Abstract
Meditation may show differential effects on stress and plasma catecholamines based on genetic polymorphisms in brain-derived neurotrophic factor (BDNF) and catechol O-methyl transferase (COMT). Eighty adults (40 men, 40 women; mean age 26 years) who practiced meditation regularly and 57 healthy control adults (35 men, 22 women; mean age 26 years) participated. Plasma catecholamines (norepinephrine (NE), epinephrine (E), and dopamine (DA)) concentrations were measured, and a modified form of the Stress Response Inventory was administered. The results were analyzed using two-way analysis of covariance (ANCOVA) with control and meditation subjects, gene polymorphism as factors, and meditation duration as the covariate. Two-way ANCOVA showed a significant interaction between control and meditation subjects, and BDNF Val66Met polymorphism on DA/NE+DA/E (p = 0.042) and NE/E+NE/DA (p = 0.046) ratios. A significant interaction was found for control and meditation subjects with COMT Val158Met polymorphism and plasma NE concentrations (p = 0.009). Post hoc ANCOVA in the meditation group, adjusted for meditation duration, showed significantly higher plasma NE concentrations for COMT Met carriers than COMT Val/Val subjects (p = 0.025). Significant differences of stress levels were found between the control and meditation subjects in BDNF Val/Met (p < 0.001) and BDNF Met/Met (p = 0.003), whereas stress levels in the BDNF Val/Val genotype did not differ between the control and meditation groups. This is the first evidence that meditation produces different effects on plasma catecholamines according to BDNF or COMT polymorphisms.
Introduction
Meditation practices have various health benefits for stress reduction (Miller et al. Citation1995; Lee et al. Citation2007), cognitive functions (Newberg et al. Citation2010), and mental disorders (Jain et al. Citation2007). Stress is considered as a condition in which an environmental demand exceeds the natural regulatory capacity of an organism, in particular situations that include unpredictability and uncontrollability (Koolhaas et al. Citation2011). Meditation decreases levels of the stress-related hormone cortisol (Jones Citation2001), and groups of subjects that meditate have significantly lower plasma norepinephrine (NE) and epinephrine (E) levels than control subjects (Infante et al. Citation2001). In addition, the meditation experience is associated with increased prefrontal cortical thickness (Lazar et al. Citation2005) and increased gray matter density in the brainstem (Vestergaard-Poulsen et al. Citation2009), which suggests potential effects of meditation on brain plasticity.
The effects of mental training on brain plasticity are evident throughout life (Slagter et al. Citation2007). Evidently, enhancement of brain health and plasticity by behavioral intervention involves increases in brain-derived neurotrophic factor (BDNF) and other growth factors that stimulate neurogenesis and increase resistance to brain insult and improved learning and mental performance (Cotman and Berchtold Citation2002), hence supporting potential roles of mind–body training in brain plasticity. Catechol O-methyl transferase (COMT) activity is especially important in the prefrontal cortex, and the valine (Val158Met (rs4680)) polymorphism is related to higher COMT activity in the prefrontal cortex, which leads to lower dopamine (DA) release (Chen et al. Citation2004). COMT is involved in the inactivation of the catecholamine neurotransmitters DA, epinephrine, and NE, and inhibition of COMT in the rat prefrontal cortex potentiates an increase in DA, but not NE levels (Tunbridge et al. Citation2004). In addition to the association of COMT with DA (Tunbridge et al. Citation2004), the neurotrophic factor BDNF is a key regulator of DA release (Goggi et al. Citation2003). Therefore, both BDNF and COMT regulate catecholamine activity, especially in the DA system, which may contribute to different effects of mind–body training on catecholamine activity and be affected by BDNF and COMT genetic polymorphisms.
Interaction between the BDNF Val66Met polymorphism and recent life stress has been reported (Kim et al. Citation2010). With regard to activation of the hypothalamic–pituitary–adrenal (HPA) axis by stress, this polymorphism is associated with HPA reactivity to psychological stress (Shalev et al. Citation2009).
