Abstract
Although the relation between stressful life events (SLEs) and risk of major depressive disorder is well established, important questions remain about the effects of stress on the course of geriatric depression. Our objectives were (1) to examine how baseline stress and change in stress is associated with course of geriatric depression and (2) to test whether polymorphisms of serotonin transporter (5-HTTLPR) and catechol-O-methyltransferase (COMT Val158Met) genes moderate this relation. Two-hundred and sixteen depressed subjects aged 60 years or older were categorized by remission status (Montgomery–Asberg depression rating scale ≤ 6) at 6 and 12 months. At 6 months, greater baseline numbers of self-reported negative and total SLEs and greater baseline perceived stress severity were associated with lower odds of remission. At 12 months, only baseline perceived stress predicted remission. When we examined change in stress, 12-month decrease in negative SLEs and level of perceived stress were associated with improved odds of 12-month remission. When genotype data were included, COMT Val158Met genotype did not influence these relations. However, when compared with 5-HTTLPR L/L homozygotes, S allele carriers with greater baseline numbers of negative SLEs and with greater decrease in negative SLEs were more likely to remit at 12 months. This study demonstrates that baseline SLEs and perceived stress severity may influence the 12-month course of geriatric depression. Moreover, changes in these stress measures over time correlate with depression outcomes. 5-HTTLPR S carriers appear to be more susceptible to both the effects of enduring stress and the benefit of interval stress reduction.
Introduction
Stressful life events (SLEs) are a well-recognized risk factor for the development of an episode of major depressive disorder (MDD) among adults (Surtees et al. Citation1986; Kessler Citation1997; Kendler et al. Citation1999; Mazure et al. Citation2000; Hammen Citation2005; Risch et al. Citation2009). The risk of developing a new MDD episode increases with both greater severity and greater number of SLEs (Kendler et al. Citation1998; Hammen Citation2005; Risch et al. Citation2009), with risk being greatest shortly after the occurrence of the event and diminishing thereafter (Kendler et al. Citation1998; Tennant Citation2002). SLEs additionally predict recurrences of MDD; however, with repeated episodes less stress may be required to trigger a new depressive episode (Kendler et al. Citation2000; Hammen Citation2005). Studies focusing on late-life depression suggest that the correlation between SLEs and onset of depressive episodes remains significant in advanced age (Murphy Citation1982; Karel Citation1997; Kraaij et al. Citation2002; Blazer and Hybels Citation2005) but is overall weaker as compared with younger adults (George Citation1994; Karel Citation1997).
The relation between stress and MDD course with treatment is more controversial. Greater number of pre-treatment SLEs has been shown to predict both worse (Monroe et al. Citation1992; Mundt et al. Citation2000) and better outcomes (Monroe et al. Citation1983), or to be a nonsignificant predictor of MDD course (Lloyd et al. Citation1981; Faravelli et al. Citation1986; Cronkite et al. Citation1998; Kivela et al. Citation2000a; Bukh et al. Citation2010). Some studies found different outcomes depending on the severity (Bulmash et al. Citation2009) and type of SLEs, such as interpersonal or achievement stressors (Mazure et al. Citation2000). Similarly, studies examining SLEs occurring during the treatment and follow-up period either report nonsignificant findings (Monroe et al. Citation1983; Gonzales et al. Citation1985; Andrew et al. Citation1993; Kivela et al. Citation2000a; Schoevers et al. Citation2003) or predict worse outcomes (Lloyd et al. Citation1981; Billings and Moos Citation1985; Cronkite et al. Citation1998; Mundt et al. Citation2000; Lethbridge and Allen Citation2008; Bulmash et al. Citation2009). By comparison, chronic stressors measured at baseline, including medical illness, family conflicts, housing, and disability almost universally predict worse outcomes (Billings and Moos Citation1985; Gonzales et al. Citation1985; Swindle et al. Citation1989; Schoevers et al. Citation2003). Finally, studies in adults show that higher levels of perceived stress both at baseline and during follow-up predict poor outcomes (Candrian et al. Citation2007; Pedrelli et al. Citation2008), whereas decrease in perceived stress during antidepressant treatment correlates with MDD remission (Reno and Halaris Citation1990).
Genetic moderators of the SLE–depression relationship have also been studied extensively, but replication (Vieland Citation2001) of polymorphism by SLE interactions has been problematic. The most studied genetic locus is the 5-HTTLPR polymorphism, a common functional polymorphism in the promoter region of the serotonin transporter gene with two variants, the S (short) and the L (long) allele. The S variant was initially reported to moderate reactivity to stress and development of depression (Caspi et al. Citation2003). However, recent meta-analyses have questioned this finding (Munafo et al. Citation2009; Risch et al. Citation2009) but not without some controversy (Lotrich and Lenze Citation2009; Rieckmann et al. Citation2009; Koenen and Galea Citation2009; Kaufman et al. Citation2010). There are fewer data in elderly cohorts, but some reports have suggested that interactions between 5-HTTLPR genotype and SLEs may predict MDD onset in advanced age (Lenze et al. Citation2005; Kim et al. Citation2007). Regarding MDD course, one study found that the S allele interacts with SLEs to predict worse outcomes (Mandelli et al. Citation2009), but a more recent study found no significant interaction (Bukh et al. Citation2010). The effect of this interaction on the course of late-life depression remains unexplored.
