Abstract
Nociceptin/orphanin FQ (N/OFQ) peptide and its receptor are not only ubiquitously expressed in mammalian brain and spinal cord but are also abundant in limbic structures, particularly in the hippocampus. The widespread distribution of N/OFQ reflects the broad spectrum of its biological actions such as nociception, food intake, spontaneous locomotor activity, and learning and memory processes. Since the hippocampus is involved in the control of adrenocortical activity, its role in stress-related phenomena is well characterized. In male Wistar rats, we first examined the effects of acute restraint stress (120 min) on the brain immunohistochemical localization of N/OFQ. The analysis carried out on sections obtained at the onset of stress revealed enhanced expression of N/OFQ in CA1, CA3, and the dentate gyrus as well as increased plasma corticosterone concentrations. Next, we examined whether endogenous glucocorticoid hormone plays a role in the modulation of hippocampal N/OFQ expression in response to stress. To this end, rats were injected with corticosterone (1 mg/kg) or subjected to restraint stress 1 week after adrenalectomy. Two hours after corticosterone administration, plasma glucocorticoid concentrations were comparable to those observed after restraint stress, while N/OFQ expression had significantly increased in all the hippocampal subfields examined. By contrast, in adrenalectomized rats, stress did not modify protein expression. These results confirm that stress can affect N/OFQ expression and that glucocorticoids may constitute hormonal mediators of this complex interplay.
Introduction
Nociceptin/orphanin FQ (N/OFQ) is a heptadecapeptide (Meunier et al. Citation1995; Reinscheid et al. Citation1995) that binds with high affinity to the opioid receptor-like (ORL-1) receptor, but does not bind to the classical mu, delta, and kappa opioid receptor types (Calò et al. Citation2000).
Numerous studies in the literature have reported that the functional role of the N/OFQ system in a wide range of physiological conditions (Calò et al. Citation2000) differs from that of opioids (Meunier et al. Citation1995; Reinscheid et al. Citation1995; Grisel et al. Citation1996; Mogil et al. Citation1996).
N/OFQ, together with its ORL-1 receptor and mRNAs, is expressed in cortical areas, in the olfactory regions, in the thalamus, in limbic structures, in the hypothalamus, in the locus coeruleus, and in the raphe complex (Neal et al. Citation1999). Its distribution covers a wide range of functions in which N/OFQ is believed to play a role, including pain modulation, nociception, motor performance, spatial learning, memory, and food intake (Meis Citation2003). The involvement of the N/OFQ system has also been demonstrated in peripheral functions (Meunier Citation1997; Calò et al. Citation2000; Kapusta et al. Citation2002), while some investigators have reported that the ORL-1 receptor plays a role in the immune system (Halford et al. Citation1995; Meunier Citation1997).
It is now recognized that exposure to a stressful event is processed in limbic areas, and that the hippocampus is one of the structures involved in mediating the stress response. The hippocampus is particularly sensitive to the effects of adrenal glucocorticoids secreted during both the diurnal rhythm and stress phenomena (Joëls Citation2008; Joëls et al. Citation2008). Corticosterone, large amounts of which are released after stress, acts in rat brain via two receptor types (De Kloet et al. Citation1998): the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR). Although both MRs and GRs are widely expressed in the brain, MRs are largely restricted to limbic brain regions (i.e. hippocampus, septal and amygdalar nuclei, and motor nuclei in the brainstem), whereas GRs, which are more ubiquitous, are found in both neurons and glial cells (De Kloet et al. Citation1998; Joëls Citation2001).
MRs are expressed in comparable amounts throughout the hippocampus and display a high affinity for corticosterone, whereas GRs are predominantly expressed in the CA1 hippocampal area and in the dentate gyrus (DG), and show a 10-fold lower affinity for corticosterone (Joëls Citation2001). Overall, corticosterone rapidly enhances the CA1/CA3 hippocampal activity after stress. By contrast, cells in the DG are less responsive to stress-induced increases in corticosterone concentrations (Joëls Citation2009).
