007). However, noninvasive methods and biomarkers to evaluate stress in animals should be established to eliminate unnecessary stress, improve animal welfare, and increase productivity. Saliva sampling has the advantage of being noninvasive, easy to perform, and stress-free in comparison with blood sampling. Therefore, saliva is considered to be a good material for evaluating the stress condition, and salivary biomarkers to evaluate stress have received a great deal of attention in humans (Yamaguchi Citation2007). Salivary cortisol level has been reported as a stress marker in pigs (Parrott and Misson Citation1989), although the same group also reported that salivary cortisol was a less sensitive marker than plasma cortisol (Parrott et al. Citation1989). Additionally, we recently showed that salivary IgA is a potentially better noninvasive marker for acute stress in pigs compared with cortisol (Muneta et al. Citation2010).
Interleukin-18 (IL-18) was originally reported as an interferon-γ (IFN-γ)-inducing factor because of its ability to induce IFN-γ production by Th1 cells (Okamura et al. Citation1995). However, IL-18 is also induced in the adrenal cortex by adrenocorticotropic hormone treatment (Conti et al. Citation1997, Citation2000; Sugama et al. Citation2000). It has been proposed that IL-18 is a possible mediator cytokine that translates stress responses (Sekiyama et al. Citation2005a). For example, plasma IL-18 level is up-regulated by immobilization stress in mice, and this increase of IL-18 in plasma is derived from the adrenal gland (Sekiyama et al. Citation2005b, Citation2006; Sugama et al. Citation2006). Moreover, IL-18 is involved in the formation of stress-induced gastric lesions (Seino et al. Citation2007) and salivary IL-18 is increased in the subjects with Sjogren's syndrome (Bombardieri et al. Citation2004). Thus, IL-18 plays important roles not only in Th1-dominated immunological reactions and inflammatory responses, but also in stress physiology (Sugama and Conti Citation2008; Alboni et al. Citation2010). However, few studies have been conducted to investigate the expression of IL-18 in stress responses in domestic animal species, except for recent papers in chickens (Shini and Kaiser Citation2009; Shini et al. Citation2010). In light of all these results, we hypothesized that IL-18 may also be involved in pig stress responses, and investigated the expression of IL-18 in pig salivary glands and saliva, and the dynamics of IL-18 during acute immobilization stress, in order to determine whether IL-18 might be utilized as a new stress marker in pigs.
Materials and methods
Animals
Four LW (Landrace breed × Large White breed) F1 male piglets (6–8 weeks olds) were used to observe the diurnal rhythm of IL-18 level in saliva. The sunrise and sunset times of the experimental days were approximately 04:00 and 19:00 h, respectively. The piglets were kept in an animal room (4.8 × 3.7 × 2.4 m) with natural light from a glass window, which was kept closed to reduce disturbance by noise and contamination by dust. They were given normal feed for growing pigs at 08:00 and 18:00 h with a free water supply. The salivary glands (submandibular glands) for the RT-PCR were taken from 12 healthy LWD (LW × Duroc breed) pigs (1-month-old, male and female) and the salivary glands for immunohistochemical staining of IL-18 were taken from four healthy LWD pigs (5-month-old, male and female) at the National Institute of Animal Health. Thirteen other 5-month-old LWD pigs (male and female) were subjected to an immobilization stress experiment at the Chiba Prefectural Livestock Research Center. All animal experiments and procedures were approved by the committees for animal ethics and experiments of Obihiro University of Agriculture and Veterinary Medicine (No. 18–59), the National Institute of Animal Health (No. 08–086) and Chiba Prefectural Livestock Research Center, respectively.
IL-18 expression in pig salivary glands by RT-PCR
Pig salivary glands were collected from twelve 1-month-old healthy LWD pigs (submandibular glands). Total RNA was isolated from the salivary glands using TRIzol® reagents (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. First-strand cDNAs were prepared from 1 μg of total RNA using a Takara RNA PCR kit (Takara Shuzo Co. Ltd., Osaka, Japan). The RT-PCR for IL-18 and β-actin was performed as described previously (Dozois et al. Citation1997; Muneta et al. Citation1999). The primers, PCR conditions, and size of the PCR products are shown in . The primers for IL-18 were specifically designed to amplify IL-18 mRNA; the forward primer (pPoIL-18-D3) was designed over two exons (exon 2 and exon 3).
