Abstract
Oxytocin facilitates pro-social behaviour and is proposed as a regulatory factor controlling stress reactivity. Previous research on oxytocin and stress has focused on achievement-related stressors among male participants. The aims of the study were to (1) examine the influence of oxytocin on the affective and cortisol response to the Yale Interpersonal Stressor (YIPS), a live social rejection paradigm, and (2) to replicate the finding that women exhibit a greater cortisol response to interpersonal stress than men (Stroud et al. 2002). Sex differences in stress responses: Social rejection versus achievement stress. Biol Psychiat 53:318–327. Ninety-six undergraduate students underwent the YIPS, where participants were excluded from two separate conversations by two same-sex confederates. Salivary cortisol concentrations and mood were repeatedly measured throughout the study. Participants were administered, in a double-blind design, a single dose of intranasal oxytocin (24 IU) or placebo prior to beginning the YIPS. The YIPS elicited a significant negative mood response that was more pronounced in females than in males. However, no significant cortisol response to the stressor and no sex difference in cortisol reactivity were observed. A significant effect of drug condition on cortisol levels was observed. Participants who were administered oxytocin exhibited a decrease in cortisol levels, relative to placebo, during the YIPS, F (4, 184) = 4.50, p < 0.05. The study failed to replicate the sex difference in the cortisol response to interpersonal stress reported by Stroud et al. (2002). Intranasal oxytocin, however, appeared to reduce cortisol concentrations during an interpersonal challenge.
Introduction
It is well established that chronic interpersonal stress contributes to the development of mental disorders (Daley et al. Citation1998) as well as physical illness (Kiecolt-Glaser et al. Citation1994). Interpersonal stress, for example, is central to current conceptualizations of major depression (Hammen Citation2003). Moreover, the health disadvantages associated with interpersonal stress are often greater than those related to non-interpersonal stress (Orth-Gomer and Leineweber Citation2005). The relationship between interpersonal stress and disease may be partly mediated through the activation of the hypothalamus–pituitary–adrenal axis (HPA), as there is evidence that poor interpersonal functioning is associated with elevated cortisol levels (Steptoe Citation1991; Decker Citation2000). Chronically elevated levels of basal cortisol have negative health consequences, such as suppressed immune function (Sapolsky Citation2004). Thus, understanding the neurobiological regulation of interpersonal stress has important implications for mental and physical health. The aim of the current study was to investigate the role that oxytocin might play in modulating the cortisol response to interpersonal stress.
Oxytocin is a mammalian hormone that also acts as a neuromodulator in the central nervous system (CNS). Although well known for its peripheral functions, which include stimulating uterine contractions during labour and milk let-down during lactation, oxytocin is directly involved in promoting social affiliation. In non-human mammals, oxytocin plays an important role in pair-bond formation (Williams et al. Citation1992; Insel and Hulihan Citation1995; Young et al. Citation2001), maternal behaviour (Pedersen et al. Citation1994; Pedersen Citation1997) and social recognition (Winslow and Insel Citation2004). Studies in humans have demonstrated that intranasal oxytocin increases interpersonal trust (Kosfeld et al. Citation2005; Baumgartner et al. Citation2008; Theodoridou et al. Citation2009) and increases positive communication during couple conflict (Ditzen et al. Citation2009). Moreover, oxytocin is reported to enhance the encoding of positive social memories (Guastella et al. Citation2008; Unkelbach et al. Citation2008), facilitate the recognition of positive facial expressions (Marsh et al. Citation2010) and improve the ability to infer the mental states of others (Domes et al. Citation2007). These lines of research underscore the importance of oxytocinergic activity in promoting pro-social behaviour.
Considering that positive social interactions attenuate subsequent physiological responses to psychosocial stressors (Heffner et al. Citation2004; Ditzen et al. Citation2007), oxytocin is proposed to comprise regulatory mechanism underlying this effect. In support of this theory, oxytocin impacts on fear conditioning and extinction in rodents (McCarthy et al. Citation1996) and attenuates HPA reactivity to prolonged social isolation in squirrel monkeys (Parker et al. Citation2005). Oxytocin also exerts anxiolytic effects in humans, by dampening activation of the amygdala in response to both threatening and positive facial stimuli (Kirsch et al. Citation2005; Domes et al. Citation2007), as well as aversely conditioned emotional responses to social stimuli (Petrovic et al. Citation2008). Few studies have examined the effects of oxytocin on the physiological stress response to interpersonal challenge. Heinrichs et al. (Citation2003) examined the effect of intranasal oxytocin on the cortisol response to the Trier Social Stress Test (TSST), an achievement-related stressor consisting of a public speech task and mental arithmetic (Heinrichs et al. Citation2003). Male participants were randomly assigned to receive intranasal oxytocin or a placebo before the TSST, with either social support from their best friend during the speech preparation period or no social support. Results of the study revealed that social support and oxytocin interacted to suppress the cortisol response to the TSST. Interestingly, the direct effect of oxytocin (i.e. in the absence of social support) on cortisol reactivity was marginal, which may be related to the fact that the study was conducted solely in male participants using a social-evaluative paradigm characterized by highly structured interactions. Recently, intranasal oxytocin was found to increase positive communication and reduce cortisol levels during a couple conflict task (Ditzen et al. Citation2009). Although these findings provide evidence that oxytocin may attenuate the biological response to interpersonal stress, the conflict was not considered to be subjectively stressful by the participants and there were no changes in cortisol level in response to the task.
