Abstract
Traumatic experiences that occur during adolescence can render individuals vulnerable to mood and anxiety disorders. A model in juvenile rats (age: 27–29 days) was developed previously to study the long-term effects of adolescent stress exposure on behaviour and physiology. This paradigm, termed juvenile stress, involves subjecting juvenile rats to different stressors on consecutive days over a 3-day period. Here, we investigated the effects of the juvenile stress paradigm on freezing behaviour and aversive 22-kHz ultrasonic vocalizations (USVs) during auditory fear conditioning in adult male rats (age: 68–90 days). We found that rats previously subjected to juvenile stress increased aversive 22-kHz USVs (total calls and time spent calling) compared with controls during fear-conditioning training. The acoustic USV parameters between control and juvenile stress rats were largely equivalent, including duration, peak frequency and amplitude. While rats did not differ in freezing behaviour during fear conditioning, juvenile stress rats exhibited greater cue-conditioned freezing upon testing 24 h later. Our results show that juvenile stress elicited different long-term changes in freezing and aversive USVs during fear conditioning. Furthermore, they highlight the importance of assessing USVs to detect experience-dependent differences between control and stress-exposed animals which are not detectable by measuring visible behaviour.
Introduction
Human and animal studies have implicated early-life stressful experiences as a key risk factor for the development of mood and anxiety disorders (Young et al. Citation1997; Kendler et al. Citation1999; Heim and Nemeroff Citation2001). The juvenile (i.e. adolescent) period is a particularly important developmental stage during which stressful experiences may lead to deficits in adulthood (Romeo and McEwen Citation2006). As such, a rat model for juvenile stress-induced depression/anxiety was developed by Avital and Richter-Levin (Citation2005) to mimic childhood exposure to early-life stress. This is a well-established model that uses a pre-defined protocol allowing for the examination of short- and long-term effects following acute stress exposure during a very specific time window of development (pre-puberty). The Richter–Levin laboratory and other groups have shown that exposure to this series of acute and variable stressors during the pre-pubescent period in rats (age: 27–29 days) caused a number of behavioural, physiological and molecular abnormalities that persisted to adulthood (Tsoory and Richter-Levin Citation2006; Tsoory et al. Citation2007, Citation2008, Citation2010; Ilin and Richter-Levin Citation2009; Maggio and Segal Citation2011). At the behavioural level, adult rats previously subjected to juvenile stress exhibited increased anxiety-related behaviour in the open field and elevated plus maze as well as impaired coping and learned helplessness in the two-way shuttle avoidance box (Ilin and Richter-Levin Citation2009; Tsoory et al. Citation2010; Yee et al. Citation2011).
The goal of this study was to examine the effects of juvenile stress on adult behaviour during an auditory fear-conditioning paradigm, focusing on freezing and aversive 22-kHz ultrasonic vocalizations (USVs). In rats, freezing is the main behavioural measurement in conditioning tests and serves to exemplify fear and defensive behaviour (Blanchard and Blanchard Citation1969; Fendt and Fanselow Citation1999; Maren Citation2001). As an additional measurement, we also sought to investigate USV production during fear conditioning, as USVs are a prominent part of rat behaviour with important communicative functions (Blanchard et al. Citation1991; Endres et al. Citation2007; Litvin et al. Citation2007; Wöhr and Schwarting Citation2007, Citation2008a), but have not been examined in previous juvenile stress studies. Juvenile and adult rats produce two distinct types of USVs. Their occurrence depends on the emotional valence of the context (Knutson et al. Citation2002). Rats produce 50-kHz USVs in appetitive situations, such as play (Knutson et al. Citation1998) or reward anticipation (Knutson et al. Citation1999), whereas 22-kHz USVs occur in aversive situations, such as inter-male fighting (Sales Citation1972), predator exposure (Blanchard et al. Citation1991) or fear conditioning (Antoniadis and McDonald Citation1999; Choi and Brown Citation2003; Jelen et al. Citation2003; Kikusui et al. Citation2003; Wöhr et al. Citation2005; Borta et al. Citation2006; Wöhr and Schwarting Citation2008a,Citationb). It is well known that in a fear-conditioning paradigm, rats emit aversive 22-kHz USVs not only in response to the aversive unconditioned stimulus (US), but also to the conditioned stimulus (CS) alone on subsequent testing days (Antoniadis and McDonald Citation1999; Choi and Brown Citation2003; Jelen et al. Citation2003; Kikusui et al. Citation2003; Wöhr et al. Citation2005; Borta et al. Citation2006; Wöhr and Schwarting Citation2008a,Citationb). Similar to freezing behaviour, 22-kHz vocalization emission in such test paradigms is thought to be associated with the emotional construct of fear (Knutson et al. Citation2002; Kikusui et al. Citation2003). USV call production was found to be significantly correlated with freezing during both conditioning (US+CS) and testing (CS only) (Wöhr et al. Citation2005; Wöhr and Schwarting Citation2008a) and was shown to be strongly dependent on the aversiveness of the situation (Wöhr et al. Citation2005). Rats exposed to higher shock intensities during fear-conditioning training emitted more 22-kHz USVs than rats exposed to lower shock intensities; in the absence of shock application, rats did not produce 22-kHz USVs. In addition to situational factors, individual differences in disposition for anxiety-related behaviour play an important role (Borta et al. Citation2006). Rats that were previously characterized as anxious, based on their anxiety-related behaviour on the elevated plus maze, emitted more 22-kHz USVs during fear-conditioning training than rats previously characterized as less anxious. Such individual differences are likely to be associated with early life experiences, e.g. maternal care (Wöhr and Schwarting Citation2008a). In general, emissions of 22-kHz USVs have been proposed to be indices of subject-dependent anxiety levels in different individuals (Jelen et al. Citation2003; Sanchez Citation2003; Borta et al. Citation2006; Schwarting et al. Citation2007).