Table I. Primers and PCR conditions for the RT-PCR of Porcine IL-18 and β-actin.
IL-18 expression in pig salivary glands by immunohistochemistry
The salivary glands (parotid gland, submandibular gland, and sublingual gland, respectively) were fixed in Zamboni fixative (Kyodo Byori, Inc., Kobe, Japan) overnight at 4°C and embedded in paraffin as described previously (Nagai et al. Citation2006). Paraffin sections were immunostained as described previously (Muneta et al. Citation2000) using an anti-pig IL-18 monoclonal antibody (clone 2-C-4, 5 μg/ml) with Histofine simple stain MAX-PO (M) solution (Nichirei Corp., Tokyo, Japan). Purified mouse IgG2b (MBL, Nagoya, Japan) was used as an isotype control.
IL-18 concentration in pig saliva
To determine the basal level and diurnal rhythm of IL-18 in saliva, we used saliva from healthy piglets taken at 09:00, 11:00, 13:00, 15:00, and 17:00 h (Muneta et al. Citation2010). The saliva was collected as described previously using medical absorbent cotton (Muneta et al. Citation2010). The IL-18 concentration in each saliva sample was evaluated by a sandwich ELISA as described previously (Muneta et al. Citation2000). An anti-pig IL-18 monoclonal antibody (clone 11-H-5) was used as a capture antibody, and a biotinylated anti-pig IL-18 monoclonal antibody (clone 5-C-5) was used as a detection antibody. The intra-assay coefficient of variation and inter-assay coefficient of variation of this ELISA were approximately 6.8 and 8.1%, respectively.
Changes in IL-18 levels during the immobilization stress
Thirteen LWD pigs were subjected to acute immobilization stress. The immobilization of the first pig was started at 10:00 h and the immobilization of the last pig ended at 16:40 h. Each pig was immobilized for 60 min within a steel cage (width 110 cm, height 120 cm, and length 180 cm), which prevented them from moving due to the pressure of the steel bars, as shown in . Saliva was collected as described previously (Muneta et al. Citation2010) using a Salivette® cotton swab (Sarstedt AG & Co., Nümbrecht, Germany). Before starting the immobilization, prestress (0 min) saliva samples were collected. The pigs were then immobilized for 60 min, and saliva was collected at 15, 30, and 60 min after the immobilization stress began. Control saliva was collected from another five LWD pigs with the same time intervals without the immobilization stress. Saliva was collected by centrifugation of the cotton swabs at 2500g for 15 min, and the collected saliva was kept at − 20°C until analysis. The IL-18 concentration in each saliva sample was evaluated by a sandwich ELISA as described previously (Muneta et al. Citation2000).
Relationship with other stress markers
We also measured the IgA concentration in the same saliva samples, as we recently reported that IgA is a salivary marker of stress induced by acute restraint (Muneta et al. Citation2010). The salivary IgA concentration was determined by ELISA using a PIG IgA ELISA quantitation kit (sensitivity: 15.625 ng/ml; Bethyl Laboratories, Inc., Montgomery, TX, USA). The intra-assay and inter-assay coefficients of variation of this ELISA were approximately 3.4 and 4.6%, respectively. The cortisol concentration in the same saliva samples was also measured by a competitive ELISA using a goat anti-mouse IgG-Fc (Bethyl Laboratories, Inc.) as a primary capture antibody and a mouse anticortisol monoclonal antibody (Millipore Corporation, Billerica, MA, USA) as a secondary capture antibody, and cortisol (Sigma Chemical Co., St. Louis, MO, USA) and HRP-labeled cortisol (Cosmo Bio Co. Ltd., Tokyo, Japan) as a standard and competitor, respectively. The intra-assay and inter-assay coefficients of variation of this EIA were approximately 4.9 and 6.5%, respectively.
