Abstract
Adolescence is a period of heightened susceptibility to anxiety disorders, yet we have little experimental evidence on what factors may lead to psychopathology in adolescence. Preclinical models of extinction are commonly used to study the treatment of anxiety symptoms. Interestingly, recent research has shown that there are fundamental changes in the process of extinction across development, which may have implications for our understanding of psychopathology across the lifespan. Specifically, this research shows that the process of extinction parallels the nonlinear function of prefrontal cortex development, such that extinction behaviour is similar in juvenile and adult rats, but involves different processes in infancy and adolescence (periods of rapid growth and pruning, respectively). Our previous studies have shown that early-life stress accelerates the transition between infant and juvenile extinction systems. In the current series of experiments, we examined whether the same early-life stress, maternal separation (MS), would lead to an earlier transition between the juvenile and adolescent extinction systems, and between the adolescent and adult extinction systems. We show that MS adolescent rats exhibit more adult-like extinction behaviour, and that adolescent-like extinction emerges earlier in development (i.e. in pre-adolescent rats). These results may have important implications for the understanding and treatment of anxiety symptoms in adolescent populations.
Introduction
Adolescence is a period of heightened susceptibility to anxiety disorders and other mental health problems; as many as half of all mental health disorders first emerge in the adolescent period (Kessler et al. Citation2005). This surge in the onset of mental health problems has prompted an increasing interest in the adolescent years as a critical period for mental health treatment and intervention. Yet, despite this increasing interest, most current research continues to focus on understanding and treating anxiety disorders in adults. Unfortunately, we still know very little about how anxiety disorders emerge and how they can be treated early in life (Pine et al. Citation2009). Importantly, recent preclinical research has begun to document substantive developmental differences in the processes by which fear is inhibited, findings that may have important implications for the understanding and treatment of anxiety disorders across the lifespan.
The development and treatment of anxiety disorders can be modelled in rats using the processes of Pavlovian fear conditioning and extinction, respectively. In a typical fear conditioning procedure, a rat is presented with pairings of an initially neutral conditioned stimulus (CS) and an aversive unconditioned stimulus (US). Following such pairings, the animal forms an association between these two stimuli and begins to exhibit fear responses to the CS. After conditioning, however, the fear elicited by that CS can be inhibited through the process of extinction, whereby the CS is repeatedly presented without the US. During extinction, the animals' fear responses to the CS progressively decrease, and it is thought that this involves the development of a new inhibitory association between the CS and US (Bouton Citation1993). The process of extinction is widely accepted as the basis for exposure-based therapies (e.g. Rothbaum and Davis Citation2003; Delgado et al. Citation2006). Interestingly, recent research has shown that there are fundamental changes in the process of extinction across development which parallel maturational changes in cortical control networks of the brain, namely the prefrontal cortex (PFC). The PFC has a protracted development and matures in a nonlinear function, first increasing in size across childhood then dipping dramatically in volume during the adolescent period before again increasing and stabilising post-adolescence (Shaw et al. Citation2008). Considering this developmental profile, it is not surprising that extinction behaviour in pre-adolescent (juvenile) and adult rats involves similar behavioural and neural mechanisms. Specifically, in postnatal day (P) 24 (juvenile) and P70+ (adult) rats, extinction is context dependent as shown by the return of fear following a change in the physical context (Bouton Citation1993; Kim and Richardson Citation2007a,Citationb). In addition, the infralimbic prefrontal cortex (ilPFC) is critically involved in extinction at these ages (Morgan et al. Citation1993; Quirk et al. Citation2000; Kim et al. Citation2009). However, extinction is fundamentally different at an earlier period of development. Specifically, it has been shown that P17 (infant) rats do not require ilPFC activity to inhibit their fear responses during extinction (Kim et al. Citation2009). Further, in animals this age-learned fear is extinguished in a way that reduces the likelihood of relapse. In other words, after extinction at P17, rats were shown to exhibit a loss of fear which was not recovered by a change in the context (Kim and Richardson Citation2007a,Citationb; Yap and Richardson Citation2007; also see Gogolla et al. Citation2009 for a similar study with mice). Hence, it has been suggested that a qualitative change in extinction takes place between the infant and juvenile periods of development, represented by the increasing involvement of the ilPFC in extinction learning and the emergence of relapse behaviours (Kim and Richardson Citation2010).