The aim of the present study was to assess the effect of oxytocin on the cortisol and affective responses to a validated interpersonal stressor in both male and female participants. Because most oxytocin administration studies have been conducted on male samples, it is imperative that similar research be conducted in women, especially in light of recent evidence suggesting that the effects of oxytocin on fear-related information processing may differ in women relative to men (Domes et al. Citation2010). We used the Yale Interpersonal Stressor (YIPS), a live social rejection paradigm known to stimulate negative mood change and activate the HPA axis (Stroud et al. Citation2000). The YIPS involves two interactions with two same-sexed confederates posed as undergraduate students, in which the participant is made to feel excluded and isolated. In contrast to the TSST, the cortisol response to the YIPS has been reported to be greater in female than in male participants, suggesting that women are more biologically reactive to interpersonal stress than men (Stroud et al. Citation2002). To the authors' knowledge, the reported sex difference in the cortisol response to interpersonal stress has never been replicated. Therefore, a second goal of the present study aimed to replicate this important finding.
In line with the studies conducted by Stroud et al. (Citation2000, Citation2002), we predicted that the YIPS would induce significant negative mood change and increase cortisol levels relative to baseline, and that these changes would be greater among female than male participants. Moreover, we hypothesized that the participants randomly assigned to receive oxytocin would report significantly less negative mood change and have significantly lower mean cortisol levels following the YIPS than the participants in the placebo group. Given the paucity of oxytocin administration studies in women, we had no specific hypotheses regarding sex differences in effects of oxytocin on stress reactivity.
Methods
Participants
One hundred undergraduate students, between the age of 18 and 35 years, were recruited to participate in the study. To recruit students, advertisements were placed in the classified advertisements posted by McGill and Concordia Universities (Montreal, Canada) and fliers were posted on both campuses. Students were also recruited through classroom visits at both universities. Students were excluded from participation if they smoked, consumed legal or illegal drugs, were not fluent English speakers, were currently ill, were suffering from either a chronic medical condition or major sensory impairment, or if they were ever diagnosed with a mental disorder. Females were excluded from participation if they were pregnant or believed they could be pregnant.
Of the 100 students who participated in the study, one provided incomplete mood ratings during the study, and three had missing cortisol data. As such, the final sample consisted of 96 participants. Forty-seven participants (24 females/23 males) were administered oxytocin and 49 were administered the placebo (24 females/25 males).
Yale Interpersonal Stressor
In accordance with the paradigm, students were informed that they were participating in a study assessing communication skills, and were asked to engage in two separate conversations with two same-sex confederates posed as fellow undergraduate students. The confederates then proceeded to gradually exclude the participant from each conversation through a series of verbal techniques and non-verbal cues. In the present study, confederates excluded participants in two 10-min conversations. Both conversations were video recorded and observed through a one-way mirror.
In the first conversation, participants and confederates were asked to introduce themselves and then to discuss ‘the advantages and disadvantages of living in Montreal’. In the first 2 min, confederates were polite in their conversation with the participant. However, as the conversation progressed, confederates began to engage with one another more than with the participant. At 3–5 min, confederates would ask each other more questions and would verbally agree with each other more than with the participant. They would also make greater eye contact with each other and smile and nod at one another more than at the participant. At 6–8 min, confederates behaved in a dismissive manner towards the participant. They would interrupt the participant, disagree with him/her and/or respond indifferently before resuming their own conversation. Confederates continued to interact positively with one another, using both verbal and non-verbal positive cues. For example, if the participant reported that she ‘really liked the parks in Montreal’, the confederate would reply ‘that's nice’ in an uninterested tone, and then continue conversing with the other confederate. In the last 2 min of the conversation, confederates ignored the participant completely. For the second conversation, participants and confederates discussed their hobbies.
Confederates were undergraduate volunteers in the laboratory and were previously unknown to participants. Twelve confederates (three males and nine females) were used in the study. All confederates took part in 6 h of training (three sessions) on the YIPS procedures. Each experimental session was observed by one of the investigators (via a one-way mirror), and confederates received feedback on their performance immediately after each session. One-hour review sessions were also performed throughout the study to maintain adherence to the YIPS protocol.
Materials
Medical history questionnaire
An in-house self-report questionnaire on participants' medical history was developed to assess past and current drug use, present medical problems, current medications, significant past illnesses and drug reactions/allergies. In females, the questionnaire was also used to assess phase of the menstrual cycle, oral contraceptive use and possible pregnancy.
Profile of mood states
The bipolar form of the profile of mood states (POMS) is a 72-item, self-report questionnaire that was designed to measure six bipolar mood states. Each mood state corresponds to a scale composed of 12 adjectives. Participants rate how they currently feel on a four-point Likert scale ranging from ‘much unlike this’ to ‘very much like this’. The POMS-bi scales are composed–anxious, agreeable–hostile, elated–depressed, confident–unsure, energetic–tired and clearheaded–confused. For each scale, a low score reflects high ratings of negative affect and low ratings of positive affect. A high score reflects the opposite. The POMS takes approximately 10–15 min to complete. The authors report good test–retest reliability and construct validity (Lorr and McNair Citation1988), and it is sensitive to mild changes in mood state (Ellenbogen et al. Citation1996).