Data and statistical analysis
The levels of IL-18, IgA, and cortisol are presented as the actual concentrations () and also as fold changes from the basal level (), which were calculated by taking the preimmobilization stress concentration as basal. Results are shown as the mean of each time point with the SE. The differences were analyzed using a repeated measures ANOVA test, and if a significant difference was seen, a Bonferroni test was used to determine the difference between each time point using the ystat 2000 program. Values of p < 0.05 were considered to indicate statistical significance.
Results
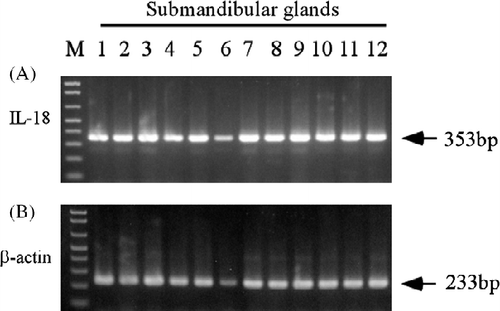
IL-18 expression in pig salivary glands by RT-PCR
As shown in , the expression of mRNA for porcine IL-18 was consistently detected with a band of 353 base pairs (bp) in the submandibular glands of all pigs examined.
Figure 2. IL-18 expression in pig salivary glands (n = 12 submandibular glands) by RT-PCR. Total RNA was isolated from the salivary glands, and the RT-PCR analyses for porcine IL-18 and β-actin were performed as described in the Materials and methods. Each PCR product size is shown at the right. M: 100 base ladder marker.
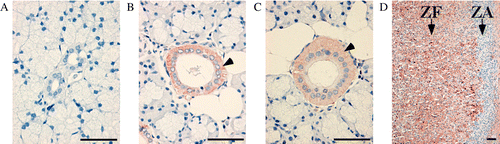
IL-18 expression in pig salivary glands by immunohistochemistry
IL-18 expression was also detected in the cytoplasm of the epithelial duct cells of the salivary gland by immunohistochemistry, as shown in . Interestingly, IL-18 immunostaining was observed in the submandibular gland and sublingual gland ducts, but no staining was observed in the parotid gland ducts (, respectively). IL-18 immunostaining was also observed in the adrenal gland () as a positive control.
Figure 3. IL-18 expression in pig salivary glands by immunohistochemistry. Results are shown for (A) parotid gland, (B) submandibular gland, and (C) sublingual glands. (D) Adrenal glands were used as a positive control. Arrows indicate zona arcuata and zona fasciculate of adrenal cortex. Arrowheads indicate the ducts of salivary glands with IL-18-positive staining. Each picture is representative of a similar pattern of staining from four different pigs. Bar = 50 μm.
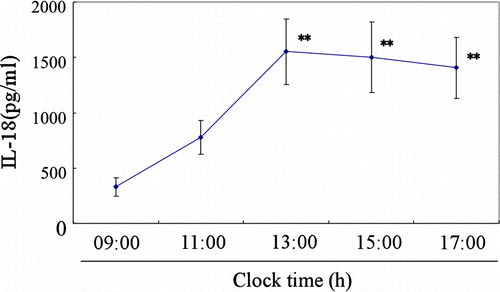
IL-18 concentrations in pig saliva
IL-18 was also detected in the saliva by ELISA, as shown in . The salivary IL-18 concentrations showed changes throughout the day (ANOVA; F = 6.265, degrees of freedom = 4, p < 0.0001). IL-18 concentration was lowest in the morning (09:00 h), increased during the day, significantly from 13:00 to 17:00 h, compared with the value at 09:00 h (Bonferroni test, p < 0.01).