As previously reported, N/OFQ is involved in a variety of behavioral and physiological functions (Bodnar Citation2008), and may play a role in physiological stress responses (Jenck et al. Citation1997; Devine et al. Citation2001). It has been shown that, when delivered intracerebroventricularly (i.c.v.), N/OFQ attenuates the behavioral inhibition of animals acutely exposed to stressful environmental conditions (Jenck et al. Citation1997). Moreover, N/OFQ precursor and ORL-1 receptor mRNA levels increase in the hippocampus of mice that are exposed to social crowding stress and are resilient to the adversive effects of the stressor (Reiss et al. Citation2007). It has also been demonstrated that N/OFQ-deficient mice display higher plasma corticosterone concentrations in both basal and stress conditions (Koster et al. Citation1999).
In view of all these findings, the first goal of our study was to investigate the effects of acute restraint stress on rat brain N/OFQ immunohistochemical localization.
Moreover, as it is clear that N/OFQ is involved in the modulation of numerous behavioral processes in which the hippocampus plays a pivotal role, we investigated whether glucocorticoids may constitute the hormonal mediators of this interaction. Thus, we conducted a series of studies aimed at elucidating the possible role played by corticosterone in the modulation of stress-related N/OFQ expression.
Materials and methods
Experimental subjects
Adult male Wistar rats (300–325 g; Harlan, Calco, Lecco Italy) were housed three per cage (42 × 27 × 16 cm) under controlled conditions (room temperature = 24°C, humidity = 50%), on a 12-h light/dark cycle (lights on at 07:00 a.m.) with food (Standard 4RF21 Diet, Charles River, Calco, Lecco, Italy) and water ad libitum. The week before the experiment, the rats were handled gently by the same operator daily between 09:30 and 10:30 a.m. to minimize stress response to manipulation. All the experiments were started at 10:00 a.m. and were carried out according to the European (86/609/EEC) and Italian (D.Lgs. 116/92) guidelines on animal care. Rats were killed by decapitation. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to use alternatives to in vivo techniques.
Restraint stress
Restraint stress was carried out as follows: the rat was removed from its cage and placed in a Plexiglas restraint device; the tail gate was adjusted to hold the rat firmly without impairing circulation to the limbs. The rat was restrained in this manner for 120 min and killed at the end of the stress application.
Adrenalectomy surgical procedures
Rats were anesthetized with an intramuscular injection of a mixture of ketamine (60 mg/kg) and xylazine (10 mg/kg). Two dorsal incisions were made caudal to the costal margin, and the adrenal glands were removed, with the capsule intact, from each side. For sham operations, approximately the same time was taken to locate the adrenal gland, which was not however removed. The muscle tissue was sutured and the skin closed using wound clips. Following adrenalectomy, the rats received a 0.9% NaCl solution as drinking water. Rats were allowed to recover for 1 week after surgery. The effectiveness of the adrenalectomy was established at autopsy and confirmed by plasma corticosterone analysis.
Drug treatment
Corticosterone (Sigma, St. Louis, MO, USA) was dissolved in sesame oil and injected (1 mg/kg s.c.); control rats received an equal volume of sesame oil s.c. The corticosterone dose was chosen on the basis of our previous findings (Scaccianoce et al. Citation2004), which indicated that the dose adopted mimics the circulating corticosterone concentrations observed in response to 120 min of restraint stress. Rats were sacrificed 120 min after treatment and trunk blood was collected.
Measurements of plasma corticosterone
Blood was collected in tubes containing 0.13 M EDTA. After centrifugation at 1900g at 4°C for 15 min, plasma samples were stored at − 20°C prior to assay. Plasma corticosterone concentrations were determined by radioimmunoassay (RIA). The cross-reactivity of the polyclonal corticosterone-antisera (MP Biomedicals, Solon, OH, USA) with related substances was negligible. The inter- and intra-assay coefficients of variation were 8.7% and 2.4%, respectively, with a detection limit of 0.0125 ng/tube. Plasma samples were diluted 1:100 with Steroid Diluent (MP Biomedicals). All measurements fell within the linear range of the standard curve (0.0125–3.0 ng/tube).
