Abstract
A fundamental goal of stress research is to understand how individuals cope with challenges. Studies on a range of vertebrate species suggest that three groups of behaviour—affiliative, aggressive and self-directed behaviours—serve as coping strategies. To date, experimental studies of coping behaviour have tended to be conducted in captive conditions; the limited number of studies in free-ranging or wild settings have been observational in nature. We investigated coping behaviours in free-ranging female Barbary macaques (Macaca sylvanus) at Trentham Monkey Forest, UK, using an experimental playback approach to quantify subjects' responses to mildly aversive threat-grunts. Compared to silent control trials, playbacks of threat-grunts increased aggressive behaviours and one of the two self-directed behaviours examined (self-scratching). No such differences were seen for self-grooming, or for any affiliative behaviour. Elevations in the rate of one measure of aggression, lunging, were positively related to an average measure of adrenocortical activity (median faecal glucocorticoid metabolite levels over the study period). Evidence from females in a variety of Old World monkey species, including Barbary macaques, indicates that affiliative behaviours have an important role in coping with stressful events in the medium to longer term. Our results suggest that, in the short term, female Barbary macaques may use aggressive rather than affiliative behaviours in response to mild stress. These findings highlight the importance of considering how coping mechanisms may vary over time after a stressor, and how coping mechanisms relate to adrenocortical activity. Playback approaches like ours provide a powerful, flexible tool to explore issues such as this in free-ranging and wild animal populations.
Introduction
Understanding why individuals vary in their susceptibility to stress-related disease is a key goal of stress research (McEwen Citation1998; Koolhaas et al. Citation2010), with implications in both ecological and biomedical contexts (Romero et al. Citation2009; McEwen and Wingfield Citation2010). Key to addressing this goal is identifying coping strategies; following Wechsler (Citation1995), these are defined here as behavioural responses to aversive, fitness-threatening situations. While it is often suggested that coping strategies serve to alleviate physiological stress responses (Weiss Citation1968; Levine and Ursin Citation1991; Cheney and Seyfarth Citation2009), evidence for a simple, causal relationship is rare, especially outside of the laboratory (Cheney and Seyfarth Citation2009). It is likely that associations between behavioural responses and stress physiology are more complex, involving feedback loops between a variety of biological and environmental components (Romero et al. Citation2009). Animal models have been widely used to explore how individuals cope with stress, with ‘fight-or-flight’ (Cannon Citation1915) and ‘freeze’ (Engel and Schmale Citation1972) responses being the first to be examined. More recently, attention has been focused on the coping role of affiliative (Taylor et al. Citation2000; Cheney and Seyfarth Citation2009), aggressive (Koolhaas Citation2008) and self-directed behaviours (Maestripieri et al. Citation1992; Higham et al. Citation2009).
Evidence for a coping function of affiliative behaviour comes from studies on a range of taxa. Albonetti and Farabollini (Citation1993), for example, demonstrated that male rats (Rattus norvegicus) groomed other males for a longer period of time after they were restrained for 30 min (severe stressor) than after an experimenter handled them briefly in a gentle manner (mild stressor). Connor et al. (Citation2006) described how wild female bottlenose dolphins (Tursiops aduncus) in male-biased groups engage often in ‘contact swimming’ during potentially distressful situations, such as in the presence of males and following harassment or herding by these males. In a study of wild chacma baboons (Papio ursinus), Engh et al. (Citation2006) found that females who had recently lost a close relative to predation had increased faecal glucocorticoid metabolite (fGCM) levels. Relative to the pre-loss period, these females subsequently increased both their grooming rates and their number of grooming partners. Following this, fGCM levels soon returned to baseline, though it is unclear whether this change was directly related to changes in grooming behaviour.
A variety of studies also indicate that animals cope with stressful situations by directing aggression towards bystanders. Clement et al. (Citation2005) found that captive African cichlid fish (Astatotilapia burtoni) reacted to a video presentation of a dominant male displaying aggressively by chasing their tank mates. Similarly, Wittig and Boesch (Citation2003) reported that wild chimpanzees (Pan troglodytes) involved in long, drawn-out conflicts redirected their aggression towards other individuals. In a meta-analysis of human studies, Marcus-Newhall et al. (Citation2000) showed that men and women exposed to distressing events, such as cold stress and negative evaluations from peers, often acted aggressively towards experimenters or other study subjects.
Finally, a number of authors have suggested that self-directed behaviours—displacement activities that appear generated by events causing motivational conflict and frustration (Tinbergen Citation1952; McFarland Citation1996)—may help individuals to cope with stressful situations (Maestripieri et al. Citation1992; Higham et al. Citation2009). Experimenter handling, restraint and exposure to novel environments increase rates of self-grooming in rats (van Erp et al. Citation1994), and exposure to a predator model induces self-scratching and self-grooming in captive male marmosets (Callithrix penicillata: Barros et al. Citation2004). Studies on free-ranging primates reveal that individuals engage in self-directed behaviours when they are in close proximity to dominant group members (olive baboons, Papio anubis: Castles et al. Citation1999) and following conflicts (e.g. Japanese macaques, Macaca fuscata: Majolo et al. Citation2009).