During adolescence, the PFC undergoes a high degree of pruning and reorganisation (Cunningham et al. Citation2002; Shaw et al. Citation2008), which has functional consequences for the extinction of learned fear. Specifically, while postnatal day (P) 35 (adolescent) rats were shown to exhibit similar learning about a CS as juvenile and adult rats, and were equally apt in extinguishing their fear responses to that CS, they were markedly impaired in the retention of the extinction memory (McCallum et al. Citation2010; Kim et al. Citation2011). That is, while 30 extinction trials were sufficient to produce good retention of extinction training in juvenile and adult rats, adolescents required twice the amount of extinction training (60 trials) to show good extinction retention the following day. In addition, while ilPFC activity was increased following 30 extinction training trials in juvenile and adult rats, ilPFC activity was not increased following this amount of extinction training in adolescents (Kim et al. Citation2011). However, adolescent rats did exhibit increased ilPFC activity when administered double the amount of extinction training (Kim et al. Citation2011). Hence, it would appear that during adolescence, PFC involvement in extinction is dramatically reduced resulting in the return of fear just 24 h later.
Taken together, these data clearly show that there are dynamic changes, both behaviourally and neurally, in the process of extinction. The development of effective exposure-based treatments for anxiety disorders during childhood and adolescence will require a greater understanding of these changes. It should also be noted that periods of rapid brain growth are often windows of vulnerability for environmental input and alteration of underlying neural systems and behaviour (e.g. Lupien et al. Citation2009). Considering the protracted development of the PFC, it is possible that alterations in the postnatal environment could affect the maturation of this structure, perhaps altering the normal changes in extinction which take place across development. Indeed, in previous research, we found that early-life adversity, in the form of postnatal maternal separation (MS), accelerated the transition between infant-like extinction and adult-like extinction in the P17 rat (Callaghan and Richardson Citation2011). In that study, rats were separated from their mother for 3 h per day across postnatal days 2–14 or were left with their mother for the same period of time. It was shown that those rats that were separated made an early transition between the infant-like, relapse-resistant, extinction system and the adult-like, relapse-prone, extinction system. In other words, infant rats that were MS early in life showed increased relapse behaviours characteristic of juvenile and adult standard-reared (SR) rats. In addition, these maternally-separated rats used a neurotransmitter [gamma-Aminobutyric acid (GABA)] that is not involved in the infant extinction system but is involved in the adult extinction system. These findings were interpreted as suggestive evidence that stress in the early postnatal environment altered the developmental trajectory of the PFC, leading to an early transition between the infant -and adult-like extinction systems. In the current study, we examined whether MS during the same postnatal period would have any effect on extinction during adolescence. Given that early-life stress caused an earlier transition from the infant- to the adult-like extinction system, we predicted that it would also cause an earlier transition into, and early emergence out of, the adolescent extinction system.
Materials and methods
Animals
The experiments were carried out in accordance with the principles of animal care and use outlined in Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (7th ed., Citation2004), and all procedures were approved by the Animal Care and Ethics Committee at The University of New South Wales. Experimentally naive Sprague-Dawley-derived rats, bred and housed at the School of Psychology, The University of New South Wales, were used. Before weaning, rats were housed with their mother and littermates in plastic boxes (24.5-cm long × 37-cm wide × 27-cm high) covered by a wire lid. At postnatal day (P) 21( ± 1), male rats that had been exposed to the same rearing condition (i.e. MS or SR) were weaned and housed in groups of eight and were maintained on a 12-h light-dark cycle (lights on at 07:00 h) with food and water available ad libitum. All experiments began when rats were in the pre-adolescent (P27) or adolescent period (P30–35). Only males were used, and no more than one rat per litter was used per group.
Maternal separation
In all experiments, rats were either exposed to MS or SR. During MS (P2-14), all pups were removed from the home cage, weighed and then placed in an incubator for 3 h (starting between 09:00 h and 12:00 h). The ambient temperature of the incubator was set at 28°C each day of separation (with daily variability ranging between 27°C and 30°C). Three cm of bedding was placed in the incubator so that animals could behaviourally thermoregulate as necessary. SR animals were briefly removed from the home-cage and weighed every day during the MS period (i.e. from P2 to 14). Using this procedure, we do not see any differences in weight between the MS and SR rats at the end of the separation period (i.e. at P14; see Callaghan and Richardson Citation2011).