Bogus social perceptions questionnaire
The Bogus social perceptions questionnaire (BSPQ) is a 12-item, in-house inventory to assess participants' perceptions of the two confederates they interacted with. It was based on the inventory used by Stroud et al. (Citation2000). Nine items were measured on a five-point Likert scale (i.e. ‘this person seems friendly’, ‘this person is interesting to talk to’, ‘this person seems extroverted’). Three items required a yes or no response (i.e. ‘I would prefer to keep this person as an acquaintance, as opposed to a friend’, ‘I would like to know this person better’, ‘this is the type of person I might include within my social circle’). Participants completed the BSPQ after each conversation and for each of the two confederates. The use of the BSPQ helped maintain the deception of participants, who believed they were assessing the communication skills of fellow participants. Moreover, it was used as a manipulation check to ascertain that the participants felt excluded by the confederates.
Salivary cortisol sampling
Saliva was expressed directly into polypropylene 6-ml vials. Samples were frozen at − 20°C until assayed for cortisol using a sensitive commercial enzyme immunoassay kit from Salimetrics (State College, PA, USA; Schwartz et al. Citation1998). The sensitivity of the assay was set at 0.012 μg/dl. The inter- and intra-assay coefficient of variation for the assays were 2.8% and 4.6% (on a range of 0.01–10 μg/dl dose), respectively. Samples were centrifuged at 1612 × g for 10 min to separate debris from saliva. Assays were conducted in the laboratory of Dr C.-D. Walker at the Douglas Hospital Research Centre (Montreal, Canada).
Procedure
Undergraduate students interested in participating in a study on ‘communication skills’ were initially screened by telephone or by email. Individuals meeting inclusion criteria were scheduled to arrive at the laboratory at either 12:00 h or 14:45 h to control for diurnal variations in cortisol secretion. Participants were asked to refrain from eating, drinking (except water) or exercising 1 h prior to the arrival at the laboratory. Upon arrival, one confederate was already in the laboratory and the other was cued (by cellular phone) to arrive several minutes after the participant. The participant and confederates were informed that they would be placed in separate rooms to complete questionnaires and undergo a relaxation phase. Each participant provided written informed consent and completed a medical history questionnaire. Eligible participants provided a first saliva sample and completed the bipolar form of the POMS questionnaire. In a double-blinded, randomized-controlled design, each participant was administered either a single intranasal dose of 24 IU oxytocin (three puffs per nostril; Syntocinon Spray, Novartis, Basel, Switzerland) or placebo (0.9% saline), via a nasal spray. Each participant then underwent a 45-min relaxation phase, which involved sitting on a reclining chair and reading magazines or books and/or listening to music. A second POMS questionnaire was completed following the relaxation phase. Afterwards, the participant was brought to a common room and underwent the YIPS (see above). The experimenter informed the participant and confederates that they would be engaging in the first of two 10-min conversations aimed at assessing communication skills in small groups. They were informed that their conversations would be recorded and observed live through a one-way mirror. At the end of the first conversation, the participant and confederates were returned to separate rooms, and the participant provided a second saliva sample and completed a third POMS questionnaire as well as the BSPQ for each of the two confederates. Next, participants took part in the second conversation, provided a third saliva sample and completed the POMS and BSPQ. Afterwards, participants provided saliva samples every 10 min during a 30-minute recovery period, for a total of six samples. Participants were fully debriefed and remunerated $50 for the time spent at the laboratory. All procedures were approved by Concordia University's Human Research Ethic Committee.
Data analysis
On the bipolar form of the POMS questionnaire, data from the six scales were summed to create a score for total mood change for each participant. A lower total mood score reflected higher ratings of negative affect and lower ratings of positive affect. Cronbach's α for the total mood scale were the following at each time point throughout the YIPS: α = 0.83 (arrival at laboratory), α = 0.85 (after relaxation phase), α = 0.86 (post-1st YIPS conversation) and α = 0.87 (post-2nd YIPS conversation). To assess changes in mood over time, a 2 (sex) × 2 (drug condition) × 4 (time) repeated measures analysis of variance (ANOVA) was conducted on total mood scores. Chi-square analyses were performed on the following three items of the BSPQ: ‘I would prefer to keep this person as an acquaintance, as opposed to a friend’, ‘I would like to know this person better’, ‘This is the type of person I might include within my social circle’, and were used to determine if the frequency of participants who reported negative perceptions of the confederates differed significantly from what was expected by chance. In order to determine if perceptions of confederates varied by sex and/or drug condition, both variables were entered in the chi-square analysis.
For the cortisol data, a paired sample t-test revealed that cortisol concentrations were significantly higher upon laboratory arrival than after the first YIPS conversation, t(95) = 4.56, p < 0.05. Because the first sample tends to be subject to outside influences (i.e. driving, public transport, novelty of the situation), it was excluded from the data analyses. Cortisol changes over time were analysed with a 2 (sex) × 2 (drug condition) × 5 (time) repeated measures analysis of covariance (ANCOVA), controlling for time of testing (12:00 h or 14:45 h). All within-subject effects were Greenhouse–Geisser corrected for violations of sphericity.