In addition to identifying behaviours that may function to assist the handling of stressful situations, a number of researchers have explored the interrelationships between these coping behaviours, stress hormone levels and social status. Among male olive baboons, individuals that tend to redirect aggression towards bystanders after losing a fight have lower plasma cortisol concentrations than males who do not show redirected aggression. Redirected aggression is not rank dependent, but rather is found in both high- (Ray and Sapolsky Citation1992) and low-ranking males (Virgin and Sapolsky Citation1997). Among male African cichlid fish, social status is an important factor in the relationship between coping behaviour and stress levels (Clement et al. Citation2005). Dominant, territory-holding males of this species show no link between a tendency to redirect aggression and blood cortisol levels. In contrast, among subordinate, non-territory holding males, males with low and high cortisol levels engage in more redirected aggression than males with mid-range cortisol concentrations (Clement et al. Citation2005).
Despite the great interest in understanding how animals cope with stressful situations, and how coping behaviours are related to stress physiology and social status, to our knowledge no studies to date have experimentally investigated behavioural coping strategies in free-ranging environments using a systematic, non-invasive approach. By far the majority of studies of coping behaviour have been conducted in captivity, with researchers relying on artificial stressors that are often highly aversive, such as placement in novel cages, resident–intruder tests and electric shocks (Tamashiro et al. Citation2005). Moreover, the animals used for such studies are effectively domesticated and often highly artificially selected and inbred, thus potentially removing much of the inter-individual variation (Koolhaas et al. Citation2010), including a naturalistic dominance hierarchy. The relatively few studies of coping behaviour that have been carried out in free-ranging or wild conditions (e.g. Sapolsky and Ray Citation1989; Engh et al. Citation2006) have used observational methods and/or been opportunistic in nature; in such contexts, potentially confounding variables cannot be controlled as tightly as is possible in an experimental design.
Here, we use an experimental playback approach, in conjunction with behavioural observation and non-invasive monitoring of stress hormone metabolites, to investigate potential coping behaviour in free-ranging adult female Barbary macaques (Macaca sylvanus). To validate the experimental method used, we first determine whether aversive playback stimuli (threat-grunt vocalisations from unknown conspecifics) elicit responses indicative of mild anxiety/stress: visual orienting towards and moving away from the loudspeaker. Next, we quantify changes in affiliative, aggressive and self-directed behaviours elicited by playbacks, in order to identify which of these may represent coping strategies. Finally, we examine relationships between potential coping behaviours, a measure of adrenocortical activity (average fGCM levels over the study period) and social status.
Materials and methods
Study site and animals
Field data were collected from February to May 2010 at Trentham Monkey Forest in Staffordshire, UK, a tourist park where two troops of Barbary macaques range in a 24 ha outdoor enclosure and visitors are restricted to paths. Barbary macaques live in multi-male, multi-female groups in which females stay in their natal groups and show a linear dominance hierarchy (Deag Citation1977). Study subjects were 12 adult females (aged 6–19) from the ‘French troop’, which comprised 70 individuals in total at the time of study (26 adult females, 27 adult males, 13 juveniles and 4 infants). All monkeys were identifiable by tattoos and individual markings, and ages and matrilineal relationships were known. This study was carried out just prior to the birth season. All females were implanted with a progestin contraceptive, Implanon (Organon, Oss, Netherlands). The active ingredient in Implanon is levonorgestrel, which has a low binding affinity (∼1%) for glucocorticoid receptors (Ouzounian et al. Citation2008). None of the study females gave birth during or after the study, so reproductive state was not controlled for in the analyses. Ethical approval was given by the Trentham Monkey Forest and the School of Human and Life Sciences at Roehampton University. Experiments are in accordance with the European Communities Council Directives.
Playback stimuli
The experimental playback stimuli consisted of three Barbary macaque threat-grunt vocalisations (mean duration ± SD: 3.87 ± 0.68 s), which were previously recorded from different individuals (two males, one female) living in Gibraltar and unknown to the study population. Vocalisations from unknown individuals were chosen instead of known individuals for two reasons. First, this avoided potential variation in different subjects' responses, due to variation in their relationship (social or genetic) with the caller. Second, this approach simulated the resident–intruder paradigm used in captivity in which the study subject is exposed to an unknown, and potentially hostile, conspecific (Miczek Citation1979; Tamashiro et al. Citation2005). Initial analyses of responses to these playback stimuli, using Mundry's (Mundry Citation1999) permutation test to take account of missing values (one female heard only two of the three experimental stimuli—see below), revealed that the time study females spent looking towards the speaker or moving in the 1 min after playback did not vary significantly across the three stimuli (looking: p = 0.994; moving: p = 0.083).
To provide a baseline measure of behaviour, while controlling for subjects' potential responsiveness to the equipment and experimenter, the control playback stimulus was an audio track of silence (4.00 s). We did not use another Barbary macaque call (e.g. a non-threatening vocalisation) as a control because we were primarily interested in responses to mildly aversive stimuli, not to threat-grunt vocalisations per se; our results (see below) support the idea that our stimuli produced the desired response in comparison to the control.
The playback stimuli were presented with a Marantz PMD670 Professional Solid State Recorder (Kanagawa, Japan) connected to a Nagra Kudelski DSM Speaker (Kudelski SA, Cheseaux-sur-Lausanne, Switzerland). Volumes of experimental stimuli were set between 75 dB and 80 dB at 3 m in a quiet indoor environment using an Adastra 952.422 Analogue Sound Level Meter (AVSL Group Ltd, Manchester, UK). During a pilot session with females from a non-study troop at Trentham Monkey Forest, threat-grunts in this volume range were found to elicit responses such as looking towards and moving away from the speaker.