Apparatus
In all experiments, fear conditioning occurred in one context (A) and extinction training occurred in a different context (B). In Experiments 1 and 3, testing took place in the extinction context, whereas in Experiment 2 testing took place in either the conditioning or the extinction context. Context A was a set of two identical chambers that were rectangular (13.5-cm long × 9-cm wide × 9-cm high), with the front wall, rear wall and ceiling made of clear Plexiglas. The floor and side walls consisted of stainless steel rods set 1 cm apart. Two high-frequency speakers were located 8 cm from either side of the chamber. A custom built constant current shock generator could deliver shock to the floor of each chamber as required. Each chamber was housed within a separate wood cabinet so that external noise and visual stimulation were minimised. A red light-emitting diode (LED) located within the camera mount was the sole source of illumination in these chambers. A low, constant background noise (50 dB, measured by a TENMA sound level meter, Type 72–860) was produced by ventilation fans located within the cabinet. These chambers were wiped clean with tap water after each session. Context B was a set of two identical chambers that were rectangular (30-cm long × 30-cm wide × 23-cm high) and wholly made of Plexiglas, with the exception of the grid floor which was the same as in context A. All the walls were transparent, except for the two side walls that consisted of vertical black and white stripes (each 5-cm wide). Two high-frequency speakers were mounted on the ceiling of each of these chambers which were housed in separate wood cabinets so that external noise and visual stimulation were minimised. A white LED and a red LED located within the camera mount were the sole sources of illumination in these chambers. A low constant background noise (50 dB) was produced by the ventilation fans in the cabinet. Thus, the two sets of contexts differed primarily in their size and their visual features. The CS was a white noise; noise level in the chamber was increased by 8 dB when the CS was presented. A computer controlled all presentations of the CS and the footshock US. The software and hardware used were custom developed at The University of New South Wales.
Behavioural procedures
On day 1, animals were placed in context A, and after a 2-min adaptation period, a white noise CS was presented for 10 s. The shock US (0.6 mA, 1 s) was administered during the last second of the CS. Three pairings of the CS and the US were given. The inter-trial interval (ITI) ranged from 85 to 135 s with a mean of 110 s. Thirty to sixty seconds after the last pairing, rats were returned to their home cages. On day 2, the animals were placed in context B and, after a 2-min adaptation period, presented with 30 non-reinforced presentations of the 10 s CS with a 10 s ITI. No shock was given during extinction. The extinction session was 12 min in duration, and 30–60 s after the last CS presentation animals were returned to their home cages. The following day, at test, the animals were placed in context A or B and their baseline level of freezing in the absence of the CS was recorded for 1 min. The CS was then presented and freezing was recorded for 2 min. No shock was given during test. All extinction and test sessions were recorded for later scoring.
Statistical analyses
The measure of learned fear in all experiments was the amount of CS-elicited freezing, which was defined as the absence of all movement except that required for respiration (Fanselow Citation1980). Freezing was scored by a time sampling procedure whereby each rat was scored every 3 s as freezing or not. These observations were then converted into a percentage score to indicate the proportion of total observations scored as freezing. A scorer blind to experimental group cross-scored approximately 30% of the rats tested in each experiment; the correlation between scorers was high in all cases (smallest r = 0.95). Extinction data were analysed using a mixed-design ANOVA and test data were analysed using independent samples t-tests. Whenever a mixed-design ANOVA was used, if the assumption of sphericity was violated, then Greenhouse-Geisser-adjusted p values and nominal df are reported. For all analyses, a p value of < 0.05 was considered statistically significant. In all experiments, rats that were statistical outliers at test (i.e. >2.5 SD away from the group mean) were excluded from analysis. Using this criterion, two exclusions were made from Experiment 3: one rat from group MS and one rat from group SR. No exclusions were made in Experiment 1 or 2. Across all experiments, baseline freezing at extinction and test was low, and there were no between-group differences in baseline freezing within any of the experiments, all p>0.09.
Results
Experiment 1: MS results in adult-like extinction learning during adolescence
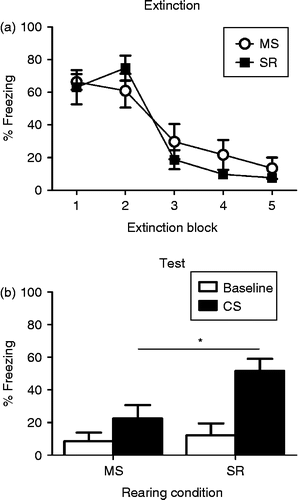
The aim of Experiment 1 was to determine whether MS would affect the retention of extinction learning in adolescent animals. Rats were between the ages of P30 and P35 at the start of the experiment. CS-elicited freezing during the five blocks of extinction training (each block comprising six CSs) is presented in . As can be seen, freezing decreased across blocks, F4,76 = 45.48, p < 0.0001. There was no main effect of rearing condition and no rearing condition by extinction block interaction, largest F(4,76) = 1.63, p = 0.19. Hence, rearing condition did not affect level of conditioned fear or the rate of extinction learning. Rearing condition did, however, affect freezing at test. While the SR rats exhibited substantial return of fear, like what has been previously reported in adolescent rats (see McCallum et al. Citation2010; Kim et al. Citation2011), MS rats exhibited significantly lower levels of CS-elicited freezing (t19 = − 2.72, p < 0.05; see ). These data show that, although MS and SR rats displayed similar levels of learning during conditioning and extinction, MS rats were significantly better at retaining extinction across a 24-h period.