Results
Validity of the interpersonal stressor
The BSPQ was administered to assess the effectiveness of the interpersonal manipulation. Following the first (second) conversation, 73% (76%) of participants reported that they had no interest in getting to know at least one of the confederates better, X2(1, N = 96) = 20.17, p < 0.05 (first conversation); X2(1, N = 96) = 26.04, p < 0.05 (second conversation), for both confederates pooled. Eighty-six percent (90%) reported that they would rather keep at least one of the confederates as an acquaintance as opposed to a friend, X2(1, N = 96) = 51.04, p < 0.05 (first conversation); X2(1, N = 96) = 60.17, p < 0.05 (second conversation), and 82% (83%) indicated that they would not include at least one of the confederates in their social circle, X2(1, N = 96) = 40.04 (first conversation), p < 0.05; X2(1, N = 96) = 42.67, p < 0.05 (second conversation). As expected, participants did not relate well to the confederates and were reluctant to get to know them better. These data support the effectiveness of the YIPS in eliciting mild social rejection. Following the first conversation, males (98%), relative to females (76%), were significantly more likely to report that they had no interest in getting to know at least one of the confederates better X2(1, N = 96) = 10.25, p < 0.05. No other sex differences were observed, and there were no significant differences by drug condition. In short, the drug manipulation did not alter how participants perceived the confederates following both YIPS conversation.
The affective response to the YIPS
The sex × drug × time repeated measures ANOVA revealed that mood ratings varied significantly across time, F(3, 276) = 21.23, p < 0.05, η2 = 0.19, and that sex was a significant predictor of total mood ratings across time, F (3, 276) = 6.66, p < 0.05, η2 = 0.07 (). No significant main effect of drug condition, or sex × drug interaction, was found. Irrespective of sex, participants who were administered oxytocin reported comparable mood ratings following the YIPS to participants who were administered the placebo.
Figure 1. Mean total mood ratings+SEM, in participants administered either oxytocin (24 females/23 males) or placebo (24 females/25 males) at four separate intervals throughout the YIPS. Total mood ratings were calculated by summing the scores of each scale of the bipolar form of the POMS questionnaire. Participants initially provided mood ratings on the POMS questionnaire and were then administered either a single dose of 24 IU oxytocin or placebo. They completed a POMS questionnaire following a 50-min relaxation phase, and then, after each 10-min YIPS conversation. A sex × drug × time repeated measures ANOVA revealed a statistically significant decrease in total mood ratings following the two YIPS conversations p < 0.05, which was more pronounced in females than in males p < 0.05. Drug did not significantly predict mood ratings across time.
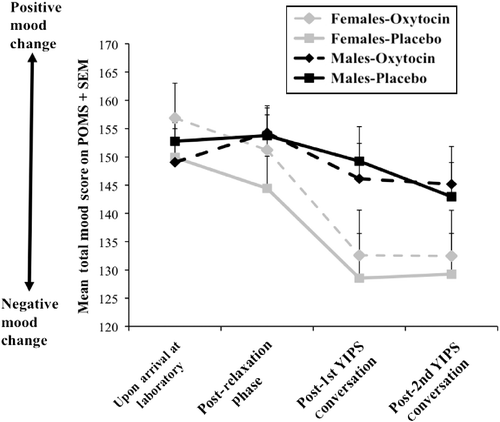
To follow up the main effect of time, Bonferroni corrected paired-sample t-tests, using an adjusted p value < 0.013, were performed between the two baseline measures and the two post-YIPS measures. Relative to baseline, mood ratings decreased significantly following the first, t(95) = 4.42, p < 0.013, d = 0.47, and second YIPS conversation, t(95) = 5.31, p < 0.013, d = 0.60. The decrease in mood ratings following each conversation was also observed with respect to mood ratings reported after the 50-min relaxation period, t(95) = 4.92, p < 0.013, d = 0.54 (first conversation); t(95) = 5.41, p < 0.013, d = 0.58 (second conversation). No other significant differences were obtained.
Secondary analyses were conducted to identify the individual mood scales that were most sensitive to the YIPS. A sex × drug × time repeated measures ANOVA was performed on individual mood scales of the POMS. Mood ratings varied significantly across time on all scales including, in the order of effect size, the agreeable–hostile scale, F (2.07, 190.11) = 44.96, p < 0.05, η2 = 0.33; elated–depressed scale, F (2.40, 219.90) = 34.01, p < 0.05, η2 = 0.27; composed–anxiety scale, F (2.33, 217.00) = 26.47, p < 0.05, η2 = 0.22; energetic–tired scale, F (2.35, 216.13) = 6.55, p < 0.05, η2 = 0.07; confident–unsure scale, F (2.40, 221.00) = 6.17, p < 0.05, η2 = 0.06 and clearheaded–confused scale, F (2.40, 220.93) = 3.86, p < 0.05, η2 = 0.04. For all scales, mood ratings became more negative following the first and second conversations relative to baseline (data not shown). Thus, mood change during the YIPS occurred across all POMS scales.
The main effect of sex was followed up with a one-way ANOVA on total mood ratings at each individual time point. Females reported significantly lower mood ratings than males after the first YIPS conversation, F (1, 94) = 5.93, p < 0.05, d = 0.50, and marginally lower mood ratings than males after the second YIPS conversation, F (1, 94) = 3.57, p>0.05, d = 0.39, although the latter fell short of statistical significance. There were no significant sex differences in the mood ratings at either of the first two time points.