Immunohistochemistry
Rats were killed by decapitation and brains were removed and rapidly transferred to frozen isopentane ( − 40°C) for 20 s and then stored at − 80°C. The N/OFQ immunoreactivity experiments were conducted on 15 μm cryostat rat brain sections using a conventional streptavidin–biotin technique. The coronal sections ( − 2.8 bregma) were fixed in 4% paraformaldehyde/0.1 M phosphate buffer for 15 min. Sections were stained using a polyclonal rabbit IgG antibody against N/OFQ (rabbit anti-Noc serum, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at a dilution of 1:500, which was determined in preliminary experiments. Briefly, sections were incubated with 3% hydrogen peroxide for 30 min, and followed by a 30-min incubation with 10% goat serum to block non-specific protein binding.
Negative control sections were treated with non-immunized goat immunoglobulin serum under the same conditions. Following careful washing with phosphate buffer saline (PBS), biotinylated goat IgG (dilution 1:1000; GE Healthcare, UK) was applied for 2 h, followed by streptavidin peroxidase conjugate (GE Healthcare, Chalfont, St. Giles, Bucks, UK) for 90 min. The color reaction was developed with a solution of 0.02% diaminobenzidine in 0.003% hydrogen peroxide for 15 min. The sections were mounted onto gelatin–chrome alum-coated glass slides and viewed through a white light microscope (Leitz, Oberkochen, Germany).
The immunohistochemistry procedures were simultaneously carried out on a number of brain sections (at least four sections/animal) from each experimental group.
Optical density (OD) analysis
Positive staining was evaluated by calculating the OD of areas of interest in each region and subregion (i.e. prefrontal cortex, hypothalamus, and hippocampus) as referenced in “The rat brain in stereotaxic coordinates” (Pellegrino et al. Citation1979).
Standard outline digital images were captured using a SPOT-RT CCD videocamera (SPOT-RT Image Software V 3.0 SPOT Diagnostic Instruments, Inc., Sterling Height, MI, USA) for each region of interest. A semiquantitative analysis of positive immunoreactivity was carried out on brain multiple sections/region. Final OD values were calculated as a function of the specific signal relative to external background measure (taken from outside the section). Constant optical conditions were maintained throughout the morphometric and densitometric evaluation (Burke et al. Citation1990).
N/ofq Ria
N/OFQ immunoreactivity levels were quantified using an RIA kit (Phoenix Pharmaceuticals, Belmont, CA, USA) as described by Lutfy et al. (Citation2008) with minor modifications. Briefly, the tissues (hippocampus, prefrontal cortex, hypothalamus, and basal forebrain) were isolated, weighted, and stored at − 80°C until required for assay. The selected brain regions were subsequently sonicated in 40 volumes of ice-cold acid acetone mixture (75% acetone, 25% 0.2 M HCl) and centrifuged for 20 min at 17,500g. After centrifugation, the supernatant was collected and lyophilized. A 1:10 dilution of each sample was prepared in RIA buffer (provided by the manufacturer) and assayed. Counts were compared with a standard curve ranging between 10 and 1280 pg/ml of N/OFQ to determine the amount of peptide immunoreactivity in samples. The inter- and intra-assay coefficients of variation were 9.8% and 5.4%, respectively.
Statistical analysis
Data were analyzed using one-way analysis of variance after Bartlett's test for the homogeneity of variances and Kolmogorov–Smirnov's test for the Gaussian distribution, and were followed by Newman–Keuls multiple-comparison test or, when appropriate, by Student's t-test.
Results
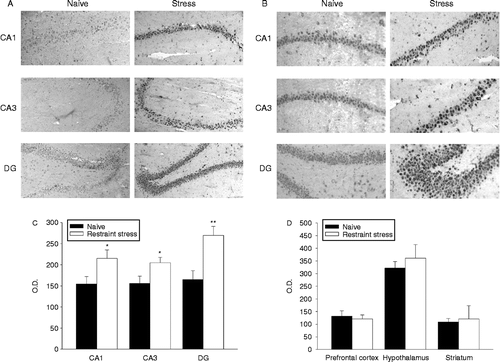
After 120 min restraint stress, N/OFQ protein expression increased in the hippocampus (). Immunoreactivity was enhanced mainly in the CA1 and CA3 areas and in the DG region, as demonstrated by the OD analysis (). Plasma corticosterone concentrations at the onset of the stress application significantly increased compared with controls (controls: 1.5 ± 0.4; restraint stress 14.9 ± 1.3 μg of corticosterone/100 ml of plasma; means ± SEM, n = 6, p < 0.001, Student's t-test). Moreover, additional areas that were examined, such as the prefrontal cortex, hypothalamus, and striatum, did not display any significant variation in response to a 120-min restraint stress application ().