Figure 1. (a) Mean ( ± SEM) levels of CS-elicited freezing during five blocks (six CS presentations in each block) of extinction training in MS (open circles; n = 11) and SR (closed squares, n = 10) adolescent rats. Regardless of rearing condition, rats showed high levels of freezing at the start of extinction that decreased across blocks. (b) Mean ( ± SEM) levels of baseline (white bars) and CS-elicited (black bars) freezing in MS and SR rats at test. * indicates a significant difference in CS-elicited freezing between groups. Baseline fear is low in both rearing conditions but higher levels of CS-elicited fear are present in the SR adolescent rats than the MS adolescent rats.
Experiment 2: MS adolescent rats exhibit renewal of fear
In Experiment 1, adolescent MS rats exhibited a more adult-like pattern of extinction than did SR rats of the same age. That is, while we replicated the recent finding that SR adolescent rats exhibit impaired retention of extinction (McCallum et al. Citation2010; Kim et al. Citation2011), we additionally showed that MS adolescents exhibit good extinction retention. There are at least two possible reasons why MS adolescent rats exhibited low levels of freezing at test in Experiment 1. First, it is possible that early-life stress accelerated the transition between adolescent and adult extinction systems, much like it accelerates the transition between infant and juvenile extinction systems. Therefore, the adolescent MS rats were exhibiting better retention of extinction, like adult rats. Alternatively, the low levels of freezing in the MS adolescent rats may merely reflect a tendency of these animals to exhibit very low levels of freezing following extinction training, regardless of the circumstances. Therefore, the aim of Experiment 2 was to determine whether MS adolescent rats always exhibit low levels of fear following extinction, or whether these rats could exhibit high levels of freezing at test 24 h later, at least in certain situations. To test this, Experiment 2 examined whether adolescent MS rats exhibit the renewal effect (i.e. a return of fear when tested in a context different from that used in extinction training). Only MS animals were used in this experiment, and animals were between ages of P30–P35 at the start of the experiment.
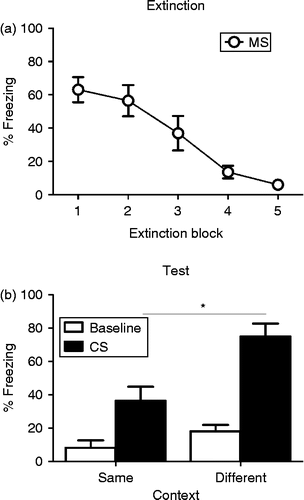
Animals were trained in Context A, extinguished in Context B and then tested in either Context A or B. CS-elicited freezing during the five blocks of extinction training is presented in . The effect of subsequent test context on behaviour during extinction was not significant, nor was the interaction, largest F(1,14) = 1.68, p = 0.22, so the groups were collapsed for this analysis. Levels of freezing were high at the beginning of extinction but decreased substantially by the conclusion of extinction training (F(4,60) = 18.1, p = < 0.0001). Freezing at test depended on the context in which animals were tested. Rats tested in the same context as extinction exhibited low levels of CS-elicited freezing (replicating Experiment 1), while rats tested outside of the extinction context exhibited much higher levels of CS-elicited freezing (t14 = − 3.36, p < 0.01). In other words, MS adolescent rats exhibited the renewal effect indicating that the failure to observe a return of fear in Experiment 1 was not merely due to MS adolescent rats always expressing low levels of CS-elicited freezing following extinction.
Figure 2. (a) Mean ( ± SEM) levels of CS-elicited freezing during five blocks (six CS presentations in each block) of extinction training in MS (open circles) adolescent rats. Rats show high levels of fear at the beginning of extinction that decreases across blocks. (b) Mean ( ± SEM) levels of baseline (white bars) and CS-elicited (black bars) freezing in MS rats tested in the same (n = 8) or different (n = 8) context as extinction training. * indicates a significant difference in CS-elicited freezing between groups. Baseline fear at test is low, regardless of context, but higher levels of CS-elicited fear are present in the MS rats tested in a different context to extinction training.