A few studies have examined the relationship between stress and USVs in rodents. However, in the majority of cases, pup vocalizations were quantified as a readout for the effects of prenatal stress on offspring (e.g. Williams et al. Citation1998; Morgan et al. Citation1999; Harmon et al. Citation2009). Only a handful of studies have examined the effects of stress on adult USVs. For example, fear-conditioned 22-kHz USVs emitted by adult male rats (age: 60 days) were decreased in animals previously exposed to neonatal isolation or maternal separation (Kosten et al. Citation2005, Citation2006). Chronic restraint stress in adulthood increased 22-kHz USVs in male rats in response to acute footshock, but chronic footshock stress had the opposite effect (Swiergiel et al. Citation2007). Long-term isolation in adult rats increased appetitive 50-kHz USVs during social interaction (Hamed et al. Citation2009). Mällo et al. (Citation2009) have also found that adult rats with inherently low positive affect (as measured by tickling-induced appetitive 50-kHz calls) emitted more 22-kHz vocalizations following chronic variable stress exposure than rats with high positive affect. Thus, there is tremendous variation in findings based on the use of different stress paradigms, the timing of stress exposure and subsequent USV recording, the type of USV measured (22 vs. 50 kHz) and the methods used to elicit USVs.
In this study, we hypothesized that juvenile stress would impact freezing and USV emission during fear conditioning in adult rats. Specifically, individual predisposition towards an anxiety-like phenotype as determined by a history of early-life stress would manifest as increased freezing and 22-kHz USVs in juvenile stress-exposed rats. To test our hypothesis, we subjected rats to juvenile stress (age: 27–29 days), then tested them in a fear-conditioning paradigm as adults (age: 68 days onward). Juvenile stress rats were compared with controls each day of the paradigm for freezing behaviour and USVs. We also conducted detailed USV analyses for all individuals.
Materials and methods
All experiments were carried out in accordance with the European Communities Council Directive of November 24, 1986 (86/EEC), including the 18 June 2007 recommendation 2007/526/EC, and approved by the Lower Saxony Federal State Office for Consumer Protection and Food Safety, Germany. A total of 48 male rats were used in this study, which was conducted on three separate occasions. Male Sprague–Dawley rats were obtained from Harlan Winkelmann (Brochen, Germany) at 21 days of age. Rats originated from 5 to 8 litters on each occasion. Upon arrival, they were randomly and equally distributed into cages designated as either ‘control’ or ‘juvenile stress’ groups. Rats from each litter were evenly divided across all experimental groups and no littermates were caged together. They were group-housed (3–4 rats/cage) in Type 4 Makrolon cages (59 × 38 × 20 cm) in a temperature- (21 ± 1°C) and humidity-controlled (55 ± 10%) facility on a 12-h reverse light cycle (red light from 6:00 to 18:00 hrs; lights on at 18:00 hrs). The rats were allowed 6 days to habituate to the animal facility. Throughout the course of the experiment, animals were unhandled aside from juvenile stress exposure, weighing and weekly cage changes.
Juvenile stress protocol
At 27–29 days of age, the juvenile stress group underwent a 3-day variable stressor protocol modified from Ilin and Richter-Levin (Citation2009), which was used previously in our laboratory (Yee et al. Citation2011). Stressors were applied under red light in a designated experiment room.
Day 1 (age: 27 days), forced swim: Rats were placed individually in circular glass water tanks (diameter 20 cm, height 40 cm, water depth 20 cm) for 10 min (temperature 22 ± 2°C). This stressor was administered between 8:00 and 9:30 hrs.
Day 2 (age: 28 days), elevated platform: Rats were placed individually on square (12 cm × 12 cm) platforms elevated to 70 cm above floor level for three trials of 30 min each (60 min intertrial interval). This stress procedure was carried out between 8:00 and 13:30 hrs.
Day 3 (age: 29 days), restraint: Rats were restrained for 2 h in well-ventilated polypropylene tubes (inner diameter 6.3 cm, length adjusted to 7.5 cm) that limit mobility without physically compressing the animal or causing harm in any way. Lateral movement was inhibited, but given their size, some animals were able to turn around within the restrainers. Restraint stress was conducted between 8:00 and 10:00 hrs.
Protocols were carried out in parallel to cagemates in the stress group so as not to isolate any rat in its home cage. Rats were returned to their home cages following each stressor. While the juvenile stress group was undergoing the stress procedures, control rats were brought in their home cages to a different experiment room and kept there under red light for the same duration as the stressors.
Fear-conditioning protocol
When rats reached adulthood (age: 68–90 days), they underwent a fear-conditioning paradigm as previously described (Wöhr et al. Citation2005). Groups of control and juvenile stress rats were tested at the same age and on the same day(s). They were tested between 8:00 and 17:00 hrs each day in alternating (between groups) and randomized order. Fear conditioning was carried out in a shock chamber (33.5 × 35 × 38 cm) made of grey and transparent plastic walls. A loudspeaker was mounted in one wall to present the tone stimulus (CS; 3-kHz sine wave tone of 20 s duration and about 72 dB). The floor of the shock chamber consisted of stainless steel rods spaced 1 cm apart. The chamber was housed within a sound-attenuating isolation cubicle (Coulbourn Instruments, Whitehall, PA, USA) equipped with a black-and-white CCD camera (Conrad Electronic, Hirschau, Germany) for video recording. USVs were recorded using a condenser ultrasound microphone (CM16; Avisoft Bioacoustics, Berlin, Germany) mounted in the shock chamber roof and connected to a computer with recording software (Avisoft Recorder) via Avisoft-UltraSoundGate hardware (USG416Hb; sampling rate: 214.285 Hz; format: 16 bit). A stand-alone shocker (Med Associates, St. Albans, VT, USA) administered the shock stimulus (US) via the rod floor. The US was a 0.5 mA scrambled shock (120 V peak-to-peak amplitude) of 500 ms duration. Stimulus delivery and timing were controlled by the Presentation program (Neurobehavioral Systems, Albany, CA, USA).
The fear-conditioning procedure was carried out over 3 days. On day 1 (habituation), a rat was placed in the shock chamber for 11 min without CS or US presentation. On day 2 (fear conditioning), the rat was placed in the shock chamber again for 11 min. After an initial 180 s (pre-shock period), six CS/US pairings were given; each pairing was followed by a 60-s inter-stimulus interval (ISI). The US (duration 500 ms) was given during the last 500 ms of the 20 s CS. On day 3 (test), the rat was again placed in the shock chamber for 11 min. After an initial phase of 180 s (context phase), the six tones (CS only) were presented again for 20 s each, with a 60-s ISI. Prior to each subject, the equipment was cleaned with 0.1% acetic acid solution.