Playback protocol
Study females took part in two (n = 1) or three (n = 11) experimental and in three (n = 12) control playback sessions, which were alternated throughout the study period. The presentation order of threat-grunt stimuli to each female was randomised. Females participated in no more than one playback session per day and were only chosen for playback sessions if the following conditions were satisfied: not engaged in social activity (i.e. was feeding or resting), was close to vegetation where the speaker could be hidden, no individuals were located between them and the speaker, and the study subject was at least 2 m from the nearest neighbour. In three cases, there was one study female 5–10 m away during the start of the session. Such females were not the focus of additional playback sessions carried out on the same day. In all other sessions, other study females were at least 10 m away. The behavioural contexts (i.e. feeding or resting activity in the minute prior to the playback stimulus) of control and experimental sessions were matched as closely as possible. During playback sessions, the speaker was concealed in vegetation 2–10 m from the study subject. Behaviour was recorded with a Panasonic NV-GS37EB Digital Video Camera (Osaka, Japan). The playback stimulus was played after 1 min of video recording if the preliminary conditions were still satisfied. Subjects' behaviour was then recorded for 20 min following presentation of playback stimuli: the first 5 min with the video camcorder and the last 15 min with a handheld Psion WorkAbout Pro (Psion Teklogix Inc., Mississauga, Canada). Video footage was later viewed in Windows Movie Maker v 6.0 (Microsoft, Redmond, WA, USA) and scored with the same behavioural ethogram configured for the Psion (see below).
Behavioural recording
Behavioural observations were carried out using ‘continuous’ and ‘point’ focal sampling (Altmann Citation1974). The immediate responses recorded were visual orienting (percentage of time in which the head was oriented at an angle judged to be ± 30° to the direction of the speaker) and percentage of time travelling. These were quantified by taking the percent of time engaged in the activity in the minute after the playback, and subtracting the percent of time engaged in the behaviour in the minute before the playback, using frame-by-frame video analysis (Windows Movie Maker). These immediate responses were used to determine whether the subjects responded to the experimental stimuli.
Data on three affiliative behaviours were collected: (i) rate of approaches to other group members (to within less than 1 m), (ii) percentage of time giving grooming and (iii) percentage time receiving grooming. Data were also recorded on three aggressive behaviours (definitions follow Hesler and Fischer (Citation2006)): (i) rate of threats (i.e. stare, rounded mouth threat, head bob or ground slap) (ii) rate of body lunges (quick and brusque movements towards another individual but pursuit is less than 5 m) and (iii) percentage of time chasing (pursuing another individual for more than 5 m) or physical aggression (biting, slapping, grabbing or pushing another individual). Finally, data were collected on two self-directed behaviours: (i) rate of self-scratching and (ii) percentage time spent self-grooming. All of these potential coping behaviours were quantified in the 0–5 and 0–20 min time periods following playback stimuli.
Frequencies of or percentage time spent in behaviours were calculated for the time study subjects were within sight. Observer XT (Noldus, Wageningen, The Netherlands) was used to configure the Psion and summarise behavioural data. Percentage time spent in a given behaviour and rates of behaviours were averaged across playback trials by stimulus, so that each female had two scores for all behaviours in all relevant time periods: one for experimental playbacks and one for controls. To determine female rank, dyadic agonistic interactions between study subjects with clear winner–loser outcomes were recorded during behavioural observations and ad libitum. These outcomes were transferred into a matrix and analysed with MatMan 1.1 (Noldus) to determine Landau's linearity index (corrected for unknown relationships), Kendall's coefficient of linearity and the directional consistency index.
Faecal sample collection and hormone analysis
Five to eight faecal samples were collected for each female (n = 81 in total) throughout the study period and analysed for fGCM levels. Faecal samples were collected opportunistically between 0900h and 1700h throughout the study period, with no more than two samples collected from each female per week. In Barbary macaques, glucocorticoid metabolites are excreted in the faeces 1 to 2 days after the original adrenocortical brain response (Wallner et al. Citation1999). Since fGCM levels therefore reflect cumulative glucocorticoid secretion, there was little reason to expect that a single playback of a threat-grunt (which is a relatively mild stressor) would greatly alter this measure of adrenocortical activity. Most of the faecal samples were collected outside of the 2-day window following playbacks with the respective animal (n = 58). A smaller number of samples were collected within 2 days following a playback session (n = 23: 13 after controls, 10 after experimental playbacks). Overall, the median fGCM levels of samples collected within 2 days of a playback did not differ from those collected outside of this time (within 2 days 472 ± 97 ng/g; outside 2 days: 404 ± 120 ng/g; Wilcoxon matched-pairs test: Z = 0.676, n = 7, 0 ties, p = 0.499). Of samples collected within 2 days, those collected after an experimental playback did not differ from those collected after a control playback (experimental: 506 ± 130 ng/g; control: 481 ± 112 ng/g; Wilcoxon matched-pairs test: Z = .944, n = 5, 0 ties, p = 0.345).
The collection process followed protocols previously described by Brent et al. (Citation2011). A thumbnail-sized portion of faeces (∼0.500 g) was placed in a labelled 30 ml high-density polyethylene (HDPE) tube and stored in an insulated bag between ice packs. Samples were transferred into a freezer at − 20°C at the end of each day. Following the collection period, all samples were transported frozen to Roehampton University, where laboratory analysis took place. Samples were freeze-dried for approximately 1 day, ground and 0.05–0.10 g of faecal powder was extracted with 3 ml of 80% methanol on a shaker (10 min). Following centrifugation (3500 g, 20 min), the supernatant was separated. Faecal extracts were analysed for glucocorticoid metabolites with a 5β-3α,11β-diol structure (hereafter referred to as fGCM) using an enzyme immunoassay for 11ß-hydroxyaetiocholanolone previously validated for monitoring adrenocortical activity in Barbary macaques (Heistermann et al. Citation2006). Samples were measured at two dilutions (1:40, N = 62; 1:80, N = 19). Sensitivity of the assays at 90% binding was 0.488 pg/50 μl. Intra- and inter-assay coefficients of variation calculated from replicates of high- and low-concentration quality controls were 2.9% (Nhigh = 17) and 6.7% (Nlow = 15), and 9.7% (Nhigh = 13) and 15.7% (Nlow = 13), respectively.