Sex differences in mood ratings across time were found on the following individual scales, in the order of effect size: energetic–tired scale, F (2.35, 216.13) = 5.51, p < 0.05, η2 = 0.06 (data not shown); composed–anxiety scale, F (2.33, 217.00) = 4.64, p < 0.05, η2 = 0.05; confident–unsure scale, F (2.40, 221.00) = 4.53, p < 0.05, η2 = 0.05 and elated–depressed scale, F (2.40, 219.90) = 3.63, p < 0.05, η2 = 0.04. Drug condition did not significantly predict mood ratings across time on any of the POMS scales. Thus, sex differences in the mood response during the YIPS occurred primarily for anxious, depressed, tired and unsure mood states.
In sum, the YIPS elicited robust negative mood change following both conversations and across all POMS scales, and mood change was more pronounced among females than males. Contrary to expectation, oxytocin did not modulate the mood response to the YIPS in either males or females.
Salivary cortisol response to the YIPS
Time of day (12:00 h vs. 14:45 h start time) effects on drug administration and salivary cortisol concentrations were examined, but none were found (data not shown). The sex × drug × time repeated measures ANCOVA, controlling for time of testing, revealed that cortisol concentrations did not vary significantly across time, F (4, 364) = 0.63, p>0.64, η2 = 0.01, and there were no significant main effect of sex, F (1, 91) = 2.97, p>0.05, η2 = 0.03, or sex × time interaction, F (4, 364) = 0.72, p>0.05, η2 = 0.01 (). In short, the YIPS failed to elicit a significant cortisol response, regardless of sex.
Table I. Mean cortisol levels (μg/dl), by gender and drug condition, across five time points throughout the YIPS.
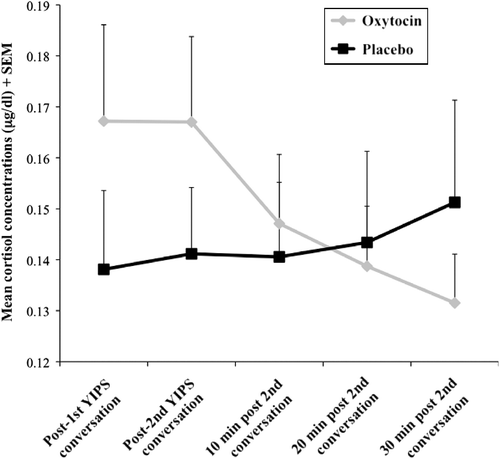
The repeated measures ANCOVA revealed a significant drug × time interaction, F (4, 364) = 2.70, p < 0.05, η2 = 0.03. Because participants receiving oxytocin unexpectedly displayed higher salivary cortisol concentrations at baseline (following first YIPS conversation; M = 0.1724, SD = 0.129) than participants receiving the placebo (M = 0.148, SD = 0.10), t(95) = 1.20, p>0.05), baseline cortisol concentrations were added as an additional covariate in these analyses. The drug × time interaction remained statistically significant after controlling for baseline cortisol concentration, F (3, 273) = 3.15, p < 0.05, η2 = 0.03 (). A follow-up repeated measures ANOVA was performed to assess variations in cortisol concentration over time in each separate drug condition. Participants in the oxytocin condition displayed a statistically significant decrease in cortisol concentrations over time, F (4, 184) = 4.50, p < 0.05, η2 = 0.09, whereas participants in the placebo condition exhibited no change in cortisol over time, F (4, 192) = 0.29, p>0.05, η2 = 0.00 (). In sum, cortisol concentrations did not increase in response to the YIPS, either in male or in female participants. Participants administered oxytocin displayed a significant decrease, relative to placebo, in cortisol concentrations throughout the YIPS. However, participants administered placebo, relative to oxytocin, displayed cortisol levels following the YIPS that were virtually unchanged from baseline.
Figure 2. Mean cortisol levels (μg/dl) + SEM, in participants receiving oxytocin (n = 47) or placebo (n = 49), and across five time points throughout the YIPS. Participants were administered either a single dose of 24 IU oxytocin or placebo upon arrival at the laboratory and then underwent a 50-min relaxation phase. They provided a cortisol sample immediately after the first and second 10-min YIPS conversations and then at three 10-min intervals following the second YIPS conversation. A sex × drug × time repeated measure ANCOVA (controlling for first cortisol measure) indicated that participants in the oxytocin condition displayed a decrease in cortisol over time, whereas participants in the placebo condition exhibited no change in cortisol concentrations over time p < 0.05.
Secondary analyses controlling for phase of menstrual cycle and oral contraceptive use
In female participants, self-reported phase of menstrual cycle (luteal or follicular) and oral contraceptive use (yes/no) were entered as covariates in drug × time repeated measures ANCOVAs conducted separately for mood ratings and cortisol concentrations. Because three females failed to report information on their menstrual cycle or oral contraceptive use on the medical history questionnaire, these data were analysed on 45 females. Twenty-two females were in the follicular phase, and 23 were in the luteal phase. Fifteen females reported using the oral contraceptive pill and 30 females reported that they did not. Neither phase of menstrual cycle nor oral contraceptive use was significantly associated with mood ratings or cortisol variations over time (data not shown).