Figure 1. Effects of acute restraint stress on hippocampal nociceptin expression. (A) Representative photomicrographs showing the increase in immunoreactivity in the CA1, CA3, and DG areas of naive rats (left panels) and rats subjected to 120 min of restraint stress (right panels). Immunohistochemical pictures are shown together with their localization in the hippocampus at level – 2.8 mm of bregma (upper panel). All images are magnified equally (200 × ). (B) Representative immunoreactivity photomicrographs of the same areas as those in (A) magnified equally (400 × ). (C) Semiquantitative analysis of N/OFQ immunoreactivity signal in hippocampal subfields. Values are expressed as means OD ± SEM (n = 6) rats per group. *p < 0.05, **p < 0.01 versus corresponding naive group (Student's t-test). (D) Semiquantitative analysis of N/OFQ immunoreactivity signal in prefrontal cortex, hypothalamus, and striatum. Values are expressed as means OD ± SEM (n = 6) rats per group.
Next, we examined whether increased plasma corticosterone concentrations affected hippocampal N/OFQ expression. To this end, rats received a corticosterone treatment (1 mg/kg s.c.) designed to mimic the circulating corticosterone concentrations observed in response to stress (vehicle: 1.9 ± 0.6; corticosterone treatment 12.5 ± 1.6 μg of corticosterone/100 ml of plasma; means ± SEM, n = 6, p < 0.001 Student's t-test).
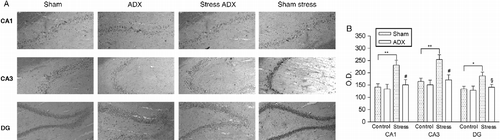
OD analysis of protein immunoreactivity revealed a significant increase in the same areas as those affected by restraint stress (i.e. CA1, CA3, and DG) (). Additional evidence indicating that endogenous corticosterone influences hippocampal N/OFQ expression after stress application was obtained using adrenalectomized rats (). While the absence of circulating glucocorticoid hormone did not modify constitutive N/OFQ expression in the hippocampus, adrenalectomy prevented any restraint stress-induced protein increase in all the regions examined.
Figure 2. Effect of corticosterone administration on hippocampal nociceptin expression. (A) Representative photomicrographs showing the increase in CA1, CA3, and DG areas in vehicle (sesame oil) (left panels) and rats injected with corticosterone (1 mg/kg s.c.) (right panels). Immunohistochemical pictures are shown together with their localization in the hippocampus at level – 2.8 mm of bregma. All images are magnified equally (200 × ). (B) Semiquantitative analysis of N/OFQ immunoreactivity signal in hippocampal subfields. Values are expressed as means OD ± SEM (n = 6) rats per group. **p < 0.01 versus corresponding vehicle-treated group (Student's t-test).
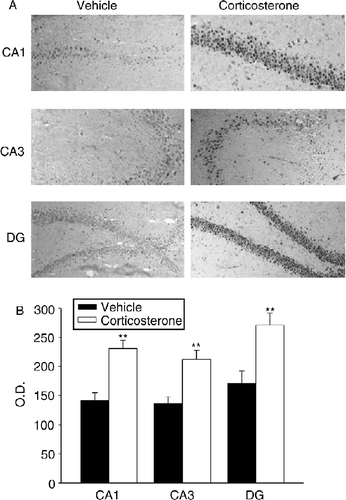
Figure 3. Effects of acute restraint stress on hippocampal nociceptin expression in rats subjected to adrenalectomy. (A) Representative photomicrographs of CA1, CA3, and DG regions in sham-operated group (SHAM) rats and adrenalectomized (ADX) rats. Control animals were left undisturbed while stressed animals were subjected to 120-min restraint stress. Immunohistochemical pictures show the hippocampus at level – 2.8 mm of bregma. All images are magnified equally (200 × ). (B) Semiquantitative analysis of N/OFQ immunoreactivity signal in hippocampal subfields. Values are expressed as means OD ± SEM (n = 6) rats per group. #p < 0.01, §p < 0.05 versus corresponding sham-adrenalectomized group. Significant differences between the stress and control group are expressed as *p < 0.05 and **p < 0.01 (Newman–Keuls multiple-comparison test).