It is long established that circulating catecholamine levels are increased by stress, as a result of activation of peripheral sympathetic nerves and the adrenal medulla. Evidently, catecholamines also act on vagal afferents to regulate sympathoadrenal system stress responses (Mravec Citation2011). Thus, differences in catecholamine activity according to BDNF or COMT polymorphisms in response to stress may be associated with different effects of mind–body training depending on the genetic variants. Higher numbers of different lifetime traumatic event types lead to a higher prevalence of post-traumatic stress disorder in a dose–response relationship, and this effect is modulated by the COMT genotype (Kolassa et al. Citation2010). Although the effects of meditation have been studied, associations of catecholamine activity with stress according to genetic polymorphisms in mind–body training have not yet been reported.
Thus, the hypothesis that this study tested was that meditation would have different effects on plasma catecholamine and stress levels among individuals depending on their BDNF or COMT genetic polymorphisms.
Materials and methods
Subjects
All participants in the control and meditation groups engaged voluntarily in this research. The meditation group consisting of 80 subjects (men and women) practiced meditation regularly were recruited from participants in the “Brain Wave Vibration” mind–body training program. The control group consisting of 57 healthy subjects (men and women) had no previous experience with meditation or similar practices and were recruited from an Internet advertisement. The control group consisted of 35 males (age: mean = 25 years, SD = 3) and 22 females (age: mean = 26 years, SD = 4), and the meditation group included 40 males (age: mean = 26 years, SD = 4) and 40 females (age: mean = 26 years, SD = 2).
The structured clinical interview for DSM-IV Non-patient Version was used (on the day of blood sampling, see below) to assess psychiatric disorders in the participants. Exclusion criteria included a lifetime history of psychosis, bipolar disorder, major depressive disorder, substance abuse or dependence, significant head injury, seizure disorder, or mental retardation. This study was approved by the Institutional Review Board at Seoul National University Hospital, and written informed consent was obtained from all the participants after the procedures had been fully explained. This study was conducted in accordance with the Declaration of Helsinki.
Subjects in the meditation group had been practicing meditation for a mean of 44 months (range 3–144 months). This study included subjects who were homogeneous in age and who practiced the same type of meditation. All subjects in the meditation group were engaged in “Brain Wave Vibration” mind–body training, which is a type of meditation with movement that is designed to facilitate relaxing both the mind, releasing negative emotions from the body through natural rhythmic movements, and focusing on bodily sensations. For this, the method places importance on heightening awareness of the movement of energy within the body by concentrating upon one's bodily sensations and emotions. Detailed methods are described in our previous report (Jung et al. Citation2010).
The Stress Response Inventory
Seventy-three meditation practitioners and 53 control participants completed the Stress Response Inventory (SRI). This study used 22 questions derived from the original SRI questionnaire (Koh et al. Citation2001). Each question was scored on a Likert scale including “not at all” (0), “somewhat” (1), “moderately” (2), “very much” (3), or “absolutely” (4). The 22 questions were categorized into three simplified stress factors: somatization, depression, and anger (Choi et al. Citation2006). Cronbach's alphas for the SRI were somatization (0.89), depression (0.88), and anger (0.87) (Choi et al. Citation2006).
Genotyping
The subjects' DNA was genotyped at the BDNF Val66Met and COMT Val158Met loci. Whole blood (12 ml) was withdrawn from each participant by venepuncture and collected into EDTA-containing tubes. The volume was measured, recorded, and a 1-ml aliquot was stored at − 20°C until genomic DNA extraction. Genomic DNA was isolated from 200 μl of whole blood using the QIAamp DNA Blood Mini kit according to the manufacturer's instructions (QIAGEN, Hilden, Germany). All polymorphisms were detected by TaqMan allelic discrimination assays on an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). BDNF Val66Met (rs6265, C__11592758_10) and COMT Val158Met (rs4680, C__25746809_50) were used for genotyping (Applied Biosystems). The PCR reaction consisted of 95°C for 10 min and 50 cycles of 92°C for 30 s and 60°C for 1 min. A total of 10 μl of PCR reaction mixture was prepared with 5 μl of 2 × TaqMan Genotyping Master mix, 0.5 μl of 20 × Drug Metabolism Genotyping Assay mix, 3.5 μl of DNase-free water, and 1 μl of genomic DNA. The ABI Prism 7500 Sequence Detection System software ver. 2.0.1 (Applied Biosystems) was used for the analysis. The genotyping procedure was screened for BDNF and COMT.