The relation between the catechol-O-methyltransferase (COMT) Val158Met polymorphism and the response to SLEs has been less well studied. The met allele variant is associated with reduced thermostability and lower enzymatic activity, resulting in slower degradation and increased availability of catecholamines in the brain, particularly dopamine (Lotta et al. Citation1995; Lachman et al. Citation1996; Chen et al. Citation2004). In addition to its role in modulating brain regions important for cognition (Savitz et al. Citation2006) and reward (Nestler et al. Citation2002), dopamine also regulates the stress system that is reciprocally connected with the mesocorticolimbic dopaminergic systems (Chrousos Citation2009). Variations in the COMT Val158Met polymorphism moderate corticolimbic responses to emotional stimuli (Smolka et al. Citation2005; Drabant et al. Citation2006), responses to physical and emotional stressors (Zubieta et al. Citation2003; Jabbi et al. Citation2007), and depressive symptoms in children with early social deprivation (Drury et al. Citation2010). To our knowledge, however, this potential interaction between COMT Val158Met polymorphism and SLEs on MDD course has not been reported.
The present study seeks to examine self-report of SLEs and their interactions with the aforementioned genetic polymorphisms as predictors of the 6- and 12-month course of geriatric depression. We hypothesized that greater numbers of SLEs at baseline and less of a decrease in SLEs over the study period would predict lower odds of remission. Moreover, in exploratory analyses, we hypothesized that SLEs and change in SLEs over the study period would interact with the 5-HTTLPR and COMT Val158Met polymorphisms, resulting in a different effect of SLEs on course of depression based on genotype.
Materials and methods
Participants
Subjects for the present study enrolled in the National Institute of Mental Health (NIMH)-sponsored Conte Center for the Neuroscience of Depression, a prospective study at Duke University Medical Center. All participants were aged 60 years or older and at enrollment met Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria for MDD, on the basis of the NIMH diagnostic interview schedule (Robins et al. Citation1981) and confirmed by clinical interview. Exclusion criteria included (1) another major psychiatric illness, (2) history of substance abuse or dependence, and (3) primary neurologic illness, including dementia. The participants were recruited through clinical referrals, self-referral, and advertisements, and provided written informed consent prior to enrollment. The study was approved by the Duke University Medical Center Institutional Review Board. Details about the sample are described below in “Results” section and .
Table I. Demographic data of participants by remission status at 6 months.
Clinical assessments and treatment
Depression severity was assessed with the Montgomery–Asberg depression rating scale (MADRS) (Montgomery and Asberg Citation1979) at baseline and during each follow-up assessment. All participants also completed the mini-mental state examination (MMSE) (Folstein et al. Citation1975) at baseline, and individuals who scored below 25 were not included in this study. Medical illness was quantified using the cumulative illness rating scale (CIRS; Miller et al. Citation1992).
Subjects were followed for a total of 12 months. During this period they were seen at least every 3 months and treated according to the Duke somatic treatment algorithm for geriatric depression (Steffens et al. Citation2002a). This algorithm engages a stepwise medication treatment approach based on past treatments and depression severity, and has a “real world” design that allows broad use of commercially available antidepressant treatments, including lithium and electroconvulsive therapy. The majority of participants were started on sertraline on study entry; however, the antidepressant regimen differed substantially across the subject population based on medication tolerability and response. Adjustments in antidepressant medications (including switching to other antidepressants or augmentation) were allowed for subjects who did not remit with initial treatments. Psychotherapy was also an option, although not routinely recommended to all participants.
Life stress was measured at baseline and at 12 months with a self-report questionnaire (Hays et al. Citation1997). This questionnaire includes 20 items assessing a variety of SLEs that occurred over the last 12 months. These include physical illness and injury to the participant and family members, separation from a loved one, resumption of a relationship after a period of separation, divorce, death of a loved one, new family members or loss of family members, relocation, work-related difficulties, legal problems, marriage, deterioration or improvement of financial situation, employment and retirement, and others. Each item has a sub-specifier that asks whether subjects experienced each SLE as negative, positive, or neutral. SLEs with “positive” or “negative” as a sub-specifier were incorporated into the “positive stress” or “negative stress” variables, respectively. All SLEs were added to form the “total stress” variable. Additionally, there is an item for evaluation of perceived or subjective stress (labeled as “average stress” in the assessment package), which asks participants to rate, on a scale of 1–10, the severity of stress that they have experienced over the last 6 months.