Median fGCM levels did not differ depending on sample collection time (0900–1230: 485 ± 95 ng/g; 1230–1700: 414 ± 104 ng/g; Wilcoxon matched-pairs test: Z = 1.362, n = 9, 0 ties, p = 0.173) nor did female age correlate with median fGCM levels (Spearman rank correlation: rs = 0.183, N = 12, p = 0.568); thus, the analysis did not control for these two factors.
Statistical analysis
Distributions of data variables were examined using one-way Kolmogorov–Smirnov tests. Based on these results, parametric or non-parametric statistical tests were employed using SPSS v. 19.0 (SPSS Inc., Chicago, IL, USA). All tests were two-tailed (α = 0.05).
Firstly, paired t-tests or Wilcoxon matched-pairs tests were used to determine whether experimental stimuli (i.e. threat-grunt playbacks) elicited visual orienting and travelling. Next, paired t-tests or Wilcoxon matched-pair tests were used to determine whether potential coping behaviours were expressed at higher frequencies after experimental than control playbacks. The expression of self-directed behaviours was investigated at the two different time intervals (0–5 min and 0–20 min) after playback stimuli. Affiliative and aggressive behaviours rarely occurred in the first 5 min following playbacks; for these only the 0–20 min period was used.
For all behaviours that occurred at significantly higher rates following experimental playbacks compared to controls, responsiveness indices were calculated for the corresponding time interval. This was done by subtracting each female's mean for control playback sessions from her mean for experimental playback sessions. Median fGCM levels were used as a measure of average adrenocortical activity. Spearman rank correlations were then used to examine relationships among the responsiveness indices, and between these indices and both median fGCM levels and dominance rank.
Results
Study subjects' initial responses to experimental playback stimuli
Females oriented longer towards the speaker (paired t-test: t11 = 5.715, p < 0.001) and travelled for a longer amount of time (Wilcoxon matched-pairs test: Z = 2.803, n = 12, 2 ties, p = 0.005) in the minute after than in the minute before experimental playbacks. Also, females oriented longer towards the speaker (paired t-test: t11 = 6.460, p < 0.0005) and travelled for a longer amount of time (paired t-test: t11 = 2.884, p = 0.015) following experimental playbacks than following control playbacks. When travelling occurred in the minute following experimental playbacks (study animals travelled in 14 of the 35 trials), the direction of travel was predominantly away from the loudspeaker (12 of the 14 occurrences).
Behavioural responses to playbacks
Affiliative behaviour
There was no difference between the 20-min periods following experimental and control playbacks in females' rates of approaches towards other group members (paired t-test: t11 = 1.211, p = 0.251), or in their time spent giving (paired t-test: t11 = 1.191, p = 0.259) or receiving grooming (Wilcoxon matched-pairs test: Z = 1.540, n = 12, 4 ties, p = 0.123).
Aggressive behaviour
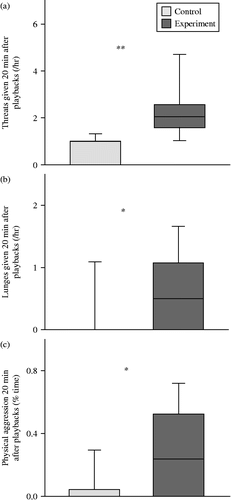
In the 20-min following experimental stimuli, females gave more threats (paired t-test: t11 = 3.311, p = 0.007), made more lunges (Wilcoxon matched-pairs test: Z = 1.992, n = 12, 6 ties, p = 0.046) and spent more time chasing/showing physical aggression towards others (Wilcoxon matched-pairs test: Z = 2.366, n = 12, 5 ties, p = 0.018) than in the 20-min period after control playbacks (see ).
Figure 1. Comparisons of (a) the rate of threats, (b) the rate of lunges and (c) the percentage of time in which physical aggression (including chases) was directed at others in the 20 min following control and experimental stimuli. One asterisk indicates significance at p < 0.05 and two asterisks indicate p < 0.01. The values shown are the interquartile range, the midline is the median and the whiskers show 10–90 percentiles.
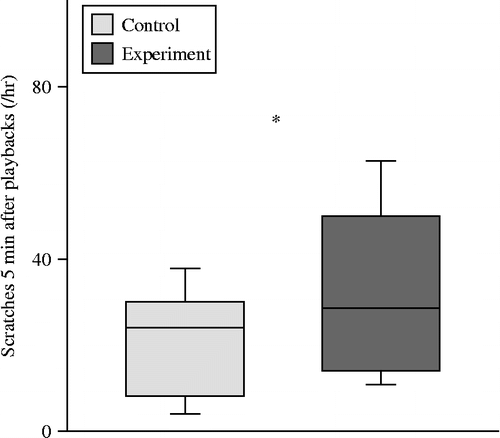
Self-directed behaviour
Self-scratching rates were higher in the 5 min following experimental playbacks compared to controls (paired t-test: t11 = 2.641, p = 0.023; ) but did not differ significantly in the 20 min (paired t-test: t11 = 1.794, p = 0.100) following experimental and control playbacks. Time spent self-grooming was not significantly different in the 5 min (paired t-test: t11 = 1.541, p = 0.151) or 20 min (Wilcoxon matched-pairs t-test: Z = .628, n = 12, 0 ties, p = 0.530) following experimental and control playbacks.