Catecholamine measurements
Blood was collected (at the same time as above) into an anticoagulant EDTA tube from seated control and meditation group subjects at random times during the day (from 09:00 to 20:00 h); the subjects were seated before sampling blood for about 30∼60 min. Subjects were told to prepare for the blood sampling by phone several days before the study (no smoking, alcohol, or severe exercise). The study was not designed to control for effects of short periods of meditation practice, hence the time of meditation practice before sampling could be different for each subject. Usually subjects meditated for 1 h each day.
After centrifugation, the separated plasma was frozen at − 80°C. Plasma catecholamine concentrations were determined by high-performance liquid chromatography, using a plasma catecholamine analysis system marketed by Chromsystem. The mobile phase was prepared according to the specification by Chromsystem. A mixture of NE, epinephrine, and DA was used at predetermined concentrations as an external standard, and dihydroxybenzylamine was used as the internal standard. A CLC-300 dosing pump with a flow rate of 1.1 ml/min was connected to a reverse-phase catecholamine C-80 (code no. 5100/K) column. A CLC-100 electrochemical detector was used, and its signal was registered and integrated by the Chromsystem Geminyx registry and the calculation terminal.
Data analyses
Plasma catecholamine concentrations for the control and meditation groups were analyzed using two different methods. The first method involved measuring the concentrations of NE, epinephrine, and DA, and the second involved analyzing the relative concentrations of these substances, yielding data such as the NE/E and DA/E concentration ratios.
The data were analyzed using two-way analysis of covariance (ANCOVA) with control and meditation subjects and gene polymorphisms as factors and meditation duration as a covariate. As meditation duration among meditation practitioners may have affected the catecholamine concentrations, their catecholamine ratios and stress levels were analyzed using ANCOVA with meditation duration as the covariate. If an interaction was found on two-way ANCOVA, the meditation group data were analyzed using post hoc ANCOVA with meditation duration as the covariate and by post hoc ANOVA in the control group. The differences between the control and meditation groups with the same genotypes were analyzed using Student's t-test. The p-values less than 0.05 were considered statistically significant.
Results
No significant differences were observed for age or gender between the meditation (n = 80, age: 18–36, mean = 26, SD = 3) and control (n = 57, age: 19–37, mean = 26, SD = 4) groups.
Associations Of The Bdnf Val66met Polymorphism With The Da/ne+da/e And Ne/e+ne/da Ratios
Data for the ratios of plasma catecholamine concentrations and statistical analyses are shown in . Two-way ANCOVA showed a significant interaction (F = 3.239, p = 0.042, ) between the control and meditation groups and the BDNF Val66Met polymorphism on the DA/NE+DA/E ratio. Post hoc ANCOVA in the meditation group, controlling for meditation duration, indicated a significantly higher DA/NE+DA/E ratio for subjects with the BDNF Val/Val polymorphism than subjects with the Met/Met polymorphism (F = 3.177, p = 0.047, ). However, one-way ANOVA for the control group showed no significant differences in catecholamine concentrations or their ratios among those with the BDNF Val66Met polymorphism. Post hoc Student's t-test showed that the meditation group had a higher DA/NE+DA/E ratio than the control group for those with the same BDNF Val/Val alleles (F = 6.345, p = 0.035, ).
Table I. Plasma catecholamine concentrations according to BDNF Val66Met and COMT Val158Met polymorphisms.
Figure 1. Different effects of meditation on plasma catecholamine concentrations according to BDNF and COMT genotype. (a) p = 0.042†: interaction effect by two-way ANCOVA, p = 0.047*: post hoc ANCOVA, p = 0.035*: post hoc Student's t-test, control group: V/V (n = 21), V/M (n = 25), M/M (n = 11), meditation group: V/V(n = 19), V/M(n = 44), M/M(n = 17). (b) p = 0.009††: interaction effects by two-way ANCOVA, control group: V/V (n = 20), V/M (n = 28), M/M (n = 9), meditation group: V/V (n = 38), V/M (n = 30), M/M (n = 12). Abbreviations: ANCOVA, analysis of covariance; V/V, valine/valine, V/M, valine/methionine, M/M, methionine/methionine polymorphism.
