Abstract
Psychological factors and stressful life events (LE) are considered to play a role in the onset of the metabolic syndrome (MS). We tested the association between LE and cortisol, a marker of chronic stress, with the risk of developing MS and their interaction. From a total number of 2906 men who completed a screening for the early detection of prostate cancer, 149 healthy men (mean ± SD age, 58.6 ± 7.7 years) were included in this study. Participants were assessed by the Holmes and Rahe questionnaire about their experience of LE during the previous 1–5 years. MS was diagnosed according to National Cholesterol Education Program-Adult Treatment Panel III (ATP-III) and International Diabetes Federation (IDF) criteria. Serum cortisol was measured at 08:00–09:00 h. Participants with MS (IDF criteria) reported significantly more past LE (p = 0.009) and greater summed weight of LE (p = 0.049) than those without MS. Furthermore, LE interacted with cortisol in relation to MS: in men with increased serum cortisol levels ( ≥ 13.7 μg/dl), number of LE significantly predicted MS-status (relative risk (RR) = 1.16, p = 0.03), whereas in men with low cortisol, LE were unrelated to MS (p = 0.52). We conclude that LE were significantly more prevalent in men with the MS than without the MS, according to IDF criteria, independent of the effects of age and body mass index, especially in men with increased serum cortisol levels.
Introduction
Metabolic syndrome (MS) is a condition characterized by several related metabolic and cardiovascular abnormalities, including visceral obesity, insulin-resistance, hypertension, altered lipoprotein and inflammatory profiles, specific neuroendocrine derangements with elevated levels of “stress” hormones (Rosmond and Björntorp Citation2000), including cortisol (Weigensberg et al. Citation2008).
The underlying causes of MS are uncertain. However, since changes in life-style (e.g. sedentary behavior and psychosocial stress) have been associated with MS, it has been suggested that MS is a life-style-induced phenomenon (Van Dijk and Buwalda, 2008). The Whitehall II study (Brunner et al. Citation1997) showed that there is a close relationship between lower social position (which reflects chronic psychosocial stress) and increased probability of developing MS. Furthermore, direct evidence that psychological factors may play a role in the development of MS is increasing (Chandola et al. Citation2006). A few prospective studies have found that life events (LE), including divorce or widowhood, predicted the onset of MS (Troxel et al. Citation2005; Räikkönen et al. Citation2007). Particular personality factors are also associated with MS, acting in synergism with stress hormones. For example, hostility is related to insulin resistance, a major element of MS, only in people with elevated circulating norepinephrine concentration (Zhang et al. Citation2006). Hostility is also associated with MS and systemic inflammation in women (Elovainio et al. Citation2011). These findings may explain the association between hostility and coronary heart disease, one important consequence of MS. Epidemiological studies reported that MS was more prevalent among individuals under psychosocial pressure or chronic stress (Brunner et al. Citation2002; Van Dijk and Buwalda Citation2008).
One major arm of the stress response is the hypothalamic–pituitary–adrenal (HPA) axis (Stratakis and Chrousos Citation1995). Some authors hypothesize that its end-product, namely cortisol, is etiological to MS (Anagnostis et al. Citation2009). Elevated cortisol secretion was found to be associated with abdominal obesity (Björntorp and Rosmond Citation2000), a main component of MS. It seems reasonable to assume that this type of obesity is centrally driven by HPA axis hyperactivity in combination with a perturbed feedback control of the HPA axis (Björntorp and Rosmond Citation2000). Cortisol may also play a role in other components of MS, such as hypertension and lipoprotein alterations (Schoorlemmer et al. Citation2009). Walker (Citation2006) reported evidence that the HPA axis is “activated” in MS and that plasma cortisol is elevated in subjects with relative glucose intolerance, hypertension, insulin resistance, and hypertriglyceridemia, all elements of MS. However, in obesity the metabolic clearance rate of cortisol is increased, tending to lower plasma cortisol concentration, thus potentially obscuring positive association between plasma cortisol and other features of MS.