Relationships among behavioural responses to playbacks
The responsiveness indices of scratches and giving lunges were positively correlated (Spearman rank correlation: rs = 0.589, n = 12, p = 0.044). None of the other correlations between responsiveness indices were significant (Spearman rank correlations: threat vs. lunge: rs = 0.067, n = 12, p = 0.835; threat vs. chase/physical aggression: rs = 0.457, n = 12, p = 0.135; lunge vs. chase/physical aggression: rs = 0.039, n = 12, p = 0.905; scratch vs. threat: rs = 0.284, n = 12, p = 0.372; scratch vs. chase/physical aggression: rs = − 0.240, n = 12, p = 0.453).
Behavioural responses, fGCM levels and social status
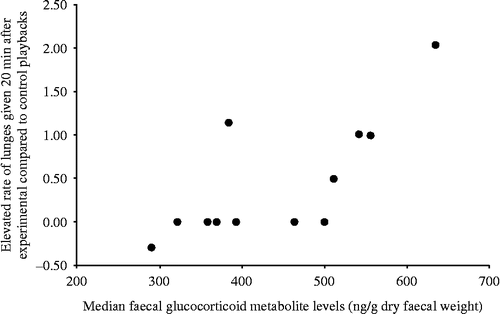
There was a positive correlation between median fGCM levels and the responsiveness index of lunges given (Spearman rank correlation: rs = 0.739, n = 12, p = 0.006; ). There were no relationships between median fGCM levels and the responsiveness indices of threats given (Spearman rank correlation: rs = 0.189, n = 12, p = 0.557), chases/physical aggression given (rs = 0.276, n = 12, p = 0.386) or self-scratching (rs = 0.308, n = 12, p = 0.330).
Figure 3. Relationship between females' median fGCM levels and the responsiveness indices of lunges directed at bystanders, i.e. mean expression in the 20 min after experimental playbacks minus mean expression in the 20 min after controls.
The dominance hierarchy constructed from the outcomes of 82 dyadic interactions between study females with the MatMan software was used in analyses. It must be noted that this hierarchy was not significantly linear (final inconsistencies = 0, h1 = 0.40, K = 0.28, DC = 0.90, p = 0.11). There were no significant relationships between dominance rank and responsiveness indices of any of the behaviours significantly elevated after experimental playbacks (Spearman rank correlations: threats given: rs = − 0.441, n = 12, p = 0.152; lunges given: rs = − 0.377, n = 12, p = 0.227; chases/physical aggression given: rs = − 0.435, n = 12, p = 0.158; self-scratch: rs = 0.210, n = 12, p = 0.512).
Finally, dominance rank and median fGCM levels were correlated, with higher ranked (i.e. more dominant) females having higher median fGCM levels (Spearman rank correlation: rs = − 0.608, n = 12, p = 0.036).
Discussion
We used playback experiments to investigate coping behaviours in free-ranging female Barbary macaques, to explore the interrelationships among these behaviours and to investigate their relationships with a measure of average adrenocortical activity over the study period (fGCM levels) and social status. Playbacks of threat vocalisations from unknown conspecifics elicited visual monitoring and movement, suggesting that these stimuli were effective as mild stressors. Females showed an increase in aggressive behaviours and self-scratching following experimental playbacks compared to controls, but no such changes were seen in levels of affiliative behaviour or self-grooming. Elevations in rates of self-scratching and aggressive lunging after the presentation of experimental stimuli were positively related; no other measures of behavioural responsiveness were correlated. Although behavioural responses to playback were unrelated to social status, fGCM levels were positively related to the magnitude of response of one aggressive behaviour—lunging. These findings present some similarities to, but also some striking contrasts with, previous studies of coping behaviour, adrenocortical activity and social status.
All three aggressive behaviours examined in this study—threats, lunges and chases/physical aggression—increased following presentation of threat-grunts compared to control trials. The increases in these behaviours, however, were not correlated with each other; this may simply reflect the fact that there is underlying variability in response between individual study females, but may also indicate these animals have different aggressive ‘coping styles’ (Koolhaas et al. Citation2010). Aggression is often stereotyped in the human literature as a predominantly male coping strategy (Neumann et al. Citation2010), and is not often explored as a coping behaviour among female non-human primates (Cheney and Seyfarth Citation2009). However, our findings concerning the potential importance of aggression are in line with a pharmacological intervention study of aggression as a response to experimentally induced physiological stress in humans. Böhnke et al. (Citation2010) found that women given exogenous cortisol showed elevated aggression compared to those given placebo, whereas no such increase was seen among men. Together, these findings from both human and non-human primates highlight the importance of exploring aggressive behavioural coping strategies in females.