Changes in IL-18 concentrations with the immobilization stress
illustrates the changes in IL-18 concentrations during the acute immobilization stress. Salivary IL-18 concentrations were significantly increased (ANOVA; F = 3.874, degrees of freedom = 3, p = 0.033) after 15 and 30 min of immobilization stress (Bonferroni test, p < 0.05) and remained increased at 60 min (Bonferroni test, p < 0.01) compared with the prestress levels. The change between the basal level of IL-18 and the level after 60 min of immobilization was about 20-fold ().
Figure 5. Changes in salivary IL-18 and IgA during immobilization stress in pigs. (A-1) The concentrations of IL-18, (B-1) IgA, and (C-1) Cortisol are shown. The fold changes from the basal values are also shown for (A-2) IL-18, (B-2) IgA, and (C-2) Cortisol. Data are the mean ± SE values from 13 different pigs. Lines and filled circles show the changes in the control samples. Asterisks indicate significant differences compared with the prestress values (Bonferroni test, *p < 0.05, **p < 0.01).
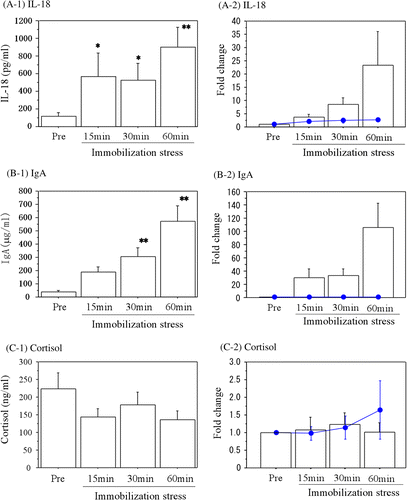
Relationship with other stress markers in saliva
We also measured IgA as a conventional stress marker in saliva during the immobilization stress. As shown in , the saliva IgA concentrations were also significantly increased (ANOVA; F = 13.232, degrees of freedom = 3, p < 0.0001) after 30 and 60 min of the acute immobilization stress (Bonferroni test, p < 0.01) compared with the prestress samples. The increase between the basal IgA concentration and the level after 60 min of immobilization was more than 100-fold (). Salivary cortisol concentrations did not change with the immobilization stress in this study (ANOVA; F = 1.599, degrees of freedom = 3, p = 0.413), as shown in .
Discussion
In this study, we examined the expression of IL-18 in porcine salivary glands and its relationship with immobilization as a promising noninvasive stress marker in pigs. Immobilization stress sometimes occurs in pig production: For example, when blood samples are taken from pigs for diagnosis or treatment, the pigs are immobilized using a pig keeper or other device. However, the saliva collection carried out in this study was easy, noninvasive, and relatively stress free, as described previously (Muneta et al. Citation2010). Moreover, unlike with blood sampling, sampling by this method is performed without special equipment or technique. Therefore, evaluation of stress using saliva samples is straightforward with respect to animal welfare issues.
IL-18 expression in the porcine salivary glands was observed at the mRNA and protein levels by RT-PCR and immunohistochemical staining, respectively. IL-18 mRNA was robustly detected in pig salivary glands (submandibular glands) by RT-PCR (). Interestingly, IL-18 showed different immunostaining patterns among the three different types of salivary glands (parotid, submandibular, and sublingual glands), as shown in . We recently reported a similar pattern of immunostaining, i.e. variation in staining patterns among the three types of salivary glands for IgA in the plasma cells of porcine salivary glands (Muneta et al. Citation2010), but the IL-18 expression pattern was more restricted to the submandibular and sublingual glands than was IgA. IL-18 is expressed as a 24-kDa precursor form and converted to an 18-kDa mature form by caspase-1 (Ghayur et al. Citation1997). Proteinase-3 is also important to induce bioactive IL-18 in the human oral epithelium (Sugawara et al. Citation2001). IL-18-binding protein (IL-18BP) is also involved in the negative regulation of IL-18 (Novick et al. Citation1999). As we did not investigate these molecules in this study, further studies must be undertaken to determine whether stressful experiences in pigs increase the level of the active form of IL-18 rather than the precursor form, and to measure the level of IL-18BP in pig salivary glands and saliva to evaluate whether a direct inhibitin of the IL-18-mediated effects by IL-18BP is triggered by stress. These studies should explain why IL-18 is differently expressed among the different types of salivary glands.