The HPA axis may react in extremes with hyperactivity in some individuals or conditions (e.g. hostility, acute stress, and depression; Tafet and Smolovich Citation2004), or hypoactivity in others (e.g. burnout, atypical depression, and posttraumatic stress disorder; Stratakis and Chrousos Citation1995; Bellingrath et al. Citation2008). Furthermore, HPA activity, either basal or stress induced, may interact with stressful LE in predicting the risk of metabolic abnormalities or disease. Recently, Gidron et al. (Citation2011) have found that cortisol interacted with LE in relation to prostate-specific antigen (PSAt), where LE were inversely correlated with PSAt levels in men with serum cortisol levels below the median, while LE were positively correlated with PSAt in men with high serum cortisol levels. Similarly, a synergistic interaction between depression and cortisol in relation to MS has been observed (Vogelzangs et al. Citation2007). Thus, several studies show that stress and LE increase the risk of MS, and that psychological factors may interact with stress hormones in relation to such health end-points.
It remains unclear whether the role of LE in MS depends on and is modified by HPA activity. The aim of this study was to test the hypotheses that there is a relationship between LE, cortisol, and MS, and that the interaction between LE and cortisol is related to the diagnosis of MS.
Materials and methods
Participants and design
A total of 2906 men (aged 45–70 years) completed an evaluation for prostatic diseases, at the Urology Division, Hospital de Clínicas “José de San Martín”, University of Buenos Aires, in May 2009, in the context of a population screening for the early detection of prostate cancer. For this study, a subsample was selected among men who were assessed between 08:00 and.09:00 h and who fulfilled all the inclusion and exclusion criteria (see below). In these men, additional measures of both LE and cortisol were included. Participants with the prostate cancer, under hormonal therapy and those with diseases that affect the HPA axis, men younger than 45 years or older than 70 years, with an alcohol intake >20 g/day, recent history of acute illness, or on medication which could modify lipid levels were excluded. This resulted in a subsample of 149 men with a mean (SD) age of 58.6 (7.7) years, which did not differ on age, body mass index (BMI), and clinical and social characteristics from the total group of men. The study used a cross-sectional design. All participants gave their written informed consent and the original screening study protocol was approved by the Ethics Committee of the hospital; the study was carried out in accordance with the Helsinki Declaration.
Measures
Background and biometrics: These included age, BMI, waist circumference, and blood pressure. In order to calculate the BMI, body weight and height were obtained for each patient. Waist circumference was measured at the level midway between the lateral lower rib margin and the superior anterior iliac crest, in a standing position and always by the same investigator. Blood pressure was registered in sitting position, after 5-min resting. The sphygmomanometer was applied to the left and right arm, and a median of the values obtained was calculated. A thorough medical examination was carried out in order to record general health, medical disorders, life-style, smoking, educational level, and marital status.
Biological tests: After 12 h fasting, blood samples (10 ml each) were obtained from peripheral vein puncture following a 15-min rest. Serum samples were separated by centrifugation at 1500g for 5 min; glucose was measured within the same day. For lipid and lipoprotein determinations, serum was kept at 4°C until processing, within 48 h. A serum aliquot was stored at − 70°C for cortisol measurement. As mentioned above, cortisol was measured in samples obtained between 08:00 and 09:00 h, taking account of the circadian rhythm of secretion of this hormone. Separated sera were aliquoted and stored at − 70°C until processing. Total serum PSAt and cortisol were determined by chemoluminescent methods (Immulite Autoanalyzer, Siemens, LA, USA). The intra-assay (CVi) and inter-assay (CVe) variation coefficients for PSAt were < 3.2% and < 4.4% respectively, and those for cortisol were < 5.0% and < 9.7%, respectively; assay sensitivity was 0.01 ng/ml for PSAt, and 1 μg/dl for cortisol. Serum triglycerides, total cholesterol, high-density lipoprotein (HDL)-cholesterol and glucose were measured in a Hitachi 917 autoanalyzer by enzymatic colorimetric methods (Roche Diagnostics GmbH, Mannheim, Germany). CVi and CVe were 1.3% and 2.4% (triglycerides), 1.1% and 2.2% (cholesterol), 1.5% and 2.6% (HDL-cholesterol), and 1.1% and 2.2% (glucose), respectively; assay sensitivity was 10 mg/dl for total cholesterol, HDL-cholesterol, and triglycerides, and 20 mg/dl for glucose. We also calculated the ratio of TG/HDL-cholesterol, which is considered a surrogate marker of insulin resistance, and a component of the allostatic load concept (Seeman et al. Citation1997).