Threat vocalisation playbacks also elicited short-term increases in rates of self-scratching, in support of the long standing (Maestripieri et al. Citation1992) but rarely tested (Watson et al. Citation1999; Pico-Alfonso et al. Citation2007) idea that self-directed behaviours serve as natural coping strategies. Understanding the role of self-directed behaviours in mitigating the impacts of stressful situations is an important goal for future research (Higham et al. Citation2009), and such work will inform studies that use self-directed behaviours as indices of anxiety, but perhaps do not consider their potential coping benefits. Majolo et al. (Citation2009), for example, found that wild Japanese macaques (M. fuscata), which showed greater increases in rates of scratching following conflicts, were less likely to take part in reconciliation. These results were interpreted as indicating that highly anxious animals were unable to repair relationships due to their emotional state. An alternative explanation is that animals showing greater elevations of scratching had already ‘coped’ with the stress of the situation and consequently had less need to reconcile. Our finding that elevations in self-scratching and aggressive lunging were positively correlated, however, indicates that these behaviours may form part of a joint response to stress-invoking situations, rather than representing alternative means to cope with a stressor (in which case no relationship or a negative correlation would be predicted).
Affiliative behaviours were unchanged following experimental compared to control playbacks; this provides no support for the ‘tend-and-befriend’ hypothesis of female coping strategies, which predicts that females respond to stressful situations by seeking positive social contact. First put forward by Taylor et al. (Citation2000) to describe human coping behaviour, this hypothesis has been supported by a number of animal studies on females in captive and wild settings (Cheney and Seyfarth Citation2009). That our subjects did not appear to seek affiliative contact following experimental playbacks is particularly surprising given the evidence from Barbary macaques (Shutt et al. Citation2007) and other closely related species (Aureli et al. Citation1999; Aureli and Yates Citation2009) that giving and receiving grooming may reduce stress among females. Work on coping mechanisms in baboons suggests that affiliating with specific partners may be the key to dealing with stress (Crockford et al. Citation2008; Wittig et al. Citation2008). In the majority of previous studies, stress and affiliative behaviour were measured over the longer term (i.e. days and weeks). We are not able to exclude the possibility that females in our study did indeed seek out social support, but on a longer timescale than was investigated here. It is also possible that females were seeking to make affiliative contact with particular kin and/or allies, but in the 20-min post playback such animals could not be found or were unavailable as social partners. Furthermore, the experimental stimulus may not have been severe enough to invoke support-seeking behaviour. A more severe stressor (e.g. potential predator attack, Engh et al. Citation2006) may have elicited very different behaviours. Nevertheless, our findings do indicate that studies of coping behaviour should look at how these behaviours may vary across time, and in relation to the availability of social partners and stressor intensity.
One index of behavioural responsiveness in this study—the elevation in rates of lunging following threat-grunts compared to control playbacks—was positively related to a measure of average adrenocortical activity (fGCM levels). This result contrasts with evidence from fish (Oncorhynchus mykiss: Øverli et al. Citation2004; A. burtoni: Clement et al. Citation2005) and primates (P. anubis: Ray and Sapolsky Citation1992; M. mulatta: Gust et al. Citation1993; Virgin and Sapolsky Citation1997) indicating that the degree to which individuals redirect aggression onto bystanders is negatively related to adrenocortical activity. In these studies, redirection of aggression occurred after a physical or simulated interaction between conspecifics, whereas in our study, levels of aggression rose after a potentially risky but ambiguous social situation (i.e. a threat from an unknown conspecific which may or may not have been directed towards the subject). Our finding that females with higher median fGCM levels show greater elevations of aggressive lunging after experimental playbacks suggests either that these individuals were responding differently to the perceived threat, or that they were interpreting the situation differently. In the case of the former, higher levels of adrenocortical activity may lead to a greater likelihood of an aggressive response (Kruk et al. Citation2004). In the latter case, higher stress levels may lead to a more negative interpretation of the potentially threatening situation (Enkel et al. Citation2010) and thus a perception of the environment as being more risky. It is unclear why lunging—but not threats or chases/physical aggression—should be linked to fGCM levels. However, it is important to note that the lack of significant correlations between fGCM levels and either threats or chases/physical aggression does not exclude the possibility that these factors are not connected in some more subtle way.
A significantly linear hierarchy was not found in our study, and therefore the results of analyses involving rank must be treated with caution. However, our finding that assigned dominance rank was not correlated with any of the four indices of behavioural responsiveness adds to the evidence from non-human primates suggesting that coping strategies are not directly linked to social status (Sapolsky Citation2000, Citation2005). Individual variation in coping style has been linked to vulnerability to stress-related disease, and identifying the factors underpinning this variation—such as rank—is therefore of applied importance (Koolhaas et al. Citation2010). Of particular value in this respect will be studies investigating the biological function of this variation in natural settings (Koolhaas Citation2008), and the type of experimental approach used here provides a means to address these issues.
Indeed, our study demonstrates the potential of the playback approach to explore the nature and consequences of behavioural coping strategies in both free-ranging and wild animal populations. Future studies could, for example, vary the nature of the stressor presented to investigate if and how coping responses differ when faced with a social stressor (as was used here) or an ecological one, such as the presence of a predator (McComb et al. Citation2011). The impact of animals' immediate social environment on their coping behaviour could also be tested, in order to investigate ‘social buffering’ (Kikusui et al. Citation2006) systematically in a natural setting. This approach could also be used to explore how coping mechanisms may vary over time after exposure to a stressor; such variation may underlie a number of the differences in the findings of this study compared with previous research. Finally, and perhaps most importantly, playback studies which also incorporate short-term, non-invasive measures of subjects' stress physiology, such as salivary alpha amylase and salivary cortisol (Higham et al. Citation2010) would help to shed light on how behavioural responses to stressors may mediate stress physiology, and how stress levels may affect coping behaviours.
Investigations of this nature will significantly enhance our understanding of animal stress biology within an ecological and evolutionary context (Koolhaas Citation2008; Koolhaas et al. Citation2010). Furthermore, as primates in wild settings arguably provide a more appropriate model for human stress than do captive animals, such studies may also provide an evolutionary framework for understanding stress in our own species (Cheney and Seyfarth Citation2009).