USV analysis
Avisoft SASLab Pro (Version 4.51; Avisoft Bioacoustics) was used for acoustic analysis of USV recordings (as described in Wöhr et al. Citation2005). A fast Fourier transform was applied to each recording (512 point FFT length; 100% frame; Hamming window; 75% time window overlap) and spectrograms were produced at a frequency resolution of 488 Hz and time resolution of 0.512 ms. Call detection was conducted by an automatic threshold-based algorithm (threshold: − 40 dB) and a hold-time mechanism (hold-time: 20 ms). A lower cut-off frequency of 18 kHz was used to reduce background noise outside the relevant frequency band to 0 dB. An experienced user verified the accuracy of call detection.
The total number of 22-kHz USVs and total time spent calling were determined automatically for each recording (subject). The temporal patterns of these parameters were analysed in detail by assessing their occurrence in 20-s time bins over the course of the 11-min fear-conditioning procedure. For rats that vocalized, the following call parameters were determined: latency to first call, mean call length, mean peak frequency (average spectrum of entire call, start point, centre and end point) and mean peak amplitude (average spectrum of entire call, start point, centre and end point; see ).
Figure 1. (A) Spectrogram of an exemplary call with the three points where peak amplitudes and frequencies were measured. These parameters were also derived from the average spectrum of the entire call (not depicted). (B) Spectrogram of two successive bouts of calls. Shown are examples for calls starting a bout (Call 1), calls within a bout (Calls 2–4), bouts (Bout 1 and Bout 2) and an inter-bout interval (IBI).
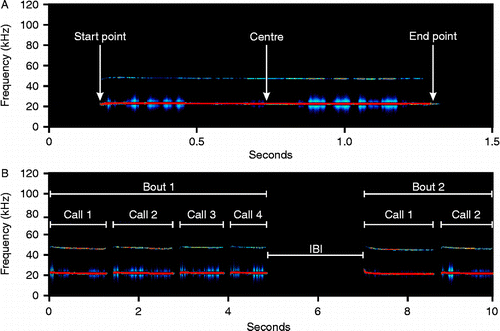
As 22-kHz USVs are emitted either as single pulses or in short bouts, calls were divided into those starting a bout versus those within a bout according to the duration of the interval between two calls. Based on detailed studies on the bout structure of aversive 22-kHz USV emission by van der Poel (van der Poel et al. Citation1989; van der Poel and Miczek Citation1991), a bout was defined as a call, or a number of calls, separated from other calls by intervals longer than 320 ms (inter-bout interval; ). The number of bouts was determined as well as some of the above-mentioned call parameters for the two different call types. In addition, and dependent on the time point of their occurrence, calls were divided into calls emitted during CS/US presentation or calls emitted within the ISIs. Call parameters (number, call length, peak frequency and peak amplitude) were determined for these two phases.
Overt behaviour
Overt behaviour was scored from video recordings for each day of the paradigm by an experienced observer blind to group assignment. The periods of 1–3 min and 4–11 min were separately scored for each animal. The time spent freezing was recorded, where freezing was defined as the lack of all somatic movement, except for respiration-related motions (Wöhr et al. Citation2005; Borta et al. Citation2006). Rearing (number of times an animal reared on its hind legs) and grooming (duration of face, body and genital grooming movements) were also scored for each period. As an additional measurement, defecation was measured by counting faecal boli excreted by each subject following test sessions.
Statistical analysis
Statistical analyses were carried out using GraphPad Prism (Version 4; GraphPad Software, La Jolla, CA, USA) or SigmaPlot (Version 11.0; Systat Software, Inc., San Jose, CA, USA) and statistical significance was set at p < 0.05. The Student's t-test (two-tailed) was used for comparisons between control and juvenile stress groups and all data are presented as mean ± SE of the mean (SEM), unless otherwise indicated. The time course of USV emission was analysed with a two-way repeated measures ANOVA followed by the Holm–Sidak post hoc test. In instances where data points (from USV or freezing data) for a subject were >2 SDs above/below the group mean, these subjects were subsequently removed as outliers from all analyses. Based on this criterion, four rats (two control, two juvenile stress) were removed completely (rats analysed: 21 control, 23 juvenile stress). Outlier rats were not cagemates.
Results
Habituation (day 1)
During the first day (habituation), none of the rats displayed signs of freezing behaviour. There were also no differences in grooming, rearing or defecation in juvenile stress compared with control rats (data not shown). Furthermore, no USVs were emitted during the entire 11-min exposure to the chamber.
Fear conditioning (day 2)
On the day of fear conditioning (day 2), minimal freezing was observed in all rats during the initial 1–3 min pre-shock phase and experimental groups did not differ (t42 = 0.79, p = 0.43; , left). There were no differences in rearing between groups (t42 = 0.61, p = 0.54). Also, none of the rats exhibited grooming behaviour and no USVs were emitted during the 1–3 min period.
Figure 2. Time spent freezing (s/min) during fear conditioning and testing. (A) Freezing during 1–3 min (pre-shock period; left) and 4–11 min (tone+shock presentation; right) during fear conditioning (day 2). (B) Freezing during 1–3 min (context period; left) and 4–11 min (tone presentation+context phase; right) during testing (day 3). n = 21 control rats, 23 juvenile stress rats; **p < 0.01 compared with respective control group.
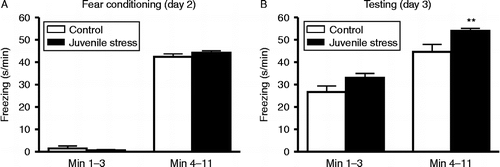
Upon the onset of shock delivery and during subsequent shocks, rats exhibited short bursts of activation with startle movements, flinches, jumps and running (not measured in detail). With repeated shock delivery, the main response during both CS/US and ISIs was a decrease in activity, as observed by an increase in freezing together with a decrease in rearing for both groups. During this phase of CS/US pairing (4–11 min), freezing time did not differ significantly between control and juvenile stress rats (t42 = 1.15, p = 0.26; , right). There were no differences in rearing (t42 = 1.73, p = 0.091) and none of the rats showed grooming behaviour. Also, there was no difference in defecation between control and juvenile stress rats following fear conditioning (t42 = 0.58, p = 0.57).