Genotyping
Subjects provided peripheral blood samples for genetic testing. Genotyping for the 5-HTTLPR polymorphism was based on a previously described method (Lesch et al. Citation1996) and its modifications (Steffens et al. Citation2002b). Extraction of genomic DNA from fresh or frozen samples of peripheral blood was performed by standard procedure (Puregene D-50K DNA Isolation Kit, Gentra, Minneapolis, MN, USA). Subsequently, a 484- and a 528-bp fragments, corresponding to the short and long alleles of the polymorphism, were generated by polymerase chain reaction. The participants could be either homozygous for the long allele (L/L genotype), heterozygous (L/S genotype), or homozygous for the short allele (S/S genotype).
For COMT genotyping, extraction and storage of DNA was based on previously reported methods (Rimmler et al. Citation1998; Taylor et al. Citation2007). An aliquot of DNA was used (Lachman et al. Citation1996), and amplification was performed by TaqMan polymerase chain reaction (Applied Biosystems, Carlsbad, California, USA) that recognized the single-nucleotide polymorphism (rs4680) specific for the COMT Val158Met polymorphism. Samples were analyzed with an AB17900 DNA analyzer and the SDS software package (Applied Biosystems). The participants could be either homozygous for the Val allele (Val/Val genotype), heterozygous (Val/Met genotype), or homozygous for the Met allele (Met/Met genotype).
Genotyping efficiency above 95% was ensured for both polymorphisms before data submission for further analysis. No significant deviation from Hardy–Weinberg equilibrium was observed for either the 5-HTTLPR or COMT Val158Met polymorphism (Taylor et al. Citation2005; Potter et al. Citation2009).
Analytic strategy
Two separate analyses were conducted for the 6- and 12-month assessments. In both analyses, participants were classified into two groups based on remission status: depressed subjects who remitted (defined as MADRS ≤ 6) and subjects who did not remit. Participants were also categorized by genotype. Given the relatively small number of 5-HTTLPR S/S homozygous subjects in our sample (39 of the total 216 participants), we dichotomized subjects as either being L allele homozygous (L/L genotype) or S allele carriers (L/S or S/S genotype). In contrast, given the approximately equal frequencies of the two COMT Val158Met alleles and their co-dominant biological effect (Drabant et al. Citation2006; Drury et al. Citation2010), we examined this genotype as a three-way variable (classifying subjects as either Met/Met, Val/Met, or Val/Val).
All statistical tests were conducted using SAS (version 9.2, Cary, NC, USA) and α = 0.05. We compared the remitted and nonremitted cohorts at the 6- and 12-month assessments for differences in demographic measures and baseline stress scores, using χ2 tests for categorical measures and t-tests for continuous variables. Logistic regression models were then constructed which examined remission status (remitted/nonremitted) as the dependent variable and controlled for age, education, and baseline MADRS score, separately for the 6- and 12-month follow-up periods. These models examined baseline stress scores, including the total number of self-reported SLEs (total stress), the number of events experienced as negative (negative stress), the number of events experienced as positive (positive stress), and the level of perceived stress severity on a scale of 1–10 (average stress), as predictors of diagnostic status at these time points. In order to assess the effect size of each baseline variable that reached statistical significance, we estimated the odds ratio (OR) for being remitted for one unit increase in stress score. Subsequently, change in stress scores was estimated by subtracting the 12-month score from the respective baseline score for each patient. These change scores (defined as delta-total, delta-negative, delta-positive, and delta-average stress) were examined as independent variables in separate models, which included age, education, baseline MADRS score, and the respective baseline stress score as covariates. Finally, in models controlling for age, education, genotype, stress scores, and baseline MADRS score, we examined interaction terms, separately for each stress variable and each genotype, as predictors of remission status.
Results
Sample characteristics
At the 6-month assessment, there were 216 subjects (84 remitted and 132 nonremitted) with clinical data included in our analyses (). These cohorts did not differ significantly in demographic measures; however, total and negative stress was significantly higher in the nonremitted cohort. There was a trend for nonremitted subjects to have higher average stress but this did not reach statistical significance.
At the 12-month evaluation, there were clinical data for 212 subjects (102 remitted and 110 nonremitted, ). The two cohorts did not differ significantly in demographic measures or in measures of total, negative, and positive stress. However, the nonremitted cohort had significantly higher average stress and a lower frequency of COMT Met homozygous individuals.
Table II. Demographic data of participants by remission status at 12 months.
To better characterize the sample, we examined the overlap of the remitted and nonremitted cohorts between the two assessments. Sixty-four subjects were remitted both at 6 and 12 months and 96 were nonremitted at both assessments. In contrast, 15 subjects were remitted at 6 months but relapsed at 12 months, and 36 were nonremitted at 6 months but remitted at 12 months. One subject had 12-month but not 6-month data.