Acknowledgements
We would like to thank Dr Sam Roberts for kindly providing the threat-grunt recordings. We thank also the many individuals who provided field and technical support: Martin Evans, Diane Floyd, Susie Hyde, Balbir Josen, Mary Mackenzie, Peter Merton, David Randell, Anna Smith and Frimpong Twum. Dr Peter Shaw kindly provided statistical advice. We are very grateful to Dr Roger Mundry, who kindly provided the software for running the permutation tests and advice on its use. Dr Caroline Ross, Dr Julia Lehmann, Dr Thore Bergman, Prof Charles Snowdon, Dr James Herman and three anonymous reviewers provided invaluable comments on an earlier version of this manuscript. This study was supported by grants from the International Primatological Society and the L.S.B. Leakey Trust.
Declaration of Interest: None of the authors report any conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Albonetti ME, Farabollini F. 1993. Effects of single and repeated restraint on the social behaviour of male rats. Physiol Behav. 53:937–942.
- Altmann J. 1974. Observational study of behaviour: Sampling methods. Behaviour. 49:227–265.
- Aureli F, Preston SD, de Waal FBM. 1999. Heart rate responses to social interactions in free-moving Rhesus macaques (Macaca mulatta): A pilot study. J Comp Psychol. 113:59–65.
- Aureli F, Yates K. 2009. Distress prevention by grooming others in crested black macaques. Biol Lett. 6:27–29.
- Barros M, de Souza Silva MA, Huston JP, Tomaz C. 2004. Multibehavioral analysis of fear and anxiety before, during, and after experimentally induced predatory stress in Callithrix penicillata. Pharmacol Biochem Behav. 78:357–367.
- Böhnke R, Bertsch K, Kruk MR, Richter S, Naumann E. 2010. Exogenous cortisol enhances aggressive behavior in females, but not males. Psychoneuroendocrinology. 35:1034–1044.
- Brent LJN, Semple S, Dubuc C, Heistermann M, MacLarnon A. 2011. Social capital and physiological stress levels in free-ranging adult female rhesus macaques. Physiol Behav. 102:76–83.
- Cannon WB. 1915. Bodily changes in pain, hunger, fear and rage. New York: D. Appleton & Company.
- Castles DL, Whiten A, Aureli F. 1999. Social anxiety, relationships and self-directed behaviour among wild female olive baboons. Anim Behav. 58:1207–1215.
- Cheney DL, Seyfarth RM. 2009. Stress and coping mechanisms in female primates. Adv Stud Behav. 39:1–44.
- Clement TS, Parikh V, Schrumpf M, Fernald RD. 2005. Behavioral coping strategies in a cichlid fish: The role of social status and acute stress response in direct and displaced aggression. Horm Behav. 47:336–342.
- Connor R, Mann J, Watson-Capps J. 2006. A sex-specific affiliative contact behavior in Indian ocean bottlenose dolphins. Tursiops sp. Ethology. 112:631–638.
- Crockford C, Wittig RM, Whitten PL, Seyfarth RM, Cheney DL. 2008. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus). Horm Behav. 53:254–265.
- Deag JM. 1977. Aggression and submission in monkey societies. Anim Behav. 25:465–474.
- Engel GL, Schmale AH. 1972. Conservation withdrawal: A primary regulatory process for organic homeostasis. In: R. Porter, J. Knight. editors. Physiology, emotions and psychosomatic illness. CIBA Foundation Symposium. Amsterdam: Elsevier57–95.
- Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, Cheney DL. 2006. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc R Soc B. 273:707–712.
- Enkel T, Gholizadeh D, von Bohlen und Halbach O, Sanchis-Segura C, Hurlemann R, Spanagel R, Gass P, Vollmayr B. 2010. Ambiguous-cue interpretation is biased under stress and depression-like states in rats. Neuropsychopharmacology. 35:1008–1015.
- Gust DA, Gordon TP, Hambright MK, Wilson ME. 1993. Relationship between social factors and pituitary: Adrenocortical activity in female Rhesus monkeys (Macaca mulatta). Horm Behav. 27:318–331.
- Heistermann M, Palme R, Ganswindt A. 2006. Comparison of different enzymeimmunoassays for assessment of adrenocortical activity in primates based on fecal analysis. Am J Primatol. 68:257–273.
- Hesler N, Fischer J. 2006. Gestural communication in Barbary macaques (Macaca sylvanus): An overview. In: Call J, Tomasello M. editors. The gestural communication of apes and monkeys. Mahwah, NJ: Lawrence Erlbaum159–195.
- Higham JP, MacLarnon AM, Heistermann M, Ross C, Semple S. 2009. Rates of self-directed behaviour and faecal glucocorticoid levels are not correlated in female wild olive baboons (Papio hamadryas anubis). Stress. 12:526–532.
- Higham JP, Vitale AB, Rivera AM, Ayala JE, Maestripieri D. 2010. Measuring salivary analytes from free-ranging monkeys. Physiol Behav. 101:601–607.
- Kikusui T, Winslow JT, Mori Y. 2006. Social buffering: Relief from stress and anxiety. Philos Trans R Soc B. 29:2215–2228.
- Koolhaas JM. 2008. Coping style and immunity in animals: Making sense of individual variation. Brain Behav Immun. 22:662–667.
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. 2010. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front Neuroendocrinol. 31:307–321.