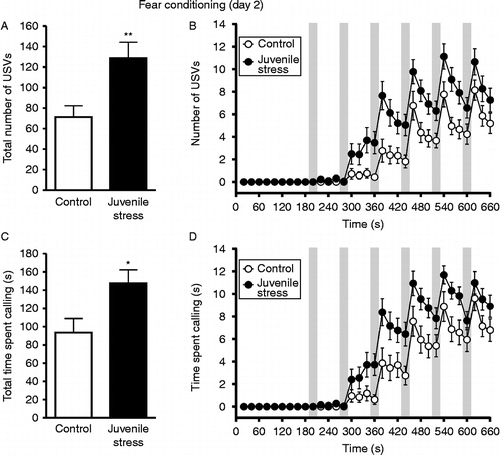
We observed USVs in 41 out of 44 total rats during CS/US delivery (fear conditioning). As hypothesized, juvenile stress rats emitted a significantly greater total number of USVs than control rats (t42 = 2.95, p = 0.005; ). When the number of USVs was analysed across time, two-way ANOVA revealed significant main effects of time [F(32,1344) = 59.48, p < 0.001] and group (control vs. juvenile stress) [F(1,1344) = 8.69, p = 0.005] as well as a significant interaction between time and group [F(32,1344) = 3.69, p < 0.001]. Juvenile stress rats emitted significantly more calls than controls at each time point between 340 and 660 s (p < 0.05; ). The total time spent calling was also significantly higher in juvenile stress rats than control rats (t42 = 2.55, p = 0.015; ). Similar to the time course of total calls, when the time spent calling for each 20-s bin was analysed across time, two-way ANOVA revealed significant effects of time [F(32,1344) = 76.19, p < 0.001] and group (control vs. juvenile stress) [F(1,1344) = 6.51, p = 0.014] and a significant interaction between time and group [F(32,1344) = 3.17, p < 0.001]. Juvenile stress rats spent significantly more time vocalizing than controls at each time point between 340 and 660 s (excluding 660 s and 620 s; p < 0.05; ).
Figure 3. USVs on the day of fear conditioning (day 2). (A) Total number of vocalizations. (B) Number of USVs emitted over time (per 20 s time bin) during the course of fear conditioning. (C) Total time spent vocalizing. (D) Time spent vocalizing during each 20-s time bin over the course of fear conditioning. Grey boxes denote occurrences of CS/US presentation. USVs, ultrasonic vocalizations; n = 21 control rats, 23 juvenile stress rats; *p < 0.05, **p < 0.01 compared with control group; statistical differences are not shown in (B) and (D) for the sake of clarity.
Several USV parameters from the rats that vocalized (n = 19 control, 22 juvenile stress) are presented in . Juvenile stress rats began calling within a shorter latency than controls (t42 = 3.35, p = 0.002). The acoustic parameters (e.g. mean peak frequency and amplitude at different call components) were not significantly different between the groups. While experimental groups did not differ in the number of bouts emitted (t42 = 1.83, p = 0.075), the number of calls within bouts was significantly greater in juvenile stress rats than in controls (t39 = 2.56, p = 0.015), which reflects the difference in total number of calls. Also, the peak frequency at the start point of calls starting a bout was significantly higher in controls than in juvenile stress animals (t39 = 2.186, p = 0.035). No such difference was observed for calls within a bout (t39 = 1.118, p = 0.270).
Table I. Characteristics of USVs during fear conditioning.
The USV parameters of vocalizing control versus juvenile stress rats according to calls occurring during CS/US presentation or ISI are presented in . As also evident in , both groups made more USVs during ISIs than CS/US presentations. In keeping with emitting more USVs in total, juvenile stress rats made significantly more calls per minute than controls during both the ISI (t39 = 2.84, p = 0.007) and CS/US (t39 = 2.60, p = 0.014) phases. Similar to the results presented in , the acoustic parameters of USVs during the specific ISI versus CS/US phases did not differ between juvenile stress and control groups.
Table II. Characteristics of USVs during fear conditioning according to their occurrence during CS/US presentation versus inter-stimulus interval phases.
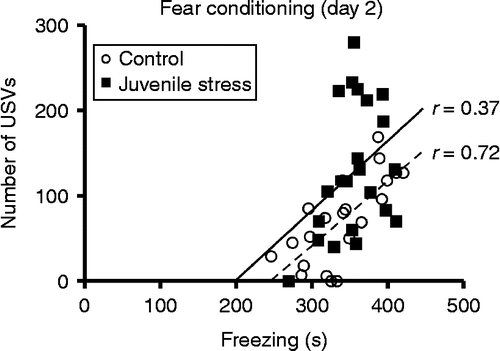
We correlated freezing time (during 4–11 min) with total number of calls on the day of fear conditioning for control and juvenile stress rats separately (). In the control group, USV emission was highly correlated with freezing (r = 0.72, p = 0.0002). However, for the juvenile stress group, USV production showed only a slight trend towards a correlation with freezing (r = 0.37, p = 0.084). Also, body weight (irrespective of group) was not correlated with USV emission (r = − 0.29, p = 0.057).
Figure 4. Scatter plots showing individual relationships between USVs (total number of calls) and freezing (s) during 4–11 min on the day of fear conditioning (day 2). Regression lines: dotted line, control group; solid line, juvenile stress group. n = 21 control rats, 23 juvenile stress rats; r = correlation coefficient.
Testing (day 3)
Juvenile stress rats froze more than controls during testing on day 3. When initially re-exposed to the context (1–3 min), there was a strong trend for juvenile stress rats to freeze longer than controls (t42 = 1.94, p = 0.059; , left). Both groups displayed minimal rearing behaviour, which did not differ significantly (t42 = 1.05, p = 0.30). None of the rats groomed during this time. During the subsequent context+CS phase (tone presentation only; 4–11 min), freezing increased in both groups, but the juvenile stress group froze for a significantly longer duration (t42 = 2.76, p = 0.008; , right). This was accompanied by a strong trend for decreased rearing in juvenile stress rats compared with controls (t42 = 1.92, p = 0.062). There was no difference in defecation (t42 = 0.15, p = 0.882) between control and juvenile stress rats. Furthermore, within-group comparisons revealed that defecation did not differ between fear conditioning (day 2) and testing (day 3) for either the juvenile stress (t42 = 0.85, p = 0.40) or control group (t42 = 1.15, p = 0.26).
Only five rats (Controls: 2 out of 21, juvenile stress: 3 out of 23) vocalized during testing. Statistics to compare USV parameters were not run on these animals due to the small sample size. However, we attempted to examine correlations between USV production during fear conditioning (day 2) and freezing during 1–3 min and 4–11 min during testing (day 3) to see whether a predictive pattern could be visible. In both control and juvenile stress groups, no correlative relationships were observed (data not shown).