Experiment 3: MS results in early emergence of ‘adolescent-like' extinction retention in pre-adolescent rats
In this experiment, we tested whether MS rats exhibit the typical ‘adolescent’ pattern of extinction (i.e. fear relapse 24 h after extinction) at an earlier developmental stage. MS and SR rats were compared; rats were P27 at the start of the experiment.
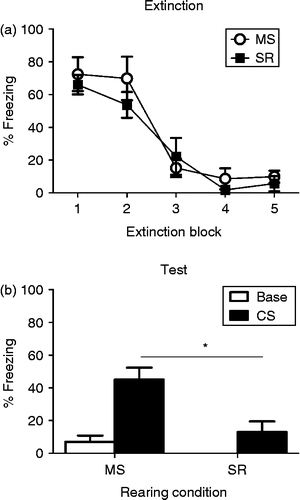
CS-elicited freezing during extinction training is presented in . As can be seen, MS and SR rats exhibited high, and similar, levels of freezing at the start of extinction, which decreased to similar low levels by the end of extinction training. There was a significant effect of extinction block, F(4, 56) = 41.21, p < 0.0001, and no effect of rearing condition or rearing condition by extinction block interaction, largest F < 1. Rearing condition did, however, affect freezing during test. MS pre-adolescent rats exhibited a higher level of CS-elicited freezing at test than did standard-reared rats of the same age (t14 = 3.51, p = < 0.01). Hence, MS rats do show the typical ‘adolescent’ pattern of extinction (i.e. fear relapse 24 h later), albeit at an earlier stage of development.
Figure 3. (a) Mean ( ± SEM) levels of CS-elicited freezing during five blocks (six CS presentations in each block) of extinction training in MS (open circles, n = 7) and SR (closed squares; n = 9) pre-adolescent rats. Rats show high, and similar, levels of fear at the beginning of extinction that decreases across blocks. (b) Mean ( ± SEM) levels of baseline (white bars) and CS-elicited (black bars) freezing in MS and SR rats during test. * indicates a significant difference in CS-elicited freezing between groups. Baseline fear is low in both rearing conditions but higher levels of CS-elicited fear are present in MS pre-adolescent rats than in SR rats of the same age.
Discussion
The results of this series of experiments demonstrate that an adverse early-life experience, MS, leads pre-adolescent and adolescent rats to exhibit extinction behaviour characteristic of older animals. Specifically, recent research has shown that adolescent rats exhibit markedly impaired extinction retention (i.e. the fear returns at test 24 h later) compared to adult rats (see McCallum et al. Citation2010; Kim et al. Citation2011). However, in the present study adolescent rats that had been maternally-separated early in life did not show this return of fear 24 h after extinction; instead, they exhibited good retention of extinction, like what is observed in adult animals. Importantly, the effect of MS on extinction retention occurred independently of changes in fear learning between the two rearing conditions, because MS had no effect on the initial amount of fear learned. Indeed, using these parameters, we have consistently failed to observe an effect of MS on fear learning (the present results; Callaghan and Richardson Citation2011). We have seen this in P17, P27 and P30–35 rats, suggesting that, using our procedures, MS specifically affects the fear inhibition system while sparing fear learning.
In the present experiments, MS did not abolish the ‘adolescent-like’ extinction profile, but simply resulted in a leftward shift in the developmental trajectory of this behaviour (i.e. emergence of the adolescent phenotype in pre-adolescence). This finding is consistent with our earlier experiments in which MS accelerated the developmental shift between infant and juvenile extinction phenotypes (Callaghan and Richardson Citation2011). The results of Experiment 2 demonstrated that the enhanced extinction retention seen in adolescent MS rats was not simply due to an inability of these animals to exhibit fear following extinction, as high levels of fear were evident when they were tested in a different context to extinction training (i.e. the renewal effect was observed).
There are various possible explanations as to why early-life adversity accelerated the developmental transition between the adolescent and adult behavioural phenotypes following extinction. One possibility is that MS accelerated the maturation of neural structures critical for adult-like extinction behaviour, namely, the PFC. As previously mentioned, the PFC is a late developing structure in the rat (e.g. Van Eden and Uylings Citation1985), and the involvement of the PFC in fear extinction varies depending on the animal's age; although the PFC is critically involved in adult extinction, it is not involved in infant extinction and is only involved in adolescent extinction when animals are given extra extinction training (Kim et al. Citation2009, Citation2011). Considering our present and previous (Callaghan and Richardson Citation2011) findings, that early-life stress results in a more ‘adult-like’ pattern of extinction in infant and adolescent rats, it is possible that MS speeds up the development of the PFC. Indeed, changes in the PFC have been shown to occur following stress in adult animals (see McEwen Citation2006, for a review). However, we know considerably less about the effects of early-life stress on the maturation of the PFC. In one recent study, however, Uchida et al. (Citation2010) found that the same MS protocol used here led to increased expression of the splicing variant of the Repressor element-1 silencing transcription factor (REST4) in the medial PFC of P14 rats. Importantly, REST4 regulates genes and microRNAs critical for synaptic plasticity and brain development, the expression of which was also increased following MS (e.g. mir124; Uchida et al. Citation2010). It is possible then that stress produces changes in the molecular machinery involved in neuronal development and plasticity, subsequently leading to accelerated PFC development and early emergence of later-developing extinction systems.