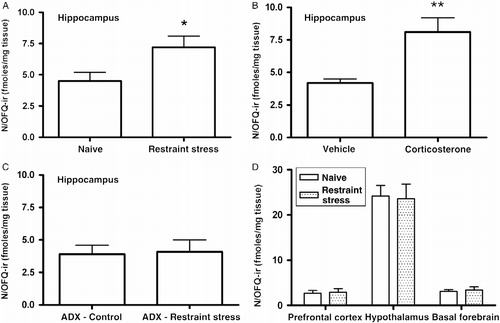
In an additional set of experiments, the effects of restrain stress on the hippocampal N/OFQ content in adrenal-intact rats, adrenalectomized rats, as well as after the administration of exogenous corticosterone (1 mg/kg s.c.), were investigated using a commercially available RIA kit. The results obtained by means of this method are comparable to those yielded by the immunohistochemistry analysis ().
Figure 4. Measurement of N/OFQ-immunoreactivity (OFQ/N-ir) in rat brain regions. Hippocampal N/OFQ immunoreactivity in adrenal-intact rats subjected to 120-min restraint stress (panel A) or corticosterone (1 mg/kg s.c.) in sesame oil (panel B). The effect of restraint stress on hippocampal N/OFQ-ir level in adrenalectomized (ADX) rats is shown in panel C. Panel D represents the effect of restraint stress on the content of N/OFQ-ir in selected brain regions. Each sample was assayed in triplicate for N/OFQ-ir. Values are expressed as mean fmol/mg tissue ± SEM; n = six rats per group. *p < 0.05, **p < 0.01 versus corresponding control group (Student's t-test).
Discussion
The current findings indicate that restraint stress increases N/OFQ expression in the hippocampus. This effect is absent in adrenalectomized rats and is mimicked by corticosterone injection, suggesting that glucocorticoids may constitute a hormonal signal involved in the relationship between stress and N/OFQ expression. A number of studies have shown that N/OFQ plays an important role in stress phenomena, though the exact function of the N/OFQ system in the stress response remains controversial. Jenck et al. (Citation1997), in a range of tests involving different sets of environmental stressors (light–dark aversion, elevated plus-maze, exploratory behavior of an unfamiliar environment, pharmacological anxiogenesis, and operant conflict), found that N/OFQ exerted attenuating effects on stress-related behavioral responses. More recently, it has been demonstrated that a peptide antagonist of the nociceptin opioid peptide (NOP) receptor that binds with high affinity to the NOP receptor exerts an anxiolytic effect in the elevated T-maze test when centrally injected in rats (Duzzioni et al. Citation2011). Clinical and experimental data have indicated that stress conditions might exacerbate both anxiety and affective disorders (for a review, see Pêgo et al. Citation2010). Although the classical pharmacological strategy to treat such disorders is considered effective, various side effects often limit the use of drugs. Thus, the possibility to pharmacologically manipulate a new, distinct, neurochemical system (i.e. N/OFQ and NOP receptors) may represent a novel approach to the treatment of a variety of mood disorders. N/OFQ-deficient mice have been found to display behavioral, sensory, and endocrine symptoms of increased stress susceptibility (Koster et al. Citation1999). In particular, mice lacking N/OFQ display impaired behavioral responses to acute stress, as revealed in the open field, plus-maze and light–dark box tests, as well as high basal and post-stress plasma corticosterone concentrations. It has also been reported that restraint stress enhances the aversive behavior demonstrated by N/OFQ female knockout mice, as indicated by the abnormal emotional reactivity revealed in the light–dark preference test (Ouagazzal et al. Citation2003).