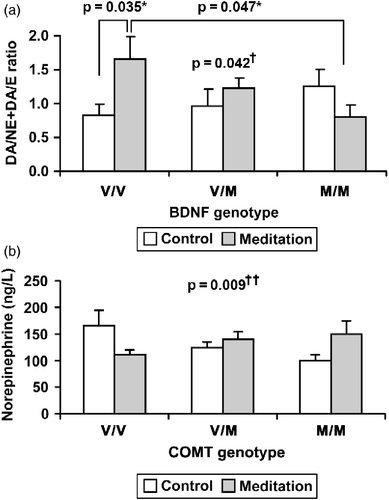
Two-way ANCOVA showed a significant interaction between control (Val/Val+Val/Met: n = 46, Met/Met: n = 11) and meditation subjects (Val/Val+Val/Met: n = 63, Met/Met: n = 17) and the BDNF Val66Met polymorphism on the NE/E+NE/DA ratios (F = 4.073, p = 0.046). Post hoc ANCOVA in the meditation group, controlling for meditation duration, showed a significantly higher NE/E+NE/DA ratio for subjects homozygous for BDNF Met/Met than BDNF Val carriers (F = 4.793, p = 0.032).
Associations of the COMT Val158Met polymorphism with plasma NE concentrations
Data for actual plasma catecholamine concentrations and statistical analyses are shown in . A significant interaction for control and meditation subjects with the COMT Val158Met polymorphism was observed for plasma NE concentrations (F = 4.924, p = 0.009, ). Post hoc ANCOVA in the meditation group, adjusted for meditation duration, showed significantly higher plasma NE concentrations for COMT Met carriers than homozygous subjects (COMT Val/Val; F = 5.260, p = 0.025). No significant interactions were found between the control and meditation subjects and the COMT Val158Met polymorphism for plasma epinephrine and DA concentrations, or the total ratios of each catecholamine ().
Associations of the BDNF Val66Met polymorphism and the COMT Val158Met polymorphism with SRI
Those with the BDNF Val/Met (p < 0.001) and BDNF Met/Met (p = 0.003, ) alleles in the meditation group had lower stress levels, measured by the SRI, than those in the control group, whereas the stress levels for the BDNF Val/Val genotype did not differ between the control and meditation groups. Different effects of mind–body training on stress reduction according to the COMT Val158Met polymorphism were not found and stress reduction in all groups was shown ().
Table II. Stress levels (SRI) according to BDNF Val66Met and COMT Val158Met polymorphisms.
Discussion
The study shows that meditation through mind–body training showed differential effects on stress and plasma catecholamine levels according to BDNF or COMT genetic polymorphisms. These are new findings that mind–body training showed different effects depending on genetic differences. Subjects with the BDNF Val/Val genotype in the meditation group had an increased DA/NE+DA/E ratio compared to those with the Val/Val allele in the control group and to those with the Met/Met allele in the meditation group (). Subjects with the COMT Met/Met allele in the meditation group showed higher NE concentrations than in the control group (). In this study, BDNF Met carriers of the meditation group reported significantly less stress than the control group, whereas Val/Val carriers did not (). This result may have been due to differences in stress levels between those with the BDNF Val/Val and Met/Met genotypes in the control group. The BDNF Met allele results in a significantly attenuated HPA axis and cardiovascular reactivity to psychosocial stressors than the Val/Val genotype (Alexander et al. Citation2010), which seems to be associated with the high stress tendency of the Met/Met allele genotype compared with other genotypes in the control group (). Although subjects with the BDNF Met/Met genotype in the control group had higher stress levels than the other genotypes, those with the BDNF Met/Met genotype showed more improvements in stress reduction than other genotypes in the control and meditation groups (), indicating greater stress-reducing effects of the BDNF Met/Met genotype through mind–body training. One possible hypothesis to explain the associations between stress reduction and the increased NE/E+NE/DA ratio in BDNF Met carriers is as follows. Stress causes the anterior pituitary to release adrenocorticotropic hormone resulting in the production of glucocorticoids (Glaser and Kiecolt-Glaser Citation2005) from the adrenal cortex, which inhibits catecholamine release (Park et al. Citation2008). Therefore, the high stress levels of the BDNF Met/Met genotype compared with other genotypes in the control group may be associated with the lower plasma NE/E+NE/DA ratio of the Met/Met genotype in the control group (). However, in the meditation group, the BDNF Met/Met genotype showed more stress reduction () and an increase in the NE/E+NE/DA ratio compared with the other genotypes (). Hence, the stress reduction of the BDNF Met/Met genotype through mind–body training may have contributed to altered regulation of the sympathetic nervous system, resulting in an increase in the NE or NE/E+NE/DA