Finally, since our objective was to examine the interaction between stress measures and genotypes, we also tested the genetic groups for differences in baseline MADRS scores and stress measures. When compared with 5-HTTLPR L/L homozygotes, S allele carriers did not differ significantly in baseline MADRS (t214 = − 0.31; p = 0.7603), or baseline total (t214 = − 1.65; p = 0.1009) and positive stress (t214 = 0.10; p = 0.9216). However, S carriers had significantly higher baseline average (t211 = − 2.53; p = 0.0123) and negative stress (t214 = − 2.04; p = 0.0421). When we compared the COMT Val158Met genotype groups, we found no statistically significant differences in baseline total (f2,199 = 1.97; p = 0.1429), negative (f2,199 = 1.73; p = 0.1791), positive (f2,199 = 1.15; p = 0.3186), or average stress (f2,197 = 1.70; p = 0.1859). However, the three genotype groups differed significantly in baseline MADRS scores (f2,199 = 3.57; p = 0.0300), with the Met/Met group exhibiting lower MADRS scores than both Met/Val (p = 0.0131) and Val/Val (p = 0.0277) groups.
Baseline stress scores as predictors of 6- and 12-month remission status: direct effects and moderation by genotypes
Logistic regression models examined the relations between baseline stress scores and remission status at the 6- and 12-month assessments, while controlling for age, education, and baseline MADRS score (). In initial models, which did not include genotype data, remission status at 6 months was significantly predicted by average (OR = 1.17; 95% confidence interval (CI) = 1.02–1.35), total (OR = 1.21; 95% CI = 1.02–1.44), and negative stress (OR = 1.23; 95% CI = 1.01–1.51). At the 12-month assessment, only average stress remained a significant predictor of remission status (OR = 1.1; 95% CI = 1.04–1.37). In all cases, greater baseline stress scores were associated with lower odds of remission.
Table III. Logistic regression models examining baseline stress measures and their interactions with genotypes as predictors of 6- and 12-month remission status.
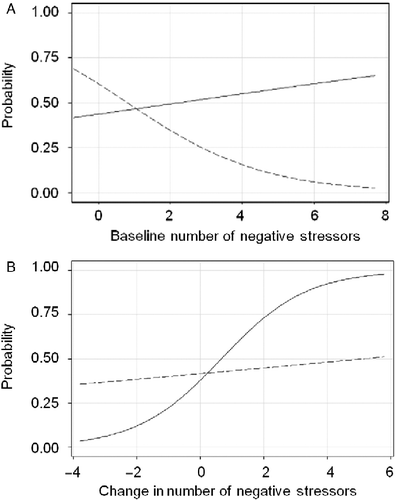
Subsequent models examined the interactions between baseline stress measures and each genotype as predictors of diagnostic status at the 6- and 12-month assessments (). After controlling for age, education, baseline MADRS score, baseline stress score, and genotype, the only interaction which attained statistical significance was the interaction between negative stress and 5-HTTLPR genotype predicting 12-month, but not 6-month, remission status. Analysis of this interaction () showed that higher baseline negative stress predicted lower odds of remission in L/L homozygotes, but slightly increased odds of remission in S allele carriers. However, as evidence of a crossover interaction between negative stress and 5-HTTLPR genotype, L/L homozygotes were also more likely to remit when there were no baseline negative events. In summary, greater numbers of negative SLEs at baseline predicted significantly worse outcomes for the L/L homozygotes, but slightly better outcomes for the S carriers at 12 months.
Figure 1. Probability of remission at the 12-month assessment by number of negative SLEs and 5-HTTLPR genotype. The solid line represents S allele carriers (L/S and S/S genotypes). The dotted line represents L/L homozygous subjects; (A) examines probability of remission with baseline number of negative stressors and (B) examines probability of remission with change in number of negative stressors.
Changes in stress scores and 12-month remission status: direct effects and moderation by genotypes
Subsequent models examined the relations between delta-stress measures and remission status. As stress scores were not obtained during the 6-month follow-up, delta-stress variables could only be estimated for the 12-month assessment. Initial models predicting remission examined change in stress measures as independent variables, while controlling for age, education, baseline MADRS scores, and baseline stress scores (). In these models, delta-average stress and delta-negative stress were significantly associated with remission status, where greater interval reductions in these stress measures correlated with increased odds of remission.
Table IV. Logistic regression models examining changes in stress scores and their interactions with genotypes as predictors of 12-month remission status.
Interaction terms between change scores and genotypes were then introduced into these models (). The only interaction that exhibited a statistically significant relation with remission status was the interaction between delta-negative stress and 5-HTTLPR genotype. Analysis of this interaction () showed that although interval reductions in negative stress correlated with greater odds of remission in both S allele carriers and L/L homozygotes, this effect was more pronounced in the S allele group. In contrast, interval increases in negative stress correlated with lower odds of remission in both genotype groups. Again this effect was greater in S carriers, demonstrating a crossover interaction between delta-negative stress and 5-HTTLPR genotype.
Discussion
In this study, we found that greater numbers of self-reported negative and total SLEs and greater perceived stress severity at baseline were associated with failure to achieve remission at 6 months, whereas perceived stress severity was the only baseline stress score that predicted 12-month remission status. Moreover, we found that interval reduction in the number of negative SLEs and the level of perceived stress during follow-up were associated with greater odds of remission at 12 months. Although COMT Val158Met genotype did not influence these relationships, 5-HTTLPR genotype did significantly interact with negative SLEs to predict the 12-month remission status. Compared with L/L homozygotes, S allele carriers were more likely to remit with greater numbers of baseline negative SLEs, and also with interval reductions in the number of negative SLEs during follow-up.