In this study, we examined, by means of a different methodological approach (i.e. immunohistochemistry and RIA), the effect of acute restraint stress on N/OFQ expression in the hippocampal area, which is involved in the control of adrenocortical activity. We subsequently investigated the role of corticosterone on stress-induced hippocampal expression of N/OFQ. We found a significant increase in stress-induced protein expression in the CA1 and CA3 regions as well as in the DG of rats subjected to restraint stress. A similar increase was observed in corticosterone-injected rats, in which hormone plasma concentrations were comparable to those observed at the onset of a 2-h period of restraint stress. Thus, although the time course of plasma corticosterone concentrations in response to stress might not exactly mirror the pharmacokinetics of the injected hormone, we may hypothesize that corticosterone secreted in response to stress is involved in stress-dependent hippocampal N/OFQ modulation. Our results are in contrast to those reported by Devine et al. (Citation2003), who examined the effects of a single acute stress application and/or chronic variable stress applications on N/OFQ neurotransmission in a variety of brain regions, and found that the N/OFQ content in the basal forebrain decreased by 25–30% in rats that were subjected to acute stress just before decapitation. The discrepancies between their work and our study might be due to differences in the restraint procedures used (i.e. 30 and 120 min, respectively).
The body of physiological data linking N/OFQ system stimulation and endocrine activity is growing steadily. For example, i.c.v. N/OFQ injections stimulate adrenocorticotropic hormone (ACTH) and corticosterone release in rats (Devine et al. Citation2001). There is, however, as yet little physiological evidence linking corticosterone or glucocorticoid concentrations to the N/OFQ system in rat brain. N/OFQ-induced hyperphagia in rat is mediated by circulating corticosterone (Nicholson et al. Citation2002). In addition, exposure to dexamethasone during the neonatal period leads to increased ORL-1 expression in the paraventricular nucleus of the hypothalamus (PVN) and DG, thus providing evidence linking early glucocorticoid exposure to ORL-1 expression (Neal et al. Citation2003).
The finding that adrenalectomized non-stressed rats did not display any variation in hippocampal N/OFQ expression suggests that endogenous corticosterone does not contribute to the maintenance of tonic expression of the protein, but does not rule out the possibility that hormonal circadian variations are in some way involved in regulating N/OFQ expression. However, stress and exogenous corticosterone experiments indicate that this hormone mediates phasic hippocampal N/OFQ expression. We may thus hypothesize that stress-induced corticosterone release activates neuronal circuits involved in N/OFQ expression and thus represents a key mediator of the N/OFQ system. One limitation of our hypothesis is that we have, within this context, interpreted stress merely as a glucocorticoid-induced phenomenon, without considering the complex response of the organism. One means of ascertaining whether the stress-induced increase in corticosterone concentrations represents the driving force on hippocampal N/OFQ would be to investigate the effects of stress in adrenalectomized rats, in which the corticosterone response is mimicked by its exogenous administration.
How corticosterone exerts these effects remains unclear. One possible explanation is that the hormone mediates hippocampal N/OFQ stress-induced expression by binding to the regulatory sequences in the preproN/OFQ gene, thus acting as a transcription factor. This hypothesis is supported by the presence of several glucocorticoid-responding elements on the preproN/OFQ human gene (Xie et al. Citation1999). Since the amino acid sequence of the N/OFQ precursor is well conserved across murine and human species (Meunier Citation1997), it remains to be seen whether the same mechanism is responsible for the role played by corticosterone in rat N/OFQ.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bodnar RJ. 2008. Endogenous opiates and behaviour: 2007. Peptides. 29:2292–2375.
- Burke RE, Cadet JL, Kent JD, Karanas AL, Jackson-Lewis V. 1990. An assessment of the validity of densitometric measures of striatal tyrosine hydroxylase-positive fibers: Relationship to apomorphine-induced rotations in 6-hydroxydopamine lesioned rats. J Neurosci Methods. 35:63–73.
- Calò G, Guerrini R, Rizzi A, Salvadori S, Regoli D. 2000. Pharmacology of nociceptin and its receptor: A novel therapeutic target. Br J Pharmacol. 129:1261–1283.
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. 1998. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 19:269–301.