An alternative possibility to stress accelerating PFC development is that adolescent MS rats may be achieving adult-like extinction behaviour via a slightly different mechanism, i.e. via different neural circuitry, than adult rats. In other words, it is possible that stress permits the utilisation of different brain regions to achieve the same behaviour. Indeed, there are examples in the literature whereby learning can occur via activation of compensatory neural pathways when the integrity of the primary learning pathway is damaged or inactive (e.g. Poulos et al. Citation2010). Although it is currently unclear which alternate brain regions may be able to compensate for PFC involvement in fear extinction, it is likely that such areas would be stress responsive and may become functional earlier in development than the PFC. Differentiation of these two possibilities should be addressed in future studies.
It is presently unclear what the functional consequences of early emerging adolescent-like extinction in MS rats might be. While it is clear that many psychological disorders emerge in the adolescent years, most adolescents transition through this stage without developing persistent problems (Kessler et al. Citation2005; Costello et al. Citation2011). This suggests that in addition to some universal factors that increase risk for the adolescent population as a whole, there are also specific factors which heighten individual risk for adolescent mental health problems (Casey et al. Citation2011). Given that recent research has shown that adolescent rats have impaired extinction retention, relative to both younger and older animals (McCallum et al. Citation2010; Kim et al. Citation2011), this could act as a universal predisposing factor to the emergence of anxiety symptoms and other mental health problems at this stage of development. However, whether additional factors (e.g. early-life stress) interact with the changes in extinction during adolescence to heighten individual risk for anxiety symptoms remains a relatively unexplored area, despite the well-known fact that early abuse, trauma and neglect increase the risk for later emerging psychopathology (e.g. Heim and Nemeroff Citation2001, Citation2002; Repetti et al. Citation2002; Chapman et al. Citation2004; Lupien et al. Citation2009). Interestingly, in the present experiments, we showed that adolescent rats exposed to early-life MS stress actually exhibited enhanced retention of extinction relative to their non-stressed peers. While high rates of fear relapse following extinction are generally considered to reflect anxious and maladapted behaviour, good extinction retention, like that seen in stressed adolescent rats, is generally considered indicative of good adjustment. What these results might indicate then is that adolescent rats are actually more resilient to adverse events following early-life adversity. Indeed, previous research has shown that exposure to stressful experiences early in life can ‘inoculate’ individuals against stress experienced later in life. For example, adolescent males who reported experiencing higher numbers of previous stressful events showed the lowest levels of cardiovascular reactivity to presently experienced stress (Boyce and Chesterman Citation1990). Also, monkeys exposed to the stressful experience of high foraging demand in infancy exhibited a diminished hypothalamic–pituitary–adrenal axis response to stress in adulthood (Parker et al. Citation2006). In fact, it has been argued that, rather than promoting resilience to all situations, early-life stress prepares individuals to function adaptively specifically in subsequent stressful situations, while impairing their ability to function under non-stressful conditions (e.g. Ricon et al. Citation2012). To illustrate that point, Ricon and colleagues exposed rats to neonatal MS (P7–14) and then exposed those rats to a learning task in adulthood which occurred after experiencing either a period of unpredictable chronic stress or after no stress. It was found that those animals tested after a second stressor in adulthood performed better on the learning task than animals not exposed to the additional stressor, and exhibited similar performance on the learning task to rats that were never stressed. In contrast, the rats exposed to the single MS stressor exhibited impaired performance relative to non-stressed controls. Hence, it is possible that MS in the current studies led to enhanced resilience to a second stressful experience (fear conditioning and extinction) in adolescence.