To our knowledge, this is the first study to examine measures of emotionally valenced SLEs and perceived stress severity, and their changes over time, as predictors of the course of geriatric depression. Although previous studies have shown that physical illness and functional disability at baseline (Murphy Citation1983; Baldwin and Jolley Citation1986; Schoevers et al. Citation2003) and deterioration of health status during follow-up (Murphy Citation1983; Kennedy et al. Citation1991; Kivela et al. Citation2000b) adversely affect the course of geriatric depression, studies examining SLEs more broadly have been mixed. While some have found that SLEs prior to treatment may predict better outcomes (Burvill et al. Citation1991) or those occurring during treatment may correlate with poor outcomes (Murphy Citation1983), the data are inconsistent and several studies report negative findings for SLEs at these time points (Murphy Citation1983; Burvill et al. Citation1991; Schoevers et al. Citation2003). These discrepancies might be partially accounted for by heterogeneity in stress measures and methodologies applied (Belsher and Costello Citation1988; Tennant Citation2002).
Our study did apply several approaches not widely used in the literature. Given the bidirectional relationship between stress and depression and the potential for MDD episodes to generate stress (Hammen Citation1991, Citation2005; Tennant Citation2002), baseline SLEs may confound the relationship between SLEs during the follow-up period and MDD course. Although past studies measured SLEs occurring during follow-up, this is the first study to assess changes in stress scores over time while controlling for the effects of baseline stress. Moreover, the majority of previous studies did not assess the emotional valence of events as done by our study. Finally, subjective reports of perceived stress severity, not assessed by the aforementioned studies, may have a more robust relation with MDD course than objective SLE measures (Reno and Halaris Citation1990; Mundt et al. Citation2000).
Our findings may help elucidate heterogeneity in the geriatric depression population, particularly a subtype of geriatric depression that is associated with both heightened perception of stress and failure to achieve remission. According to previous reports, certain subtypes of depression might be associated with heightened levels of perceived stress (Farabaugh et al. Citation2004), differential stress sensitivity (Harkness and Monroe Citation2006), and resistance to treatment (Ravindran et al. Citation2002). The prediction of 6- and 12-month remission status by baseline stress scores might be partially explained by the mediation of the effects of baseline stress by chronic stressors, which may occur as enduring sequelae of SLEs (Kessler Citation1997), and have been shown to predict MDD course more strongly and with a longer “at risk” period than acute stressors (McGonagle and Kessler Citation1990; Kendler et al. Citation1998; Tennant Citation2002; Hammen Citation2005). Although we did not assess for chronic stressors per se, we did find that patients who had less reduction in stress scores over the study period, and thus enduring levels of stress, had decreased odds of remission.
Interestingly, S allele carriers reported significantly higher numbers of baseline negative SLEs and greater perceived stress severity. This is consistent with previous studies showing that genetic factors may increase the risk for exposure to stressors (Kendler et al. Citation1999; Tennant Citation2002; Hammen Citation2005). In addition to this association with stress exposure and increased risk of a depressive response to stress (Caspi et al. Citation2003), our study further demonstrates that the S allele moderates the effects of stressors on the course of depression.
This interaction should be interpreted in conjunction with the main effects of SLEs. Baseline negative SLEs significantly predicted 6-month remission status for both L/L and S allele carriers, which did not differ significantly at this time point (). However, baseline negative SLEs predicted lower odds of 12-month remission only for the L/L homozygotes and the interaction term reached statistical significance at this time point (, ). Furthermore, changes in the number of negative SLEs over time correlated with more pronounced effects in remission status for S carriers, as shown by the significantly steeper slope for this group (, ). Notably, both figures show evidence of crossover interaction. When taken together, these findings are consistent with previous reports supporting a “differential susceptibility” hypothesis for the 5-HTTLPR genotype (Taylor et al. Citation2006; Belsky et al. Citation2009). Elderly L/L homozygotes may be less negatively affected by enduring levels of stress, but they are also more “rigid” to environmental changes and require more time to recover when the effects of SLEs wear off. On the other hand, S carriers may be more affected by enduring levels of SLEs, but they are also more “susceptible” to environmental changes and tend to improve faster with treatment or when the effects of SLEs cease.