ratio, which could mediate beneficial cognitive functions, such as attention and alertness (Lapiz and Morilak Citation2006). The increased tendency for higher NE concentrations in subjects with the COMT Met/Met allele in the meditation group than in the control group () was similar to the high NE/E+NE/DA ratio in those with the BDNF Met/Met genotype, and these similar results seem to support the above hypothesis in the case of BDNF Met/Met. That is, COMT Val allele carriers report greater perceived social acceptance than homozygous Met allele carriers, and homozygous Val allele carriers report greater maintenance of positive emotions during stress (Waugh et al. Citation2009). Unlike subjects with the COMT Val/Val genotype, those with the COMT Met/Met genotype seem to have more vulnerability to socially stressful situations based on the trend toward higher stress levels compared with other genotypes such as BDNF Met/Met (). In contrast with the control group, the tendency for increased NE level in those with the COMT Met/Met allele in the meditation group compared with other genotypes () may stem from stress reduction. Interestingly, the same Met/Met polymorphism, although in different genes (BDNF and COMT), showed similar results in increased NE/E+NE/DA ratio for the BDNF Met/Met genotype and increased plasma NE concentrations for the COMT Met/Met allele in the meditation group. These results suggest that although BDNF and COMT are different genes, changes in valine or methionine in these genes that alter protein functions mediate effects of mind–body training on catecholamine activity.
The BDNF Val/Val genotype in the meditation group showed an increased DA/NE+DA/E ratio compared with those with the Val/Val allele in the control group and to those with the Met/Met allele in the meditation group (). In the control group, although BDNF Val/Val carriers had a low DA/NE+DA/E ratio compared with Met/Met carriers, the actual DA concentrations of the Val/Val and Met/Met subjects were similar (). The tendency for a low DA/NE+DA/E ratio in the BDNF Val/Val carriers seemed to result from the tendency for high actual NE and epinephrine concentrations in BDNF Val/Val carriers compared with Met/Met carriers. This finding may have implications for the sympathetic–adrenal–medullary (SAM) axis. Experiencing a stressful situation affects the SAM axis as well as the HPA axis, and this involves increased epinephrine and NE secretion (Glaser and Kiecolt-Glaser Citation2005). The tendency for high plasma NE and epinephrine concentrations in subjects with the BDNF Val/Val genotype compared with the Met/Met genotype in the control group () may be associated with SAM axis activity. Therefore, lower actual NE concentrations in the meditation group than those of the control group for subjects with the BDNF Val/Val genotype () evidently increased the DA/NE+DA/E ratio in the meditation group ().
Brain regions including the prefrontal cortex and right anterior insula associated with attention, interoception, and sensory processing are thicker in meditation participants than matched controls (Lazar et al. Citation2005), suggesting that meditation enhances brain plasticity (Xiong and Doraiswamy Citation2009). We hypothesize that thicker brain regions in meditation participants may be related to neurogenesis and brain plasticity through mind–body training depending on genetic polymorphisms. The effects of meditation may be associated with adaptation of existing neural connections and neurogenesis to accommodate new information and experiences. BDNF is required for neurogenesis (Lee et al. Citation2002), and adult hippocampal neurogenesis is not only affected by internal growth factors including BDNF, but also regulated by external stimuli (Lee and Son Citation2009). Enriched environments and physical activity stimulate hippocampal neurogenesis (Brown et al. Citation2003). Therefore, psychological well-being from stress reduction and physical movements during meditation practice may produce beneficial effects on brain plasticity according to BDNF genotype. Mind–body practices elicit specific gene expression changes in short- and long-term practitioners, suggesting that consistent, constitutive changes in gene expression resulting from the relaxation response may relate to long-term physiological effects (Dusek et al. Citation2008). Therefore, gene expression changes in the BDNF and COMT genotypes through mind–body training may contribute to regulating plasma concentrations or ratios of catecholamines.