Discussion
The present study examined the effect of intranasal oxytocin on the affective and cortisol response to the YIPS, a social rejection paradigm aimed at eliciting interpersonal stress. As expected, the YIPS elicited a robust lowering of mood in the full study sample, and the negative mood response was greater in women than in men. Moreover, we assessed the validity of the social rejection manipulation by having participants rate the likeableness of the study confederates. As expected, 80% or more of the study participants reported negative ratings of the confederates. Both of these findings attest to the effectiveness of the interpersonal stress manipulation. In contrast to its robust effects on social and emotional measures, the YIPS did not elicit an increase in cortisol concentrations.
We had hypothesized that the participants receiving oxytocin would report significantly less negative mood change and have significantly lower mean cortisol levels following the YIPS than participants in the placebo group. Contrary to the hypothesis, oxytocin did not influence the mood response on the POMS or any of its subscales. With one exception (Heinrichs et al. Citation2003), most studies have failed to find an effect of oxytocin on self-report ratings of emotion (Kosfeld et al. Citation2005; Buchheim et al. Citation2009; Di Simplicio et al. Citation2009). Each of the above studies investigated a single dose of 24 IU of oxytocin, indicating that dosage effects cannot account for the inconsistencies between studies. Despite the negative findings in this study, it is possible that the effects of oxytocin on mood are limited to participants who are vulnerable to mood change (Buchheim et al. Citation2009; CitationCardoso et al. in press). These types of interactions are rarely assessed in studies of exogenous oxytocin, and therefore warrant further research.
In accordance with our hypothesis on HPA functioning, intranasal oxytocin was associated with decreasing cortisol concentrations during the YIPS as compared with the placebo. The findings are consistent with other laboratory stress induction methods (Heinrichs et al. Citation2003), and suggest that oxytocin dampens the functioning of the HPA axis during different kinds of psychological challenge. The stress-attenuating effects of oxytocin may serve as a mechanism to facilitate pro-social behaviour (Taylor Citation2006; Heinrichs et al. Citation2009). It will be important to determine if oxytocin attenuates HPA reactivity to non-psychological challenges (e.g. physical stress), and to ascertain whether oxytocin actually reaches the CNS (and if so, how). Although passage of oxytocin from nose to brain is likely through extraneuronal intercellular clefts in the olfactory epithelium, the exact process is not well delineated (Born et al. Citation2002).
A second goal of the current study was to replicate previous findings reported by Stroud et al. (Citation2000, Citation2002), which demonstrated that the YIPS induced higher cortisol concentrations among female compared with male participants. This finding was particularly influential in the literature, as it suggested that women were biologically more sensitive to interpersonal stress than men, with implications for understanding the sex difference in prevalence rates of depression (Young and Korszum Citation1999; Hyde et al. Citation2008). The present study failed to replicate the sex difference in the cortisol response to interpersonal stress. To our knowledge, no published study has directly replicated the result. In a study conducted by Zwolinkski (Citation2008), only females in the luteal phase of their menstrual cycle, and with a history of high relational victimization, displayed a significant cortisol response to a modified version of the YIPS. The lack of observed sex differences in HPA axis reactivity to laboratory stress is consistent with several previous studies that used other types of stress induction (Stoney et al. Citation1987; Kirschbaum et al. Citation1999; Kelly et al. Citation2008).
Although no sex difference in salivary cortisol concentrations was found, female participants reported greater negative mood change in response to the YIPS than male participants. These findings are consistent with research demonstrating that females display more negative emotional reactions to interpersonal stress in the natural environment than males (Rudolph Citation2002; Hankin et al. Citation2007). Interestingly, Stroud et al. (Citation2000, Citation2002) observed no sex differences in affected ratings over time. The discrepancy in findings may be due to the fact that Stroud et al. (Citation2000, Citation2002) employed visual analogue scales to measure effect, whereas the present study relied on the POMS. The original POMS questionnaire has been identified as a more sensitive measure of mood change than visual analogue scales (Little and Penman Citation1989). Alternatively, the sample size in the present study (n = 96) was larger than the samples in the studies by Stroud et al. (Citation2000, Citation2002; n = 25; n = 50, respectively), allowing for more power to detect group differences. It is possible that this sex difference was related to perceptions of confederates. Male and female participants were exposed to different sets of same-sex confederates, which may have contributed to sex differences in the mood response to the YIPS. Males reported a greater disinterest in the confederates than females. Perhaps males engaged in external attributions of confederates (i.e. ‘the confederates are jerks’), whereas females engaged in internal attributions of confederates (i.e. ‘the confederates do not like me’). If so, females would be expected to be more sensitive to the rejection of confederates and to report a greater negative mood response to the YIPS.
A number of study limitations warrant discussion. First, the YIPS failed to elicit a significant cortisol response, and therefore the present study may relate to daytime cortisol levels but cannot readily be interpreted as demonstrating an effect of oxytocin on biological stress reactivity per se. Second, although participants were randomly assigned to receive oxytocin or placebo, a non-significant group difference in salivary cortisol concentration was observed at baseline. Therefore, it is possible that the observed decrease in cortisol over time, in participants who were administered oxytocin, reflected a regression towards the mean as opposed to an actual attenuation of HPA axis activity. We addressed this possibility by statistically controlling for the first cortisol measure and found that the drug by time interaction remained statistically significant. Third, the HPA axis was the only physiological system that was investigated in the present study, and it is possible that the YIPS activated other stress-related physiological systems. Stroud et al. (Citation2000) had observed a significant increase in systolic and diastolic blood pressure in response to the YIPS. Although oxytocin has previously been shown to dampen sympathetic nervous system activity in rats (Holst et al. Citation2002), it was recently found to have no effect on blood pressure or heart rate measures during a public speech task (Citationde Oliveira et al. in press).