Discussion
Overall, this study showed that differences in anxiety-related behaviour due to previous exposure to juvenile stress affected overt behaviour and affective ultrasonic calling in a standard auditory fear-conditioning paradigm. Specifically, juvenile stress rats exhibited more aversive 22-kHz USVs in response to tone/shock pairings on the fear-conditioning training day and increased freezing during tone presentation on the testing day.
Extensive research with different animal models has demonstrated that acute or chronic stress exposure during adolescence has an immense impact on development at various levels. In terms of endocrine functioning, adolescent stress causes pronounced changes in hypothalamic–pituitary–adrenal (HPA) axis responsivity and corticosterone levels, both basally and in response to subsequent stressors (Romeo et al. Citation2006; Romeo and McEwen Citation2006; Bazak et al. Citation2009; McCormick et al. Citation2010a; Weintraub et al. Citation2010; Jankord et al. Citation2011). Studies investigating changes in molecular and neuronal mechanisms have demonstrated that adolescent stress induces major changes in brain structure and morphology, molecular mechanisms as well as neuronal and neurotransmitter signalling (Toth et al. Citation2008; Tsoory et al. Citation2008, Citation2010; Bingham et al. Citation2010; McCormick et al. Citation2010b; Weintraub et al. Citation2010; Maggio and Segal Citation2011; Oztan et al. Citation2011). Adolescent stress also causes profound behavioural deficits related to anxiety- or depressive-like behaviour, emotionality and learning (Tsoory and Richter-Levin Citation2006; Pohl et al. Citation2007; Tsoory et al. Citation2007; Vidal et al. Citation2007; Toth et al. Citation2008; Ilin and Richter-Levin Citation2009; Bingham et al. Citation2010; Weintraub et al. Citation2010; Oztan et al. Citation2011; Ricon et al. Citation2012; Yee et al. Citation2011). Furthermore, it appears that adolescent stress may exert sex-dependent effects before, during and after puberty. In studies utilizing male and female rodents, many of the adolescent stress-related effects are gender dependent, with females exhibiting similar and/or opposite responses to males in terms of endocrine (McCormick and Mathews Citation2007; McCormick et al. Citation2008; Bourke and Neigh Citation2011) and/or behavioural effects (Pohl et al. Citation2007; Toledo-Rodriguez and Sandi Citation2007; McCormick et al. Citation2008; Jacobson-Pick and Richter-Levin Citation2010; Weintraub et al. Citation2010; Bourke and Neigh Citation2011).
There is inconsistency in results from previous studies investigating stress exposure during adolescence and subsequent conditioned freezing behaviour in response to fear conditioning in rats. Toledo-Rodriguez and Sandi (Citation2007) found that a pre-puberty stress paradigm enhanced cue-conditioned fear in male rats during adolescence but not in adulthood, whereas cue-conditioned fear responses were not affected by stress in females. Another study by Morrissey et al. (Citation2011) showed that adolescent chronic social stress in males impaired adulthood context- and cue-conditioned freezing. Differences in our results and the above-mentioned impairment in fear-conditioning response found by Morrissey et al. (2011) are likely due to the timing, nature (stressor type) and duration (acute vs. chronic) of the protocol. The Morrissey study used a 16-day social instability paradigm beginning at 30 days of age. This protocol spanned periods of both pre-pubescent and pubescent development, which are markedly different in terms of developmental trajectory and windows of opportunity for insult (Spear Citation2000; Andersen Citation2003; Foilb et al. Citation2011). Puberty is associated with pronounced neuroendocrine changes that can impact brain structure, emotionality and stress responsiveness differently relative to the pre-pubescent period (McCormick and Mathews Citation2007; Romeo Citation2010). On the other hand, our protocol was limited to stress exposure during the pre-pubescent period (Spear Citation2000). In agreement with our findings, another juvenile stress study utilizing a similar pre-pubescent stress protocol also demonstrated that auditory fear conditioning in adult male rats exposed to juvenile stress increased freezing behaviour during testing compared with control rats (Tsoory et al. Citation2010). In addition, they found that freezing was not different between groups on the conditioning day, which is again in line with our findings. In other stress paradigms (e.g. maternal deprivation and acute stress in adulthood), results from fear conditioning tests have also yielded discrepancies. Rats subjected to different maternal deprivation protocols and later tested in fear conditioning as adults exhibited either no differences (Lehmann et al. Citation1999; Pryce et al. Citation2003; Kosten et al. Citation2006) or impairments in fear-conditioning responsiveness (e.g. decreased freezing) (Lehmann et al. Citation1999; Meerlo et al. Citation1999). In contrast, adulthood stress paradigms, such as acute (Cordero et al. Citation2003b) and chronic restraint stress (Conrad et al. Citation1999; Sandi et al. Citation2001; Cordero et al. Citation2003a), caused potentiation of context- or cue-conditioned freezing. Thus, it is apparent that results from fear-conditioning tests depend largely on gender, the nature and timing of the stress protocol, as well as the timing of testing.
The literature regarding the relationship between stress exposure and USVs has been largely limited to prenatal stress and the quantification of pup vocalizations during maternal separation (e.g. Williams et al. Citation1998; Morgan et al. Citation1999; Harmon et al. Citation2009). Yet, a few studies have looked at USV emission as a readout for adult behaviour following stress. Most relevant to this study is the major work conducted by Kosten and colleagues, where they demonstrated that context-induced 22-kHz USVs and cue-induced 22-kHz USVs were impaired (i.e. reduced time spent calling) during fear conditioning in adult male rats previously subjected to neonatal isolation (Kosten et al. Citation2005) or maternal separation (Kosten et al. Citation2006), respectively. To the best of our knowledge, this is the first study to examine USV production in detail during a testing paradigm in adult male rats previously exposed to juvenile stress. We found that the experience of juvenile stress exposure strongly impacted affective USV emission during fear-conditioning training (day 2). Rats subjected to juvenile stress emitted more aversive 22-kHz USVs than control rats. On average, emissions began after the third CS/US presentation in both groups, but the juvenile stress group maintained higher levels of vocalizations, making more calls than control rats during both ISIs and CS/US presentation. While the number of bouts emitted did not differ between groups, stressed rats emitted more USVs within bouts. This specificity indicates that USVs within bouts are more sensitive to changes in affect, which is in line with a previous study where it was shown that USVs within bouts, but not USVs starting bouts, were affected by the intensities of foot-shocks applied in a fear-conditioning paradigm (Wöhr et al. Citation2005). Notably, bout structure is known to be changed by pharmacological agents, such as benzodiazepines (van der Poel et al. Citation1989). Except for a reduction in peak frequency at the beginning of USVs starting a bout in stressed rats, the acoustic USV parameters between control and juvenile stress rats were largely equivalent. Although changes in peak frequency have been related to a subject's affective state (van der Poel and Miczek Citation1991; Borta et al. Citation2006), very little is known about the functional significance.