- Kruk MR, Halasz J, Meelis W, Haller J. 2004. Fast positive feedback between the adrenocortical stress response and a brain mechanism involved in aggressive behavior. Behav Neurosci. 118:1062–1070.
- Levine S, Ursin H. 1991. What is stress?. In: Brown MR, Koob GF, Rivier C. editors. Stress: Neurobiology and neuroendocrinology. New York: Marcel Dekker, Inc3–21.
- Maestripieri D, Schino G, Aureli F, Troisi A. 1992. A modest proposal: Displacement activities as an indicator of emotions in primates. Anim Behav. 44:967–979.
- Majolo B, Ventura R, Koyama NF. 2009. Anxiety level predicts post-conflict behaviour in wild Japanese macaques (Macaca fuscata yakui). Ethology. 115:986–995.
- Marcus-Newhall A, Pedersen WC, Carlson M, Miller N. 2000. Displaced aggression is alive and well: A meta-analytic review. J Pers Soc Psychol. 78:670–689.
- McComb K, Shannon G, Durant SM, Sayialel K, Slotow R, Poole J, Moss C. 2011. Leadership in elephants: The adaptive value of age. Proc R Soc B. 278:3270–3276.
- McEwen BS. 1998. Protective and damaging effects of stress mediators. N Engl J Med. 33:171–179.
- McEwen BS, Wingfield JC. 2010. What's in a name? Integrating homeostasis, allostasis and stress. Horm Behav. 57:105–111.
- McFarland DJ. 1996. On the causal and functional significance of displacement activities. Z Tierpsychol. 23:217–235.
- Miczek KA. 1979. A new test for aggression in rats without aversive stimulation: Differential effects of d-amphetamine and cocaine. Psychopharmacology. 60:253–259.
- Mundry R. 1999. Testing related samples with missing values: A permutation approach. Anim Behav. 58:1143–1153.
- Neumann ID, Veenema AH, Beiderbeck DI. 2010. Aggression and anxiety: Social context and neurobiological links. Front Behav Neurosci. 4:1–16.
- Ouzounian S, Verstraete L, Chabbert-Buffet N. 2008. Third-generation oral contraceptives: Future implications of current use. Expert Rev Obstet Gynecol. 3:189–201.
- Øverli Ø, Korzan WJ, Larson ET, Winberg S, Lepage O, Pottinger TG, Renner KJ, Summers CH. 2004. Behavioral and neuroendocrine correlates of displaced aggression in trout. Horm Behav. 45:324–329.
- Pico-Alfonso MA, Mastorci F, Ceresini G, Ceda GP, Manghi M, Pino O, Troisi A, Sgoifo A. 2007. Acute psychosocial challenge and cardiac autonomic response in women: The role of estrogens, corticosteroids, and behavioral coping styles. Psychoneuroendocrino. 32:451–463.
- Ray JC, Sapolsky RM. 1992. Styles of male social behavior and their endocrine correlates among high-ranking wild baboons. Am J Primatol. 28:231–250.
- Romero LM, Dickens MJ, Cyr NE. 2009. The reactive scope model—A new model integrating homeostasis, allostasis, and stress. Horm Behav. 55:375–389.
- Sapolsky RM. 2000. Physiological correlates of individual dominance style. In: Aureli F, de Waal FBM. editors. Natural conflict resolution. Berkeley, CA: University of California Press114–116.
- Sapolsky RM. 2005. Social hierarchy and primate health. Science. 308:648–652.
- Sapolsky RM, Ray JC. 1989. Styles of dominance and their endocrine correlates among wild olive baboons (Papio anubis). Am J Primatol. 18:1–13.
- Shutt K, MacLarnon A, Heistermann M, Semple S. 2007. Grooming in Barbary macaques: Better to give than to receive?. Biol Lett. 3:231–233.
- Tamashiro KLK, Nguyen MNN, Sakai RR. 2005. Social stress: From rodents to primates. Front Neuroendocrinol. 26:27–40.
- Taylor SE, Cousino Klein C, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. 2000. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol. Rev.. 107:411–429.
- Tinbergen N. 1952. “Derived” activities: Their causation, biological significance, origin, and emancipation during evolution. Q Rev Biol. 27:1–32.
- van Erp AMM, Kruk MR, Meelis W, Willekens-Bramer DC. 1994. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: Handling, social contact, defeat, novelty, restraint and fur moistening. Behav Brain Res. 65:47–55.
- Virgin CE, Sapolsky RM. 1997. Styles of male social behavior and their endocrine correlates among low-ranking baboons. Am J Primatol. 42:25–39.
- Wallner B, Möstl E, Dittami J, Prossinger H. 1999. Fecal glucocorticoids document stress in female Barbary macaques (Macaca sylvanus). Gen Comp Endocr. 113:80–86.
- Watson SL, Ward JP, Davis KB, Stavisky RC. 1999. Scent-marking and cortisol response in the Small-eared bushbaby (Otolemur garnettii). Physiol Behav. 66:695–699.
- Wechsler B. 1995. Coping and coping strategies: A behavioural view. Appl Anim Behav Sci. 43:123–134.
- Weiss JM. 1968. Effects of coping responses on stress. J Comp Physiol Psychol. 65:251–260.
- Wittig RM, Boesch C. 2003. The choice of post-conflict interactions in wild chimpanzees (Pan troglodytes). Behaviour. 140:1527–1559.
- Wittig RM, Crockford C, Lehmann J, Whitten PL, Seyfarth RM, Cheney DL. 2008. Focused grooming networks and stress alleviation in wild female baboons. Horm Behav. 54:170–177.