The secretion of water and components of saliva are controlled by the parasympathetic and sympathetic nervous systems (PNS and SNS). The parotid gland secretes mainly fluid and electrolyte components of saliva and is regulated by PNS impulses (Garrett Citation1987). The submandibular and sublingual glands are mixed glands, and secretion of the protein component of saliva is enhanced by SNS impulses (Sabbadini and Berczi Citation1995). The involvement of IL-18 in stress-induced microglial activation has been reported in the rat brain (Sugama et al. Citation2007). Moreover, IL-18 expression was increased by restraint stress in the neurons of the medial habenula (Sugama et al. Citation2002). As the habenula is a potential site for the interaction between neuro-endocrine and immune functions, these results suggest that IL-18 might be involved in interactions between the central nervous system and the periphery during stress (Alboni et al. Citation2010). Although the relationship between IL-18 in the brain and in saliva is unknown, it is proposed that IL-18 secretion by the submandibular and sublingual glands in response to stress may be a result of stimulation by the SNS activation.
A diurnal rhythm of IL-18 concentration in saliva was observed. Circadian oscillations of TNF-α, IL-6 and cortisol in serum and saliva have been reported in the subjects with advanced neoplasm or post-traumatic stress disorder in children (Baranowski et al. Citation1999; Pervanidou et al. Citation2007). We recently observed a similar diurnal rhythm of salivary IgA concentration in pigs (Muneta et al. Citation2010). This diurnal variation in salivary IL-18 concentrations should be taken into account in future studies using IL-18 as a marker for stress evaluation in pigs.
The salivary IL-18 levels were significantly increased by immobilization. IgA level was also significantly increased after the immobilization, as previously reported (Muneta et al. Citation2010). Moreover, the increase in IL-18 level after the immobilization stress was more rapid than the increase in IgA, although the fold change in IgA level from basal was greater than that of IL-18 in this study. As IL-18 has been reported to have mucosal adjuvant activity to increase the IgA response in saliva (Bradney et al. Citation2002), these results indicate that IL-18 in saliva, produced in response to an SNS stimulus, plays some role in enhancing IgA production in saliva to increase host immunity during acute stress. However, further studies must be carried out to reveal the relationship between IL-18 expression and IgA in pig saliva during stress responses. Salivary cortisol concentrations were not increased during immobilization in this study. This difference may be attributable to the fact that cortisol is secreted by the adrenal cortex as a result of activation of the hypothalamo–pituitary–adrenocortical axis, while IL-18 and IgA in saliva are produced from the salivary glands and may be released as a result of SNS activation during acute immobilization. Moreover, previous reports have indicated that salivary cortisol is a less sensitive marker than plasma cortisol (Parrott et al. Citation1989), and is highly variable and responsive to a wide range of factors (Weibel Citation2003; Hanrahan et al. Citation2006). We conclude that salivary IL-18 and IgA may be more sensitive noninvasive stress markers than cortisol in pigs.
In conclusion, the data described in this study provide the first evidence that salivary IL-18 concentration is related to acute stress in pigs. These results indicate that salivary IL-18 may be used as a noninvasive acute stress marker in the assessment of the welfare status of pigs. We are now investigating the salivary IL-18 levels in pigs during exposure to other stressors.
Acknowledgements
We would like to thank Mr Masaru Kobayashi for his excellent technical assistance with the immunohistochemistry. We would also like to thank Dr Ituro Yamane for his help with the statistical analysis. This work was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan.
Declaration of interest: The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alboni S, Cervia D, Sugama S, Conti B. 2010. Interleukin-18 in the CNS. J Neuroinflammation. 7:9.
- Baranowski M, Muc-Wierzgon M, Madej K, Wierzgon J, Zubelewicz B. 1999. The estimation of endogenous tumor necrosis factor alpha and cortisol levels in serum in advanced neoplasm. J Exp Clin Cancer Res. 18:241–245.