The difference in USV emission is particularly remarkable given that no overt behavioural differences between groups were detected. Despite a very detailed behavioural analysis, including the assessment of freezing, rearing and grooming behaviour, combined with an assessment of defecation, no effect of juvenile stress on visible behavioural responses during fear-conditioning training could be detected. As the time spent freezing was rather high in both experimental groups during fear conditioning (controls: 42 s/min; juvenile stress: 44 s/min), it is possible that juvenile stress-induced changes could not be detected due to a ceiling effect, although higher freezing values have been obtained in response to more intense foot-shocks (47 s/min, Wöhr et al. Citation2005; 50 s/min, unpublished observations). In any case, it is apparent that measuring overt behaviour in an attempt to discern large differences between control and juvenile stress groups in a fear-conditioning paradigm may be limited by ceiling effects in behavioural parameters. This underscores the significance and usefulness in assessing USVs. Overall, this study showed that measuring affective USV emission can provide insight into experience-dependent changes in affective processing in motivationally relevant situations that are not detectable by classic behavioural measures typically applied in fear-conditioning studies.
Individual differences in the disposition to exhibit anxiety-related behaviour play an important role in the production of aversive 22-kHz USVs during fear conditioning. As suggested previously (Sanchez Citation2003), we propose that the differences in aversive 22-kHz USVs observed here represent distinct individual differences in terms of anxiety. Several other studies have also utilized aversive 22-kHz USVs as behavioural determinants for anxiety-related phenotypes and/or as a screening method for anti-anxiety drugs (De Vry et al. Citation1993; Molewijk et al. Citation1995; Jelen et al. Citation2003; Borta et al. Citation2006; Mällo et al. Citation2009). The validity of measuring aversive USVs emitted specifically during fear conditioning to assess anxiety-like behaviour in rats was demonstrated in a study by Borta et al. (Citation2006). Here, rats that previously exhibited anxiety-like behaviour in the elevated plus maze, a classic test of anxiety (Pellow et al. Citation1985), also emitted more aversive 22-kHz USVs during fear-conditioning training than rats previously exhibiting less anxiety-like behaviour (Borta et al. Citation2006). Such individual differences are likely to be associated with the individual's early life experiences, e.g. maternal care (Wöhr and Schwarting Citation2008a). Our present study adds to this by demonstrating that exposure to traumatic experiences during juvenility induces anxiety-like behaviour in rats that can be clearly measured in a fear-conditioning paradigm.
As in previous studies (Wöhr et al. Citation2005; Wöhr and Schwarting Citation2008a,Citationb), we observed a clear relationship between freezing and aversive 22-kHz USVs in control animals not subjected to juvenile stress. Remarkably, while a highly positive correlation between freezing and 22-kHz USVs was found in control animals during fear conditioning training (day 2), no such correlation was found in juvenile stress rats. It is difficult to interpret the lack of a significant correlation between freezing and aversive 22-kHz USVs in juvenile stress animals. As mentioned above, the possible ceiling effect could confine the freezing time values to a narrow range at the high end of freezing time, since freezing values were very high in all juvenile stress rats.
Juvenile stress exposure did not increase freezing behaviour during fear conditioning on day 2, despite increasing USV production. Furthermore, during testing (day 3), juvenile stress increased freezing, but USVs were not observed in the majority of rats (controls: 2 out of 21; juvenile stress: 3 out of 23). This relatively low number of vocalizing rats was somewhat unusual, but actually in line with most of the previous studies using this paradigm (Borta et al. Citation2006: 9 out of 20 rats; Wöhr and Schwarting Citation2008a: 10 out of 18; Wöhr and Schwarting Citation2008b: 5 out of 12; Wöhr et al. Citation2005: 3 out of 7). As in this study, freezing is typically higher during testing (day 3) than during conditioning (day 2), whereas the opposite pattern can be found for USV production, with relatively low levels of 22-kHz USVs during testing (Wöhr et al. Citation2005; Borta et al. Citation2006; Wöhr and Schwarting Citation2008a,Citationb). Thus, decoupling of USV emission and freezing can be observed over time, as well as in response to stress exposure. In fact, a similar decoupling was also observed in a maternal separation paradigm, whereby cue-conditioned 22-kHz USVs were impaired in stressed rats, but freezing was not (Kosten et al. Citation2006). The decoupling phenomenon points to distinct adaptive functions of 22-kHz USV production and freezing behaviour along with a separation of underlying neuronal mechanisms.
As Kosten et al. (Citation2006) have pointed out, presentation of the cue/context associated with shock delivery does not activate all fear measures to the same extent. These measurements are governed by different neuronal mechanisms of motor output. Conditioned fear is notably controlled by the hippocampus and amygdala (Fendt and Fanselow Citation1999; Maren Citation2001), whereas 22-kHz USVs are initiated in the laterodorsal tegmental nucleus (Brudzynski Citation2001). Therefore, the dissociation between freezing and USV production during fear conditioning in stressed animals could be due to the fact that the mechanisms of fear responsiveness within these brain structures, as well as the sensitivity in measuring such outputs, may be affected or regulated differently following stress exposure. While the Kosten group observed stress-induced impairment in USVs with no effect on freezing during conditioned testing following early life postnatal stress (Kosten et al. Citation2006), we observed the opposite decoupling phenomenon (increased freezing but minimal vocalizations) following juvenile stress. This discrepancy points to obvious differences in the developmental stage during which stress exposure occurs, since stress-related and fear-conditioning-related brain areas change markedly during development in terms of functionality and vulnerability (Andersen Citation2003).