The findings of our study should be viewed in the context of its limitations. First, this is a secondary data analysis; the methods used to gather our data were not developed for the purpose of this report and confounding of our results cannot be excluded. For instance, we did not control for previous history of depression and SLEs during childhood, which might be important moderators of the relations between recent SLEs and episodes of MDD (Kessler Citation1997; Kraaij and de Wilde Citation2001; Hammen Citation2005). Second, although we followed our sample prospectively and identified the subjects that remitted at different time points, our analyses did not discern between relapses and chronicity of MDD during the 1-year study period, outcomes that might be predicted by different factors (Alexopoulos et al. Citation1989). For example, Kivela et al. (Citation2000a) found that SLEs during treatment did not predict relapses of geriatric depression; however, the same authors (Kivela et al. Citation2000b) found that SLEs during treatment might predict chronicity of depression in elderly patients. Third, stress measures were gathered retrospectively and may be subject to recall bias. Although all models controlled for baseline MADRS score and thus for the level of depression concurrent with baseline stress measurements, the severity of depression at 12 months might have influenced the self-report of stress measures at that time, especially perceived stress. Consequently, it is not clear if nonremission contributed to greater perceived stress at 12 months, or if high perceived stress contributed to nonremission. Furthermore, one artifact with perceived stress measurements might have been its single-item questionnaire, which has not been previously validated. Fourth, information about specific treatments each subject received prior to enrollment and during the study period was not available. Our study engaged an algorithm-based stepwise approach, which mimics “real-world” treatment, but also results in extreme heterogeneity in antidepressant agents and doses that cannot be controlled for during analyses. Finally, although we did not perform a power analysis, we considered our sample to be underpowered to draw any conclusions regarding epistatic effects. Thus, three-way interactions between the two polymorphisms and stress measures were not examined.
Although the current results do not explain the mechanisms of the relation between stress and geriatric depression, they do suggest that measurements of emotionally valenced SLEs and perceived stress severity are important in the prediction and monitoring of the course of MDD in old age. Moreover, efforts that aim at stress reduction might be a useful adjunct in the treatment of geriatric depression, especially when targeted to “susceptible” individuals. The S allele of the 5-HTTLPR polymorphism may be one such factor of susceptibility. Although this finding remains to be replicated by larger studies, other genetic loci should also be explored.
Acknowledgements
This project was supported by NIMH grants R01 MH078216-04 and R01 MH054846-15.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alexopoulos GS, Young RC, Abrams RC, Meyers B, Shamoian CA. 1989. Chronicity and relapse in geriatric depression. Biol Psychiatry. 26 6: 551–564.
- Andrew B, Hawton K, Fagg J, Westbrook D. 1993. Do psychosocial factors influence outcome in severely depressed female psychiatric in-patients?. Br J Psychiatry. 163:747–754.
- Baldwin RC, Jolley DJ. 1986. The prognosis of depression in old age. Br J Psychiatry. 149:574–583.
- Belsher G, Costello CG. 1988. Relapse after recovery from unipolar depression: A critical review. Psychol Bull. 104 1: 84–96.
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. 2009. Vulnerability genes or susceptibility genes?. Mol Psychiatry. 14 8: 746–754.
- Billings AG, Moos RH. 1985. Life stressors and social resources affect posttreatment outcomes among depressed patients. J Abnorm Psychol. 94 2: 140–153.
- Blazer DG, Hybels CF. 2005. Origins of depression in later life. Psychol Med. 35 9: 1241–1252.
- Bukh JD, Bock C, Vinberg M, Werge Y, Gether U, Kessing LV. 2010. No association between genetic polymorphisms and stressful life events on outcomes of antidepressant treatment. Eur Neuropsychopharmacol. 20 5: 327–335.
- Bulmash E, Harkness KL, Stewart JG, Bagby RM. 2009. Personality, stressful life events, and treatment response in major depression. J Consult Clin Psychol. 77 6: 1067–1077.
- Burvill PW, Hall WD, Stampfer HG, Emmerson JP. 1991. The prognosis of depression in old age. Br J Psychiatry. 158:64–71.
- Candrian M, Farabaugh A, Pizzagalli DA, Baer L, Fava M. 2007. Perceived stress and cognitive vulnerability mediate the effects of personality disorder comorbidity on treatment outcome on major depression: A path analysis. J Nerv Ment Dis. 195 9: 729–737.
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. 2003. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 301 5631: 386–389.
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. 2004. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Human Genetics. 75 5: 807–821.
- Chrousos GP. 2009. Stress and disorders of the stress system. Nat Rev Endocrinol. 5 7: 374–381.
- Cronkite RC, Moos RH, Twohey J, Cohen C, Swindle RJr. 1998. Life circumstances and personal resources as predictors of the ten-year course of depression. Am J Community Psychol. 26 2: 255–280.
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. 2006. Catechol O-methyltransferase Val158Met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 63 12: 1396–1406.
- Drury SS, Theall KP, Smyke AT, Keats BJB, Egger HL, Nelson CA, Fox NA, Marshall PJ, Zeanah CH. 2010. Modification of depression by COMT val158met polymorphism in children exposed to early severe psychosocial deprivation. Child Abuse Negl. 34 6: 387–395.
- Farabaugh AH, Mischoulon D, Fava M, Green C, Guyker W, Alpert J. 2004. The potential relationship between levels of perceived stress and subtypes of major depressive disorder (MDD). Acta Psychiatr Scand. 110 6: 465–470.
- Faravelli C, Ambonetti A, Pallanti S, Pazzagli A. 1986. Depressive relapses and incomplete recovery from index episode. Am J Psychiatry. 143 7: 888–891.