The BDNF and COMT genetic polymorphisms are related to mood disorders, schizophrenia (Rybakowski Citation2008), and pain (Zubieta et al. Citation2003). Just as the treatment efficacy and side effects of selective serotonin reuptake inhibitors (fluoxetine) in patients with major depressive disorder are associated with the BDNF Val66Met polymorphism (Zou et al. Citation2010), mind–body training in this study showed different effects on catecholamine activity or psychological state according to the BDNF Val66Met polymorphism. Therefore, mind–body training may complement therapeutic limitations of medication and may enhance the therapeutic effects for stress-related disorders as an alternative compensatory approach and contribute to individual therapy based on genetic polymorphisms. Furthermore, mind–body training is free from any harmful and undesired side effects resulting from medication.
However, this study had some limitations. The samples were collected during the daytime. They were taken in similar conditions at random times during the day in both the control and meditation groups. Any effects of diurnal variations of the parameters measured in this research need to be investigated in a further study. As this study was limited by its cross-sectional design, a longitudinal study to investigate the same subjects before starting meditation and after such mind–body training is needed. Plasma catecholamine concentrations do not necessarily reflect central nervous system catecholamine levels, which are difficult to measure in humans. Nevertheless, as the BDNF and COMT genotypes are not different between central and peripheral systems, the findings from this study might reflect interactions between catecholamine metabolism and meditation in the brain.
In conclusion, this is the first report showing that meditation has different effects on stress and plasma catecholamines according to BDNF and COMT genetic polymorphisms. These findings suggest the potential for adjunct therapies for stress-related disorders based on the different effects of mind–body training between individuals according to genetic polymorphisms.
Acknowledgements
The authors give special thanks to Korean Institute of Brain Science for the assistance in the current study. This work was supported by Cognitive Neuroscience Program of the Korean Ministry of Science and Technology (M10644020003-08N4402-00310).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexander N, Osinsky R, Schmitz A, Mueller E, Kuepper Y, Hennig J. 2010. The BDNF Val66Met polymorphism affects HPA-axis reactivity to acute stress. Psychoneuroendocrinology. 35:949–953.
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. 2003. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 17:2042–2046.
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. 2004. Functional analysis of genetic variation in catechol-O-Methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 75:807–821.
- Choi SM, Kang TY, Woo JM. 2006. Development and validation of a modified form of the stress response inventory for workers. J Korean Neuropsychiatr Assoc. 45:541–553.
- Cotman CW, Berchtold NC. 2002. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 25:295–301.
- Dusek JA, Otu HH, Wohlhueter AL, Bhasin M, Zerbini LF, Joseph MG, Benson H, Libermann TA. 2008. Genomic counter-stress changes induced by the relaxation response. PLoS One. 3:e2576.
- Glaser R, Kiecolt-Glaser JK. 2005. Stress-induced immune dysfunction: Implications for health. Nat Rev Immunol. 5:243–251.
- Goggi J, Pullar IA, Carney SL, Bradford HF. 2003. Signalling pathways involved in the short-term potentiation of dopamine release by BDNF. Brain Res. 968:156–161.
- Infante JR, Torres-Avisbal M, Pinel P, Vallejo JA, Peran F, Gonzalez F, Contreras P, Pacheco C, Roldan A, Latre JM. 2001. Catecholamine levels in practitioners of the transcendental meditation technique. Physiol Behav. 72:141–146.