As pointed out above, the decoupling of freezing behaviour and USVs might also be linked to differences in the adaptive value of freezing and the emission of aversive 22-kHz USVs. While the former is a defensive behaviour which reduces the likelihood of being detected by a predator (Blanchard and Blanchard Citation1971), the production of 22-kHz USVs may serve a communicative function, namely as alarm calls to warn conspecifics about external danger (Blanchard et al. Citation1991; Litvin et al. Citation2007; Wöhr and Schwarting Citation2009). Aversive 22-kHz USVs elicited freezing behaviour in conspecifics (Blanchard et al. Citation1991; Endres et al. Citation2007; Wöhr and Schwarting Citation2007, Citation2008b), and conspecifics were found to be predisposed to associate the occurrence of 22-kHz USVs with aversive events (Endres et al. Citation2007). However, as the emission of 22-kHz alarm calls can probably be detected by predators (Litvin et al. Citation2007), there is a conflict between producing alarm calls to warn conspecifics and remaining silent to avoid being detected by predators. That is, similar to freezing behaviour, remaining silent could also represent an adaptive response and might explain why high levels of freezing behaviour observed during testing of learned fear were seldom accompanied by 22-kHz USV emission.
In summary, this study showed that juvenile stress exposure increased anxiety-related behaviour in an auditory fear-conditioning paradigm in terms of freezing behaviour and aversive 22-kHz USV emission. Furthermore, we demonstrate that measurement of USVs is valid and useful in determining differences between stress and control animals relative to anxiety/fear in different behavioural paradigms, especially in cases where differences in visible behavioural measurements are not apparent.
Acknowledgements
The authors wish to thank Cornelia Heckmann for technical assistance and Dr Gabriele Flügge for intellectual input and guidance. Nicole Yee is financially supported by the Kurt Lange Stiftung.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Notice of CorrectionThis article was originally published online ahead of print on 10 January 2012; however, a change was made after being published online. The following sentence in the Results section:Juvenile stress rats spent significantly more time vocalizing than controls at each time point between 340 excluding 660 s and 620 s; p < 0.05; .was changed to:Juvenile stress rats spent significantly more time vocalizing than controls at each time point between 340 and 660 s (excluding 660 s and 620 s; p < 0.05; ).
References
- Andersen SL. 2003. Trajectories of brain development: Point of vulnerability or window of opportunity?. Neurosci Biobehav Rev. 27:3–18.
- Antoniadis EA, McDonald RJ. 1999. Discriminative fear conditioning to context expressed by multiple measures of fear in the rat. Behav Brain Res. 101:1–13.
- Avital A, Richter-Levin G. 2005. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol. 8:163–173.
- Bazak N, Kozlovsky N, Kaplan Z, Matar M, Golan H, Zohar J, Richter-Levin G., Cohen H.. 2009. Pre-pubertal stress exposure affects adult behavioral response in association with changes in circulating corticosterone and brain-derived neurotrophic factor. Psychoneuroendocrinology. 34:844–858.
- Bingham B, McFadden K, Zhang X, Bhatnagar S, Beck S, Valentino R. 2010. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology. 36:896–909.
- Blanchard RJ, Blanchard DC. 1969. Crouching as an index of fear. J Comp Physiol Psychol. 67:370–375.
- Blanchard RJ, Blanchard DC. 1971. Defensive reactions in the Albino rat. Learn Motiv. 2:351–362.
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. 1991. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 50:967–972.
- Borta A, Wöhr M, Schwarting RKW. 2006. Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behav Brain Res. 166:271–280.
- Bourke CH, Neigh GN. 2011. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 60:112–120.
- Brudzynski SM. 2001. Pharmacological and behavioral characteristics of 22 kHz alarm calls in rats. Neurosci Biobehav Rev. 25:611–617.
- Choi JS, Brown TH. 2003. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. J Neurosci. 23:8713–8721.
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. 1999. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 113:902–913.
- Cordero MI, Kruyt ND, Sandi C. Modulation of contextual fear conditioning by chronic stress in rats is related to individual differences in behavioral reactivity to novelty. Brain Res. 2003a; 970:242–245.
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav. 2003b; 44:338–345.
- De Vry J, Benz U, Schreiber R, Traber J. 1993. Shock-induced ultrasonic vocalization in young adult rats: A model for testing putative anti-anxiety drugs. Eur J Pharmacol. 249:331–339.
- Endres T, Widmann K, Fendt M. 2007. Are rats predisposed to learn 22 kHz calls as danger-predicting signals?. Behav Brain Res. 185:69–75.
- Fendt M, Fanselow MS. 1999. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 23:743–760.
- Foilb A, Lui P, Romeo R. 2011. The transformation of hormonal stress responses throughout puberty and adolescence. J Endocrinol. 210:391–398.
- Hamed A, Jaroszewski T, Maciejak P, Szyndler J, Lehner M, Kamecka I, . 2009. The effects of buspirone and diazepam on aversive context- and social isolation-induced ultrasonic vocalisation. Physiol Behav. 98:474–480.
- Harmon KM, Greenwald ML, McFarland A, Beckwith T, Cromwell HC. 2009. The effects of prenatal stress on motivation in the rat pup. Stress. 12:250–258.
- Heim C, Nemeroff CB. 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 49:1023–1039.
- Ilin Y, Richter-Levin G. 2009. Enriched environment experience overcomes learning deficits and depressive-like behavior induced by juvenile stress. PLoS ONE. 4:e4329.
- Jacobson-Pick S, Richter-Levin G. 2010. Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav Brain Res. 214:268–276.
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. 2011. Stress vulnerability during adolescent development in rats. Endocrinology. 152:629–638.
- Jelen P, Soltysik S, Zagrodzka J. 2003. 22-kHz ultrasonic vocalization in rats as an index of anxiety but not fear: Behavioral and pharmacological modulation of affective state. Behav Brain Res. 141:63–72.
- Kendler KS, Karkowski LM, Prescott CA. 1999. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 156:837–841.
- Kikusui T, Nishizawa D, Takeuchi Y, Mori Y. 2003. Conditioned fear-related ultrasonic vocalizations are emitted as an emotional response. J Vet Med Sci. 65:1299–1305.
- Knutson B, Burgdorf J, Panksepp J. 1998. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 112:65–73.
- Knutson B, Burgdorf J, Panksepp J. 1999. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 66:639–643.
- Knutson B, Burgdorf J, Panksepp J. 2002. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 128:961–977.
- Kosten TA, Lee HJ, Kim JJ. 2006. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 1087:142–150.
- Kosten TA, Miserendino MJ, Bombace JC, Lee HJ, Kim JJ. 2005. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav Brain Res. 157:235–244.
- Lehmann J, Pryce CR, Bettschen D, Feldon J. 1999. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 64:705–715.
- Litvin Y, Blanchard DC, Blanchard RJ. 2007. Rat 22 kHz ultrasonic vocalizations as alarm cries. Behav Brain Res. 182:166–172.