Fourth, our failure to replicate the biological sex difference in cortisol reactivity to interpersonal stress reported by Stroud et al. (Citation2000, Citation2002) may be attributed to the methodological differences between studies, such as the administration of oxytocin. Because oxytocin attenuated cortisol levels, separate analyses were performed in the placebo group (n = 49), but this revealed no significant sex differences in cortisol reactivity to the YIPS. Several procedures were implemented to replicate the YIPS, as reported by Stroud et al. (Citation2000, Citation2002). Confederates received extensive training on the YIPS. The vast majority of participants (93%) reported that the confederates had deceived them. Because each conversation was observed live through a one-way mirror, confederates were given regular feedback on their performance during and after sessions to prevent deviations from the YIPS protocol over time. Overall, the ratings on the BSPQ were comparable to those reported by Stroud et al. (Citation2000, Citation2002) and supported the efficacy of the paradigm in eliciting mild social rejection.
Fifth, the phase of menstrual cycle varied among female participants, and participants using oral contraceptives were included in the study. Statistical analysis revealed that menstrual cycle phase had no significant influence on mood ratings or cortisol concentrations over time. However, menstrual cycle phase data were based on self-report and may have been inaccurate. It is possible that levels of circulating oestrogen modulate the effects of oxytocin on behaviour and physiology (Gimpl and Fahrenholz Citation2001). Finally, the placebo nasal spray used in the control condition contained a saline solution without the inactive compounds present in the oxytocin solution. Thus, it is possible that some of the findings observed in the present study could be due to the effects of a non-active compound administered with oxytocin.
In conclusion, women reported a greater increase in negative affect in response to an interpersonal stressor than men. Although women may be more emotionally responsive to interpersonal rejection than men, they exhibited no evidence of heightened adrenocortical reactivity to interpersonal stress, in contrast to what was previously reported by Stroud et al. (Citation2002). In addition, the results of the present study indicate that oxytocin attenuates HPA axis functioning during an interpersonal challenge. These results support the mediating role of oxytocin in the stress-protective effects of positive social interactions. In view of the finding that positive social relationships protect against stress-related disease (Uchino et al. Citation1999), results of the study reveal important implications for better understanding the neurobiology that regulates this phenomenon.
Acknowledgements
Over the course of the research project, Anne-Marie Linnen was supported by a scholarship from les Fonds québécois de la recherche sur la nature et les technologies (FQRNT). Dr Ellenbogen is currently supported by a Canada Research Chair appointment from the Social Sciences and Humanities Research Council of Canada. We thank Claire-Dominique Walker at the Douglas Hospital Research Laboratories for conducting the cortisol assays. We thank Pfeiffer of America, and in particular Danielle Petrow, for generously supplying us with nasal spray bottles and pumps. We thank members of the Stress and Developmental Psychopathology Laboratory at Concordia University, supervised by Dr Ellenbogen, for their help on this research project. We would also like to thank Dustin Washburn, Cory Cooperman, Janet Kaldas, Brigitte Hanna and Anne-Sophie Ouellette for their work on this research project.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. 2008. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 58:639–650.
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. 2002. Sniffing neuropeptides: A transnasal approach to the human brain. Nat Neurosci. 5:514–516.
- Buchheim A, Heinrichs M, George C, Pokorny D, Koops E, Henningsen P, O'Connor M-F, Gnndel H. 2009. Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology. 34:1417–1422.
- Cardoso C, Linnen A-M, Joober R, Ellenbogen MA. Coping style moderates the effect of intranasal oxytocin on the mood response to interpersonal stress. Exp Clin Psychopharmacol in press.
- Daley SE, Hammen C, Davila J, Burge D. 1998. Axis II symptomatology, depression, and life stress during the transition from adolescence to adulthood. J Consult Clin Psychol. 66:595–603.
- Decker SA. 2000. Salivary cortisol and social status among Dominican men. Horm Behav. 38:29–38.
- Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ. 2009. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. J Psychopharmacol. 23:241–248.
- Ditzen B, Neumann ID, Bodenmann G, von Dawans B, Turner RA, Ehlert U, Heinrichs M. 2007. Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology. 32:565–574.
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. 2009. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiat. 65:728–731.
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. 2007. Oxytocin improves ‘mind-reading’ in humans. Biol Psychiat. 61:731–733.
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. 2010. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 35:83–93.
- Ellenbogen MA, Young SN, Dean P, Palmour RM, Benkelfat C. 1996. Mood response to acute tryptophan depletion in healthy volunteers: Sex differences and temporal stability. Neuropsychopharmacology. 15:465–474.
- Gimpl G, Fahrenholz F. 2001. The oxytocin receptor system: Structure, function and regulation. Physiol Rev. 81:629–683.
- Guastella AJ, Mitchell PB, Mathews F. 2008. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiat. 64:256–258.
- Hammen C. 2003. Interpersonal stress and depression in women. J Affect Dis. 74:49–57.
- Hankin BL, Mermelstein R, Roesch L. 2007. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Dev. 78:279–295.