- Devine DP, Watson SJ, Akil H. 2001. Nociceptin/orphanin FQ regulates neuroendocrine function of the limbic hypothalamic-pituitaryadrenal axis. Neuroscience. 102:541–553.
- Devine DP, Hoversten MT, Ueda Y, Akil H. 2003. Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J Neuroendocrinol. 15:69–74.
- Duzzioni M, Duarte FS, Leme LR, Gavioli EC, De Lima TC. 2011. Anxiolytic-like effect of central administration of NOP receptor antagonist UFP-101 in rats submitted to the elevated T-maze. Behav Brain Res. 222:206–211.
- Grisel JE, Mogil JS, Belknap JK, Grandy DK. 1996. Orphanin FQ acts as a supraspinal, but not a spinal, anti-opioid peptide. Neuroreport. 7:2125–2129.
- Halford WP, Gebhardt BM, Carr DJ. 1995. Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol. 59:91–101.
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJJr, Nothacker HP, Civelli O. 1997. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci USA. 94:14854–14858.
- Joëls M. 2001. Corticosteroid actions in the hippocampus. J Neuroendocrinol. 13:657–669.
- Joëls M. 2008. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 583:312–321.
- Joëls M, Karst H, DeRijk R, de Kloet ER. 2008. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 31:1–7.
- Joëls M. 2009. Stress, the hippocampus, and epilepsy. Epilepsia. 50:586–597.
- Kapusta DR, Dayan LA, Kenigs VA. 2002. Nociceptin/orphanin FQ modulates the cardiovascular, but not renal, responses to stress in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 29:254–259.
- Koster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK. 1999. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci USA. 96:10444–10449.
- Lutfy K, Lam H, Narayanan S. 2008. Alterations in the level of OFQ/N-IR in rat brain regions by cocaine. Neuropharmacology. 55:198–203.
- Meis S. 2003. Nociceptin/orphanin FQ: Actions within the brain. Neuroscientist. 9:158–168.
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monserrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. 1995. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 377:532–535.
- Meunier JC. 1997. Nociceptin/orphanin FQ and the opioid receptor-like ORL-1 receptor. Eur J Pharmacol. 340:1–15.
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. 1996. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 75:333–337.
- Neal CRJr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJJr. 1999. Localization of Orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 406:503–547.
- Neal CRJr, VanderBeeka BL, Vázquez DM, Watson SJJr. 2003. Dexamethasone exposure during the neonatal period alters ORL1 mRNA expression in the hypothalamic paraventricular nucleus and hippocampus of the adult rat. Dev Brain Res. 146:15–24.
- Nicholson JR, Akil H, Watson SJJr. 2002. Orphanin FQ-induced hyperphagia is mediated by corticosterone and central glucocorticoid receptors. Neuroscience. 115:637–643.
- Ouagazzal AM, Moreau JL, Pauly-Evers M, Jenck F. 2003. Impact of environmental housing conditions on the emotional responses of mice deficient for nociceptin/orphanin FQ peptide precursor gene. Behav Brain Res. 144:111–117.
- Pêgo JM, Sousa JC, Almeida OF, Sousa N. 2010. Stress and the neuroendocrinology of anxiety disorders. Curr Top Behav Neurosci.. 2:97–117.
- Pellegrino LJ, Pellegrino AS, Cushman AJ. 1979. A stereotaxic atlas of the rat brain. New York: Plenum Press.
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJJr, Civelli O. 1995. Orphanin FQ: A neuropeptide that activates an opioid like G protein-coupled receptor. Science. 270:792–794.
- Reiss D, Wolter-Sutter A, Krezel W, Ouagazzal AM. 2007. Effects of social crouwding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav Brain Res. 184:167–173.
- Scaccianoce S, Mattei V, Del Bianco P, Gizzi C, Sorice M, Hiraiwa M, Misasi R. 2004. Hippocampal prosaposin changes during stress: A glucocorticoid-independent event. Hippocampus. 14:275–280.
- Xie GX, Ito E, Maruyama K, Suzuki Y, Sugano S, Sharma M, Pietruck C, Palmer PP. 1999. The promoter region of human prepro-nociceptin gene and its regulation by cyclic AMP and steroid hormones. Gene. 238:427–436.