- Maggio N, Segal M. 2011. Persistent changes in ability to express long-term potentiation/depression in the rat hippocampus after juvenile/adult stress. Biol Psychiatry. 69:748–753.
- Mällo T, Matrov D, Koiv K, Harro J. 2009. Effect of chronic stress on behavior and cerebral oxidative metabolism in rats with high or low positive affect. Neuroscience. 164:963–974.
- Maren S. 2001. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 24:897–931.
- McCormick CM, Mathews IZ. 2007. HPA function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 86:220–233.
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010a; 72:73–85.
- McCormick CM, Nixon F, Thomas C, Lowie B, Dyck J. Hippocampal cell proliferation and spatial memory performance after social instability stress in adolescence in female rats. Behav Brain Res. 2010b; 208:23–29.
- McCormick CM, Smith C, Mathews IZ. 2008. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 187:228–238.
- Meerlo P, Horvath KM, Nagy GM, Bohus B, Koolhaas JM. 1999. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J Neuroendocrinol. 11:925–933.
- Molewijk HE, van der Poel AM, Mos J, van der Heyden JA, Olivier B. 1995. Conditioned ultrasonic distress vocalizations in adult male rats as a behavioural paradigm for screening anti-panic drugs. Psychopharmacology (Berl). 117:32–40.
- Morgan KN, Thayer JE, Frye CA. 1999. Prenatal stress suppresses rat pup ultrasonic vocalization and myoclonic twitching in response to separation. Dev Psychobiol. 34:205–215.
- Morrissey MD, Mathews IZ, McCormick CM. 2011. Enduring deficits in contextual and auditory fear conditioning after adolescent, not adult, social instability stress in male rats. Neurobiol Learn Mem. 95:46–56.
- Oztan O, Aydin C, Isgor C. 2011. Stressful environmental and social stimulation in adolescence causes antidepressant-like effects associated with epigenetic induction of the hippocampal BDNF and mossy fibre sprouting in the novelty-seeking phenotype. Neurosci Lett. 501:107–111.
- Pellow S, Chopin P, File SE, Briley M. 1985. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 14:149–167.
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. 2007. Repeated exposure to stress across the childhood-adolescent period alters rats' anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci. 121:462–474.
- Pryce CR, Bettschen D, Nanz-Bahr NI, Feldon J. 2003. Comparison of the effects of early handling and early deprivation on conditioned stimulus, context, and spatial learning and memory in adult rats. Behav Neurosci. 117:883–893.
- Ricon T, Toth E, Leshem M, Braun K, Richter-Levin G. 2012. Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress. 15:11–20.
- Romeo RD. 2010. Adolescence: A central event in shaping stress reactivity. Dev Psychobiol. 52:244–253.
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, . 2006. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 147:1664–1674.
- Romeo RD, McEwen BS. 2006. Stress and the adolescent brain. Ann NY Acad Sci. 1094:202–214.
- Sales GD. 1972. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav. 20:88–100.
- Sanchez C. 2003. Stress-induced vocalisation in adult animals. A valid model of anxiety?. Eur J Pharmacol. 463:133–143.
- Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. 2001. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 102:329–339.
- Schwarting RKW, Jegan N, Wöhr M. 2007. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav Brain Res. 182:208–222.
- Spear L. 2000. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 24:115–123.
- Swiergiel AH, Zhou Y, Dunn AJ. 2007. Effects of chronic footshock, restraint and corticotropin-releasing factor on freezing, ultrasonic vocalization and forced swim behavior in rats. Behav Brain Res. 183:178–187.
- Toledo-Rodriguez M, Sandi C. 2007. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007:71203.
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, . 2008. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 107:522–532.
- Tsoory M, Cohen H, Richter-Levin G. 2007. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 17:245–256.
- Tsoory M, Guterman A, Richter-Levin G. 2008. Exposure to stressors during juvenility disrupts development-related alterations in the PSA-NCAM to NCAM expression ratio: Potential relevance for mood and anxiety disorders. Neuropsychopharmacology. 33:378–393.
- Tsoory M, Richter-Levin G. 2006. Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress. Int J Neuropsychopharmacol. 9:713–728.
- Tsoory MM, Guterman A, Richter-Levin G. 2010. “Juvenile stress” alters maturation-related changes in expression of the neural cell adhesion molecule L1 in the limbic system: Relevance for stress-related psychopathologies. J Neurosci Res. 88:369–380.
- van der Poel AM, Miczek KA. 1991. Long ultrasonic calls in male rats following mating, defeat and aversive stimulation: Frequency modulation and bout structure. Behaviour. 119:127–142.
- van der Poel AM, Noach EJ, Miczek KA. 1989. Temporal patterning of ultrasonic distress calls in the adult rat: Effects of morphine and benzodiazepines. Psychopharmacology (Berl). 97:147–148.
- Vidal J, Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. 2007. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 92:824–830.
- Weintraub A, Singaravelu J, Bhatnagar S. 2010. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 1343:83–92.
- Williams MT, Hennessy MB, Davis HN. 1998. Stress during pregnancy alters rat offspring morphology and ultrasonic vocalizations. Physiol Behav. 63:337–343.
- Wöhr M, Borta A, Schwarting RKW. 2005. Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: A dose-response study in the rat. Neurobiol Learn Mem. 84:228–240.
- Wöhr M, Schwarting RKW. 2007. Ultrasonic communication in rats: Can playback of 50-kHz calls induce approach behavior?. PLoS ONE. 2:e1365.
- Wöhr M, Schwarting RKW. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav Neurosci. 2008a; 122:310–330.
- Wöhr M, Schwarting RKW. Ultrasonic calling during fear conditioning in the rat: No evidence for an audience effect. Anim Behav. 2008b; 76:749–760.
- Wöhr M, Schwarting RKW. 2009. Activation of limbic system structures by replay of ultrasonic vocalization in rats. In: Brudzynski SM. editors. Handbook of mammalian vocalization. Oxford: Academic Press113–124.
- Yee N, Plassmann K, Fuchs E. 2011. Juvenile stress impairs body temperature regulation and augments anticipatory stress-induced hyperthermia responses in rats. Physiol Behav. 104:408–416.
- Young EA, Abelson JL, Curtis GC, Nesse RM. 1997. Childhood adversity and vulnerability to mood and anxiety disorders. Depress Anxiety. 5:66–72.