While it is possible that MS rats in the current experiments exhibited increased resilience to stress, such an explanation fails to account for the results of Experiment 3. In that experiment, it was shown that the adolescent profile of extinction is not abolished, but is merely brought forward in development to be expressed in pre-adolescence. Those findings suggest that rather than promoting ‘resilience’ to a second stressor in adolescence, early-life stress was instead accelerating the normal developmental trajectory of extinction behaviour, such that adult-like extinction was apparent in adolescent rats. Based on those results, perhaps a more likely alternative is that early-life stress is maladaptive because it alters the developmental timing of the fear inhibition system. Indeed, precise developmental timing has been shown to be critical for the proper functioning of perceptual systems. For example, early visual stimulation of the quail embryo was shown to accelerate development of the visual system, allowing hatched quails to utilise visual information at earlier stages of development than non-stimulated chicks (Lickliter Citation1990a,Citationb). However, in those studies, the premature development of the visual system came at a cost; chicks that received the prenatal visual stimulation were impaired in their responsiveness to auditory maternal cues. Such results suggest that the staggered development of different perceptual modalities is actually adaptive for the animal, serving to limit sensory load and reduce the competition between developing sensory systems (Turkewitz and Kenny Citation1982, Citation1985; Lickliter Citation2000). Hence, the early maturation of the adolescent extinction system in postnatally stressed pre-adolescent rats, and possibly accelerated maturation of the brain regions which support mature extinction (i.e. the PFC), may have negative outcomes for concurrently developing neural and behavioural systems which could ultimately increase risk for psychopathology. Although speculative, there are many intriguing potential implications of this idea. For example, while early-life stress accelerates the development of extinction in aversive learning procedures, it may negatively affect extinction learning of appetitive conditioning. This is an important question to examine, because substance abuse disorders are a common diagnosis during adolescence (Costello et al. Citation2011), and substance abuse is highly comorbid with mood and anxiety disorders (Merikangas and Kalaydjian Citation2007; Kessler and Wang Citation2008).
One limitation of the current set of experiments is that the lack of adult comparison groups prevents us from making conclusive statements regarding the behavioural phenotype of MS adolescent rats as ‘adult-like’. However, across numerous experiments, using similar conditioning procedures as ours, adult rats have been shown to exhibit good extinction retention when tested 24 h following extinction training, evidenced by low levels of conditioned fear at test (McCallum et al. Citation2010; Kim et al. Citation2011; Quirk Citation2002). Further, there are now several studies which show that adolescent rats are impaired in retaining an extinction memory across a 24-h period relative to adult animals, i.e. the adolescent rats display higher levels of freezing than adult rats at test (Kim et al. Citation2011; McCallum et al. Citation2010). Importantly, in the current experiments, MS adolescent rats were freezing at low levels during test indicating good extinction retention, which is what would be expected in adult rats under normal conditions. Hence, the higher levels of freezing in adolescent SR rats can be considered as relatively poor extinction retention, which is what would be expected in typically developing adolescent animals.
We have provided clear evidence that MS early in life leads to an early emergence of adolescent-like extinction behaviours while preserving the ability of these animals to exhibit fear relapse following extinction. While it is still unclear whether these altered behaviours in MS adolescent animals are adaptive or maladaptive, ultimately, what these studies illustrate is that early-life environments have a strong and lasting impact on the developing organism, affecting behaviours which are important for the function and survival of the animal across the lifespan. Further research is clearly needed to quantify the effect of MS on the neural development of the PFC in adolescent animals and the functionality of altered extinction behaviours in different learning procedures (i.e. appetitive learning). Through further research into these areas we may be able to gain greater insight into how the early-life environment programmes the animal to develop along a trajectory towards health or illness.
Acknowledgements
This research was supported by Australian Research Council Discovery Project Grant (DP0985554) to RR and by an Australian Postgraduate Award to BLC. Financial disclosures: Neither of the authors have any financial disclosures.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Australian code of practice for the care and use of animals for scientific purposes. 2004. Canberra (Australia): Australian Government Publishing Service.
- Bouton ME. 1993. Context, time and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 114:80–99.
- Boyce WE, Chesterman E. 1990. Life events, social support, and cardiovascular reactivity in adolescence. J Dev Behav Paediatr. 11:105–111.
- Callaghan BL, Richardson R. 2011. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci. 125:20–28.
- Casey BJ, Ruberry EJ, Libby V, Glatt CE, Hare T, Soliman F, Duhoux S, Frielingsdorf H, Tottenham N. 2011. Transitional and translational studies on risk for anxiety. Depress Anxiety. 28:18–28.
- Chapman D, Whitfield C, Felitti V, Dube S, Edwards V, Anda R. 2004. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disorders. 82:217–225.
- Costello EJ, Copeland W, Angold A. 2011. Trends in psychopathology across the adolescent years: What changes when children become adolescents, and when adolescents become adults?. J Child Psychol Psychiatry. 52:1015–1025.