- Heffner KL, Kiecolt-Glaser JK, Loving TJ, Glaser R, Malarkey WB. 2004. Spousal support satisfaction as a modifier of physiological responses to marital conflict in younger and older couples. J Behav Med. 27:233–254.
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. 2003. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiat. 54:1389–1398.
- Heinrichs M, von Dawans B, Domes G. 2009. Oxytocin, vasopressin, and human social behavior: Hormones & Social Behavior. Front Neuroendocrinol. 30:548–557.
- Holst S, Uvnas-Moberg K, Petersson M. 2002. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Auton Neurosci. 99:85–90.
- Hyde JS, Mezulis AH, Abramson LY. 2008. The ABCs of depression: Integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. 115:291–313.
- Insel TR, Hulihan TJ. 1995. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 109:782–789.
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. 2008. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psychiat. 39:87–98.
- Kiecolt-Glaser JK, Malarkey WB, Cacioppo JT, Glaser R. 1994. Stressful personal relationships: Immune and endocrine function. In: Glaser R, Kiecolt-Glaser JK, Glaser R, Kiecolt-Glaser JK. editors. Handbook of human stress and immunity. San Diego, CA: Academic Press321–339.
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. 2005. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 25:11489–11493.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamic–pituitary–adrenal axis. Psychosom Med. 61:154–162.
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. 2005. Oxytocin increases trust in humans. Nature. 435:673–676.
- Little K, Penman E. 1989. Measuring subacute mood changes using the profile of mood states and visual analogue scales. Psychopathology. 22:42–49.
- Lorr M, McNair DM. 1988. Manual: Profile of mood states: Bipolar form. Educational and Industrial Testing ServiceSan Diego, CA.
- Marsh AA, Yu HH, Pine DS, Blair RJR. 2010. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology. 209:225–232.
- McCarthy M, McDonald CH, Brooks PJ, Goldman D. 1996. An anxioyltic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 60:1209–1215.
- de Oliveira DC, Zuardi AW, Graeff FG, Queiroz RH, Crippa JA. Anxiolytic-like effect of oxytocin in the simulated public speaking test. J Psychopharmacol in press.
- Orth-Gomer K, Leineweber C. 2005. Multiple stressors and coronary disease in women. The stockholm female coronary risk study. Biol Psychol. 69:57–66.
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. 2005. Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology. 30:924–929.
- Pedersen CA. 1997. Oxytocin control of maternal behavior: Regulation by sex steroids and offspring stimuli. In: Carter CS, Lederhendler II, Kirkpatrick B. editors. The integrative neurobiology of affiliation. New York: New York Academy of Sciences126–145.
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. 1994. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 108:1163–1171.
- Petrovic P, Kalisch R, Singer T, Dolan RJ. 2008. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 28:6607–6615.
- Rudolph KD. 2002. Gender differences in emotional responses to interpersonal stress during adolescence. J Adolesc Health. 30:3–13.
- Sapolsky RM. 2004. Social status and health in human and other animals. Annu Rev Anthropol. 33:393–418.
- Schwartz EP, Granger DA, Susman EJ, Gunnar MR, Laird B. 1998. Assessing salivary cortisol in studies of child development. Child Dev. 69:1503–1513.
- Steptoe A. 1991. The links between stress and illness. J Psychosom Res. 35:633–644.
- Stoney CM, Davis MC, Matthews KA. 1987. Sex differences in physiological responses to stress and in coronary heart disease: A causal link?. Psychophysiology. 24:127–131.
- Stroud LR, Tanofsky-Kraff M, Wilfley DE, Salovey P. 2000. The Yale Interpersonal Stressor (YIPS): Affective, physiological, and behavioral responses to a novel interpersonal rejection paradigm. Ann Behav Med. 22:204–213.
- Stroud LR, Salovey P, Epel ES. 2002. Sex differences in stress responses: Social rejection versus achievement stress. Biol Psychiat. 53:318–327.
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. 2009. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Horm Behav. 42:128–132.
- Taylor SE. 2006. Tend and befriend: Biobehavioral bases of affiliation under stress. Curr Dir Psychol Sci. 15:273–277.
- Uchino BN, Uno D, Holt-Lunstad J. 1999. Social support, physiological processes, and health. Curr Dir Psychol Sci. 8:145–148.
- Unkelbach C, Guastella AJ, Forgas JP. 2008. Oxytocin selectively facilitates recognition of positive sex and relationship words. Psychol Sci. 19:1092–1094.
- Williams JR, Catania KC, Carter CS. 1992. Development of partner preferences in female prairie voles (Microtus ochrogaster): The role of social and sexual experience. Horm Behav. 26:339–349.
- Winslow JT, Insel TR. 2004. Neuroendocrine basis of social recognition. Curr Opin Neurobiol. 14:248–253.
- Young E, Korszum A. 1999. Women, stress, and depression: Sex differences in hypothalamic–pituitary–adrenal axis regulation. In: Leibenluft E. editors. Gender differences in mood and anxiety disorders: From bench to bedside. Washington, DC: American Psychiatric Press31–52.
- Young LJ, Lim MM, Gingrich B, Insel TR. 2001. Cellular mechanisms of social attachment. Horm Behav. 40:133–138.
- Zwolinski J. 2008. Biopsychosocial responses to social rejection in targets of relational aggression. Biol Psychol. 79:260–267.