- Folstein MF, Folstein SE, McHugh PR. 1975. Mini-mental state a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12 3: 189–198.
- George IK. 1994. Social factors and depression in late life. In: Schneider LS, Reynolds CF, Lebowitz BD, Friedhoff AJ. editors. Diagnosis and treatment of depression in late life. Washington DC: American Psychiatric Press131–153.
- Gonzales LR, Lewinsohn PM, Clarke GN. 1985. Longitudinal follow-up of unipolar depressives: An investigator of predictors of relapse. J Consult Clin Psychol. 53 4: 461–469.
- Hammen C. 1991. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 100 4: 555–561.
- Hammen C. 2005. Stress and depression. Annu Rev Clin Psychol. 1:293–319.
- Harkness KL, Monroe SM. 2006. Severe melancholic depression is more vulnerable than non-melancholic depression to minor precipitating life events. J Affect Disord. 91 2–3: 257–263.
- Hays JC, Krishnan KRR, George LK, Pieper CF, Flint EP, Blazer DG. 1997. Psychosocial and physical correlates of chronic depression. Psychiatry Res. 72 3: 149–159.
- Jabbi M, Kema IP, van der Pompe G, te Meermann GJ, Ormel J, den Boer JA. 2007. Catechol-O-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr Genetics. 17 3: 183–193.
- Kaufman J, Gelernter J, Kaffman A, Caspi A, Moffitt T. 2010. Arguable assumptions, debatable conclusions. Biol Psychiatry. 67 4: e19–e20.
- Karel MJ. 1997. Aging and depression: Vulnerability and stress across adulthood. Clin Psychol Rev. 17 8: 847–879.
- Kendler KS, Karkowski LM, Prescott CA. 1998. Stressful life events and major depression: Risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment dis. 186 11: 661–669.
- Kendler KS, Karkowski LM, Prescott CA. 1999. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 156 6: 837–841.
- Kendler KS, Thornton LM, Gardner CO. 2000. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. Am J Psychiatry. 157 8: 1243–1251.
- Kennedy GJ, Kelman HR, Thomas C. 1991. Persistence and remission of depressive symptoms in late life. Am J Psychiatry. 148 2: 174–178.
- Kessler RC. 1997. The effects of stressful life events on depression. Ann Rev Psychol. 48:191–214.
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. 2007. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiatry. 62 5: 423–428.
- Kivela SL, Viramo P, Pahkala K. Factors predicting the relapse of depression in old age. Int J Geriatr Psychiatry. 2000a; 15 2: 112–119.
- Kivela SL, Viramo P, Pahkala K. Factors predicting chronicity of depression in elderly primary care patients. Int Psychogeriatr. 2000b; 12 2: 183–194.
- Koenen KC, Galea S. 2009. Gene–environment interactions and depression. JAMA. 302 17: 1859.
- Kraaij V, de Wilde EJ. 2001. Negative life events and depressive symptoms in the elderly: A life span perspective. Aging Ment Health. 5 1: 84–91.
- Kraaij V, Arensman E, Spinhoven P. 2002. Negative life events and depression in elderly persons: A meta-analysis. J Gerontol Psychol Sci. 57B 1: 87–94.
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. 1996. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 6 3: 243–250.
- Lenze EJ, Munin MC, Ferrell RE, Pollock BG, Skidmore E, Lotrich F, Rogers JC, Quear T, Houck P, Reynolds CF. 2005. Association of the serotonin transporter gene-linked polymorphic region (5-HTTLPR) genotype with depression in elderly persons after hip fracture. Am J Geriatr Psychiatry. 13 5: 428–432 3rd.
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. 1996. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 274 5292: 1527–1531.
- Lethbridge R, Allen NB. 2008. Mood induced cognitive and emotional reactivity, life stress, and the prediction of depressive relapse. Behav Res Ther. 46 10: 1142–1150.
- Lotrich FE, Lenze E. 2009. Gene–environment interactions and depression. JAMA. 302 17: 1859–1860.
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, Taskinen J. 1995. Kinetics of human soluble and membrane-bound catechol-O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 34 13: 4202–4210.
- Lloyd C, Zisook S, Click MJr, Jaffe KE. 1981. Life events and response to antidepressants. J Human Stress. 7 1: 2–15.
- Mandelli L, Marino E, Pirovano A, Calati R, Zanardi R, Colombo C, Serretti A. 2009. Interaction between SERTPR and stressful life events on response to antidepressant treatment. Eur Neuropsychopharmacol. 19 1: 64–67.
- Mazure CM, Bruce ML, Maciejewski PK, Jacobs SC. 2000. Adverse life events and cognitive-personality characteristics in the prediction of major depression and antidepressant response. Am J Psychiatry. 157 6: 896–903.
- McGonagle KA, Kessler RC. 1990. Chronic stress, acute stress, and depressive symptoms. Am J Community Psychol. 18 5: 681–706.
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF. 1992. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the cumulative illness rating scale. Psychiatry Res. 41 3: 237–248 3rd.