- Jain S, Shapiro SL, Swanick S, Roesch SC, Mills PJ, Bell I, Schwartz GE. 2007. A randomized controlled trial of mindfulness meditation versus relaxation training: Effects on distress, positive states of mind, rumination, and distraction. Ann Behav Med. 33:11–21.
- Jones BM. 2001. Changes in cytokine production in healthy subjects practicing Guolin Qigong: A pilot study. BMC Complement Altern Med. 1:8.
- Jung YH, Kang DH, Jang JH, Park HY, Byun MS, Kwon SJ, Jang GE, Lee US, An SC, Kwon JS. 2010. The effects of mind–body training on stress reduction, positive affect, and plasma catecholamines. Neurosci Lett. 479:138–142.
- Kim SJ, Cho SJ, Jang HM, Shin J, Park PW, Lee YJ, Cho IH, Choi JE, Lee HJ. 2010. Interaction between brain-derived neurotrophic factor Val66Met polymorphism and recent negative stressor in harm avoidance. Neuropsychobiology. 61:19–26.
- Koh KB, Park JK, Kim CH, Cho S. 2001. Development of the stress response inventory and its application in clinical practice. Psychosom Med. 63:668–678.
- Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ. 2010. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 67:304–308.
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. 2011. Stress revisited: A critical evaluation of the stress concept. Neurosci Biobehav Rev. 35 5: 1291–1301.
- Lapiz MD, Morilak DA. 2006. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 137:1039–1049.
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B. 2005. Meditation experience is associated with increased cortical thickness. Neuroreport. 16:1893–1897.
- Lee E, Son H. 2009. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep. 42:239–244.
- Lee J, Duan W, Mattson MP. 2002. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 82:1367–1375.
- Lee SH, Ahn SC, Lee YJ, Choi TK, Yook KH, Suh SY. 2007. Effectiveness of a meditation-based stress management program as an adjunct to pharmacotherapy in patients with anxiety disorder. J Psychosom Res. 62:189–195.
- Miller JJ, Fletcher K, Kabat-Zinn J. 1995. Three-year follow-up and clinical implications of a mindfulness meditation-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiatry. 17:192–200.
- Mravec B. 2011. Role of catecholamine-induced activation of vagal afferent pathways in regulation of sympathoadrenal system activity: Negative feedback loop of stress response. Endocr Regul. 45:37–41.
- Newberg AB, Wintering N, Khalsa DS, Roggenkamp H, Waldman MR. 2010. Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: A preliminary study. J Alzheimers Dis. 20:517–526.
- Park YS, Ha Choi Y, Park CH, Kim KT. 2008. Nongenomic glucocorticoid effects on activity-dependent potentiation of catecholamine release in chromaffin cells. Endocrinology. 149:4921–4927.
- Rybakowski JK. 2008. BDNF gene: Functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics. 9:1589–1593.
- Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Ebstein RP, Kaitz M. 2009. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 34:382–388.
- Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. 2007. Mental training affects distribution of limited brain resources. PLoS Biol. 5:e138.
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. 2004. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 24:5331–5335.
- Vestergaard-Poulsen P, van Beek M, Skewes J, Bjarkam CR, Stubberup M, Bertelsen J, Roepstorff A. 2009. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport. 20:170–174.
- Waugh CE, Dearing KF, Joormann J, Gotlib IH. 2009. Association between the catechol-O-methyltransferase Val158Met polymorphism and self-perceived social acceptance in adolescent girls. J Child Adolesc Psychopharmacol. 19:395–401.
- Xiong GL, Doraiswamy PM. 2009. Does meditation enhance cognition and brain plasticity?. Ann N Y Acad Sci. 1172:63–69.
- Zou YF, Wang Y, Liu P, Feng XL, Wang BY, Zang TH, Yu X, Wei J, Liu ZC, Liu Y, Tao M, Li HC, Li KQ, Hu J, Li M, Zhang KR, Ye DQ, Xu XP. 2010. Association of brain-derived neurotrophic factor genetic Val66Met polymorphism with severity of depression, efficacy of fluoxetine and its side effects in Chinese major depressive patients. Neuropsychobiology. 61:71–78.
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. 2003. COMT Val158Met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 299:1240–1243.