- Becker BA, Nienaber JA, Christenson RK, Manak RC, DeShazer JA, Hahn GL. 1985. Peripheral concentrations of cortisol as an indicator of stress in the pig. Am J Vet Res. 46:1034–1038.
- Bombardieri M, Barone F, Pittoni V, Alessandri C, Conigliaro P, Blades MC, Priori R, McInnes IB, Valesini G, Pitzalis C. 2004. Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjogren's syndrome: Relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate. Arthritis Res Ther. 6:447–456.
- Bradney CP, Sempowski GD, Liao HX, Haynes BF, Staats HF. 2002. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J Virol. 76:517–524.
- Breinekova K, Svobora M, Smutna M, Volrova L. 2007. Markers of acute stress in pigs. Physiol Res. 56:323–329.
- Calcagni E, Elenkov I. 2006. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. 1069:62–76.
- Caporale V, Alessandrini B, Dalla Villa P, Del Papa S. 2005. Global perspectives on animal welfare: Europe. Rev Sci Tech. 24:567–577.
- Carstensen L, Rontved CM, Nielsen JP. 2005. Determination of tumor necrosis factor-α responsiveness in piglets around weaning using an ex vivo whole blood stimulation assay. Vet Immunol Immunopathol. 105:59–66.
- Conti B, Jahng JW, Tinti C, Son JH, Joh TH. 1997. Induction of interferon-gamma inducing factor in the adrenal cortex. J Biol Chem. 272:2035–2037.
- Conti B, Sugama S, Kim Y, Tinti C, Kim H, Baker H, Volpe B, Attardi B, Joh T. 2000. Modulation of IL-18 production in the adrenal cortex following acute ACTH or chronic corticosterone treatment. Neuroimmunomodulation. 8:1–7.
- Dozois CM, Oswald E, Gautier N, Serthelon JP, Fairbrother JM, Oswald IP. 1997. A reverse transcription-polymerase chain reaction method to analyze porcine cytokine gene expression. Vet Immunol Immunopathol. 58:287–300.
- Einarsson S, Brandt Y, Lundeheim N, Madej A. 2008. Stress and its influence on reproduction in pigs: A review. Acta Vet Scand. 50:48.
- Garrett JR. 1987. The proper role of nerves in salivary secretion: A review. J Dent Res. 66:387–397.
- Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 386:619–623.
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. 2006. Strategies for salivary cortisol collection and analysis in research with children. Appl Nurs Res. 19:95–101.
- Mitchell GB, Clark ME, Siwicky M, Caswell JL. 2008. Stress alters the cellular and proteomic compartments of bovine bronchoalveolar lavage fluid. Vet Immunol Immunopathol. 125:111–125.
- Muneta Y, Shimoji Y, Yokomizo Y, Mori Y. 1999. Molecular cloning of porcine interleukin-1β converting enzyme and differential gene expression of IL-1β converting enzyme, IL-1β, and IL-18 in porcine alveolar macrophages. J Interferon Cytokine Res. 19:1289–1296.
- Muneta Y, Mikami O, Shimoji Y, Nakajima Y, Yokomizo Y, Mori Y. 2000. Detection of porcine interleukin-18 by sandwich-ELISA and immunohistochemical staining using its monoclonal antibodies. J Interferon Cytokine Res. 20:331–336.
- Muneta Y, Yoshikawa T, Minagawa Y, Shibahara T, Maeda R, Omata Y. 2010. Salivary IgA as a useful non-invasive marker for restraint stress in pigs. J Vet Med Sci. 72:1295–1300.
- Nagai Y, Watanabe K, Aso H, Ohwada S, Muneta Y, Yamaguchi T. 2006. Cellular localization of IL-18 and IL-18 receptor in pig anterior pituitary gland. Domest Anim Endocrinol. 30:144–154.
- Niekamp SR, Sutherland MA, Dahl GE, Salak-Johnson JL. 2007. Immune responses of piglets to weaning stress: Impact of photoperiod. J Anim Sci. 85:93–100.
- Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. 1999. Interleukin-18 binding protein: A novel modulator of the Th1 cytokine response. Immunity. 10:127–136.
- Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. 1995. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 378:88–91.
- Parrott RF, Misson BH. 1989. Changes in pig salivary cortisol in response to transport stimulation, food and water deprivation, and mixing. Br Vet J. 145:501–505.
- Parrott RF, Misson BH, Baldwin BA. 1989. Salivary cortisol in pigs following adrenocorticotrophic hormone stimulation: Comparison with plasma levels. Br Vet J. 145:362–366.
- Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP. 2007. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 32:991–999.
- Sabbadini E, Berczi I. 1995. The submandibular gland: A key organ in the neuro-immuno-regulatory network?. Neuroimmunomodulation. 2:184–202.
- Seino H, Ueda H, Kokai M, Tsuji NM, Kashiwamura S, Morita Y, Okamura H. 2007. IL-18 mediates the formation of stress-induced, histamine-dependent gastric lesions. Am J Physiol Gastrointest Liver Physiol. 292:G262–G267.
- Sekiyama A, Ueda H, Kashiwamura S, Nishida K, Kawai K, Teshima-Kondo S, Rokutan K, Okamura H. IL-18; a cytokine translates a stress into medical science. J Med Invest. 2005a; 52:236–239.
- Sekiyama A, Ueda H, Kashiwamura S, Sekiyama R, Takeda M, Rokutan K, Okamura H. A stress-induced, superoxide-mediated caspase-1 activation pathway causes plasma IL-18 upregulation. Immunity. 2005b; 22:669–677.
- Sekiyama A, Ueda H, Kashiwamura S, Nishida K, Yamaguchi S, Sasaki H, Kuwano Y, Kawai K, Teshima-Kondo S, Rokutan K, Okamura H. 2006. A role of the adrenal gland in stress-induced up-regulation of cytokines in plasma. J Neuroimmunol. 171:38–44.
- Shini S, Kaiser P. 2009. Effect of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress. 12:388–399.
- Shini S, Shini A, Kaiser P. 2010. Cytokine and chemokine gene expression profiles in heterophils from chickens treated with corticosterone. Stress. 13:185–194.
- Spencer BT, Howell PG. 1989. Some husbandry factors influencing weaning stresses in piglets. J S Afr Vet Assoc. 60:62–64.
- Southern KJ, Rasekh JG, Hemphil FE, Thaler AM. 2006. Conditions of transfer and quality of food. Rev Sci Tech. 25:675–684.
- Sugama S, Conti B. 2008. Interleukin-18 and stress. Brain Res Rev. 58:85–95.
- Sugama S, Kim Y, Baker H, Tinti C, Kim H, Joh TH, Conti B. 2000. Tissue-specific expression of rat IL-18 gene and response to adrenocorticotropic hormone treatment. J Immunol. 165:6287–6292.
- Sugama S, Cho BP, Baker H, Joh TH, Lucero J, Conti B. 2002. Neurons of the superior nucleus of the medial habenula and ependymal cells express IL-18 in rat CNS. Brain Res. 958:1–9.
- Sugama S, Wang N, Shimokawa N, Koibuchi N, Fujita M, Hashimoto M, Dhabhar FS, Conti B. 2006. The adrenal gland is a source of stress-induced circulating IL-18. J Neuroimmunol. 172:59–65.
- Sugama S, Fujita M, Hashimoto M, Conti B. 2007. Stress-induced morphological microglial activation in the rodent brain: Involvement of interleukin-18. Neuroscience. 146:1388–1399.
- Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, Hanzawa K, Kumagai K, Okamura H, Takeda H. 2001. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 167:6568–6575.
- Weibel L. 2003. Methodological guidlines for the use of salivary cortisol as biological marker of stress. Presse Med. 32:845–851.
- Yamaguchi M. 2007. Stress evaluation using a biomarker in saliva. Nippon Yakurigaku Zasshi. 129:80–84 (in Japanese).