- Monroe SM, Bellack AS, Hersen M, Himmelhoch JM. 1983. Life events, symptom course, and treatment outcome in unipolar depressed women. J Consult Clin Psychol. 51 4: 604–615.
- Monroe SM, Kupfer DJ, Frank E. 1992. Life stress and treatment course of recurrent depression: 1. Response during index episode. J Consult Clin Psychol. 60 5: 718–724.
- Montgomery SA, Asberg M. 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry. 134:382–389.
- Munafo MR, Durrant C, Lewis G, Flint J. 2009. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 65 3: 211–219.
- Mundt C, Reck C, Backenstrass M, Kronmuller K, Fiedler P. 2000. Reconfirming the role of life events for the timing of depressive episodes. A two-year prospective follow-up study. J Affect Disord. 59 1: 23–30.
- Murphy E. 1982. Social origins of depression in old age. Br J Psychiatry. 141:135–142.
- Murphy E. 1983. The prognosis of depression in old age. Br J Psychiatry. 142:111–119.
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. 2002. Neurobiology of depression. Neuron. 34 1: 13–25.
- Pedrelli P, Feldman GC, Vorono S, Fava M, Petersen T. 2008. Dysfunctional attitudes and perceived stress predict depressive symptoms severity following antidepressant treatment in patients with chronic depression. Psychiatry Res. 161 3: 302–308.
- Potter GG, Taylor WD, McQuoid DR, Steffens DC, Welsh-Bohmer KA, Krishnan KR. 2009. The COMT Val158Met polymorphism and cognition in depressed and nondepressed older adults. Int J Geriatr Psychiatry. 24 10: 1127–1133.
- Ravindran AV, Matheson K, Griffiths J, Merali Z, Anisman H. 2002. Stress, coping, uplifts, and quality of life in subtypes of depression: A conceptual frame and emerging data. J Affect Disord. 71 1–3: 121–130.
- Reno RM, Halaris AE. 1990. The relationship between life stress and depression in an endogenous sample. Compr Psychiatry. 31 1: 25–33.
- Rieckmann N, Rapp MA, Muller-Nordhorn J. 2009. Gene–environment interactions and depression. JAMA. 302 17: 1861.
- Rimmler J, McDowell JG, Slotterback BD, Haynes CS, Menold MM, Rogala A, Speer MC, Gilbert JR, Hauser ER, Vance JM, Pericak-Vance MA. 1998. Development of a data coordinating center (DCC): Data quality control for complex disease studies. Am J Hum Genet Suppl. 62:A240.
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. 2009. Interaction between the berotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 301 23: 2462–2471.
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. 1981. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 38 4: 381–389.
- Savitz J, Solms M, Ramesar R. 2006. The molecular genetics of cognition: Dopamine, COMT, and BDNF. Genes Brain Behav. 5 4: 311–328.
- Schoevers RA, Beekman ATF, Deeg DJH, Hooijer C, Jonker C, van Tilburg W. 2003. The natural history of late-life depression: Results from the Amsterdam study of the elderly (AMSTEL). J Affect Disord. 76 1–3: 5–14.
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Büchel C, Heinz A. 2005. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 25 4: 836–842.
- Steffens DC, McQuoid DR, Krishnan KRR. The Duke somatic treatment algorithm for geriatric depression (STAGED) approach. Psychopharmacol Bull. 2002a; 36 2: 58–68.
- Steffens DC, Svenson I, Marchuk DA, Levy RM, Hays JC, Flint EP, Krishnan KR, Siegler IC. Allelic differences in the serotonin transporter-linked polymorphic region in geriatric depression. Am J Geriatr Psychiatry. 2002b; 10 2: 185–191.
- Surtees PG, Miller PM, Ingham JG, Kreitman NB, Rennie D, Sashidharan SP. 1986. Life events and the onset of affective disorder: A longitudinal general population study. J Affect Disord. 10 1: 37–50.
- Swindle RWJr, Cronkite RC, Moos RH. 1989. Life stressors, social resources, coping, and the 4-year course of unipolar depression. J Abnorm Psychol. 98 4: 468–477.
- Taylor WD, Steffens DC, Payne ME, MacFall JR, Marchuk DA, Svenson IK, Krishnan KR. 2005. Influence of serotonin transporter promoter region polymorphisms on hippocampal volumes in late-life depression. Arch Gen Psychiatry. 62 5: 537–544.
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. 2006. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 60 7: 671–676.
- Taylor WD, Zuchner S, Payne ME, Messer DF, Doty TJ, MacFall JR, Beyer JL, Krishnan KR. 2007. The COMT Val158Met polymorphism and temporal lobe morphometry in healthy adults. Psychiatry Res. 155 2: 173–177.
- Tennant C. 2002. Life events, stress and depression: A review of recent findings. Aust N Z J Psychiatry. 36 2: 173–182.
- Vieland VJ. 2001. The replication requirement. Nat Genet. 29 3: 244–245.
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. 2003. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 299 5610: 1240–1243.