- Cunningham MG, Bhattacharyya S, Benes FM. 2002. Amygdalocortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J Compar Neurol. 453:116–130.
- Delgado MA, Olsson A, Phelps EA. 2006. Extending animal models of fear conditioning to humans. Biol Psychiatry. 73:39–48.
- Fanselow MS. 1980. Signalled shock-free periods and preference for signalled shock. J Exp Psychol; Animal Behav Process. 6:65–80.
- Gogolla N, Caroni P, Lüthi A, Herry C. 2009. Perineuronal nets protect fear memories from erasure. Science. 325:1258–1261.
- Heim C, Nemeroff CB. 2001. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 49:1023–1039.
- Heim C, Nemeroff CB. 2002. Neurobiology of early-life stress: Clinical studies. Semin Clin Neuropsychol. 7:147–159.
- Kessler RC, Berglund R, Demler O, Jin R, Merikangas KR, Walters EE. 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 62:593–602.
- Kessler RC, Wang CS. 2008. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health. 29:115–129.
- Kim JH, Hamlin AS, Richardson R. 2009. Fear extinction across development: The involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 29:10802–10808.
- Kim JH, Richardson R. A developmental dissociation in reinstatement of an extinguished fear response in rats. Neurobiol Learn Mem. 2007a; 88:48–57.
- Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007b; 121:131–139.
- Kim JH, Richardson R. 2010. New findings on extinction of conditioned fear early in development: Theoretical and clinical implications. Biol Psychiatry. 67:297–303.
- Kim JH, Li S, Richardson R. 2011. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb Cortex. 21:530–538.
- Lickliter R. 2000. The role of sensory stimulation in perinatal development: Insights from comparative research of the high-risk infant. Dev Behav Paediatrics. 21:437–447.
- Lickliter R. Premature visual stimulation accelerates intersensory functioning in bobtail quail neonates. Dev Psychobiol. 1990a; 23:15–27.
- Lickliter R. Premature visual experience facilitates visual responsiveness in bobtail quail neonates. Infant Behav Dev. 1990b; 13:487–496.
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. 2009. Effects of stress throughout the lifespan on brain, behaviour and cognition. Nat Rev Neurosci. 10:434–445.
- McCallum J, Kim JH, Richardson R. 2010. Impaired extinction retention in adolescent rats: Effects of D-cycloserine. Neuropsychopharmacology. 35:2134–2142.
- McEwen BS. 2006. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 87:873–904.
- Merikangas KR, Kalaydjian A. 2007. Magnitude and impact of comorbidity of mental disorders from epidemiologic surveys. Curr Opin Psychiatry. 20:353–358.
- Morgan MA, Romanski LM, LeDoux JE. 1993. Extinction of emotional learning: Contribution of the medial prefrontal cortex. Neurosci Lett. 163:109–113.
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. 2006. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance and primates. Proc Natl Acad Sci. 103:3000–3005.
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, Fox NA. 2009. Challenges in developing novel treatments for childhood disorders: Lessons from research on anxiety. Neuropsychopharmacology. 34:213–228.
- Poulos AM, Ponnusamy R, Dong H-W, Fanselow MS. 2010. Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proc Natl Acad Sci USA. 107:14881–14886.
- Quirk GJ. 2002. Memory for extinction of conditioned fear is long lasting and persists following spontaneous recovery. Learn Mem. 9:361–363.
- Quirk GJ, Russo GK, Barron JL, Lebron K. 2000. The role of the ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 20:6225–6231.
- Repetti RL, Taylor SE, Seeman TE. 2002. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 128:330–366.
- Ricon T, Toth E, Leshem M, Braun K, Richter-Levin G. 2012. Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress. 15:11–20.
- Rothbaum BO, Davis M. 2003. Applying learning principals to the treatment of post-trauma reactions. Ann New York Acad Sci. 1008:112–121.
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. 2008. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 28:3586–3594.
- Turkewitz G, Kenny PA. 1982. Limitations on input as a basis for neural organization and perceptual development: A preliminary theoretical statement. Dev Psychobiol. 15:357–368.
- Turkewitz G, Kenny PA. 1985. The role of developmental limitations of sensory input on sensory/perceptual organization. J Dev Behav Paediatrics. 6:302–306.
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y. 2010. Early-life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 30:15007–15018.
- Van Eden CG, Uylings HBM. 1985. Cytoarchitectonic development of the prefrontal cortex of the rat. J Comp Neurol. 241:253–267.
- Yap CSL, Richardson R. 2007. Extinction in the developing rat: An examination of renewal effects. Dev Psychobiol. 49:565–575.