MS diagnosis: MS was diagnosed according to the National Cholesterol Education Program (NCEP), Adult Treatment Panel-III (ATP III) (Citation2001), and the International Diabetes Federation (IDF) criteria (Alberti et al. Citation2006). Patients were classified as having MS, according to ATP-III, if they met three or more of the following criteria: waist circumference >102 cm, triglycerides ≥ 150 mg/dl (1.7 mmol/l), HDL-cholesterol < 40 mg/dl (1.03 mmol/l), systolic and/or diastolic blood pressure ≥ 130/85 mmHg, and impaired fasting serum glucose concentration ≥ 110 mg/dl (6.1 mmol/l). Using the IDF definition, patients were classified as having MS if they presented with waist circumference >94 cm plus two of the following criteria: serum triglycerides ≥ 150 mg/dl (1.7 mmol/l), HDL-cholesterol < 40 mg/dl (1.03 mmol/l), and impaired fasting serum glucose concentration ≥ 100 mg/dl (5.6 mmol/l), and systolic and/or diastolic blood pressure ≥ 130/85 mmHg. Hence, the study population was divided into two groups, with and without MS, using two sets of criteria.
Life events: These were assessed using the Holmes and Rahe (Citation1967) schedule of readjustment scale which was self-administered. This included 40 items, where men indicated which event they experienced during the past 1–5 years. Two LE indices were derived as the total number of LE experienced by a participant, and the total weight of LE each participant had selected. The weights reflected their mean degree of stressfulness as perceived by the participants: in the development of this scale for large samples, Grant et al. (Citation1978) found that both types of score have similar correlations with a criterion of psychiatric symptoms.
Statistical analysis
We first tested the distribution of variables, and then performed transformations to normalize the data using normality tests (kurtosis and skewness). Thus, some results are presented as log-transformed data. As MS was a categorical variable, its bivariate relationship with continuous variables was tested using t-tests, and its relationship with categorical variables was tested using χ2 tests. We then tested with a multiple logistic regression, whether LE, cortisol, and the LE × cortisol interaction predicted MS status, controlling for necessary confounders. In this analysis, LE, cortisol concentrations, and the LE × cortisol interaction across the entire sample and confounders were the independent variables, and MS was the dichotomous-dependent variable. Concentrations of PSAt were considered as well, since in a previous study on the same population, we observed a cortisol × LE interaction in relation to PSAt (Gidron et al. Citation2011) and PSAt is associated with markers of MS (Grosman et al. Citation2010). We then divided the sample at the median cortisol concentration into men with cortisol concentration above and below the median. A multivariate analysis was then done, at each level of cortisol separately, testing whether LE correlated with MS, independent of other factors related to MS, as shown below. Data are presented as mean ± SD.
Results
depicts the general characteristics of the entire sample of men. The mean age was slightly below 60 years; overall the men were moderately overweight and reported a mean of five LE, 12.8% were smokers and approximately half the sample had hypertension. Using the IDF classification for MS, 54.4% had MS, and according to the ATP criteria this percentage was reduced to 37.6%.
Table I. General characteristics of the entire sample of men (N = 149).
presents scores of participants with and without MS, using the IDF classification, on the main continuous and categorical study variables. Of all variables, beyond the expected significant differences in the MS components, participants with MS reported significantly more past LE (t(141) = 2.6, p = 0.009) and a greater summed weight of LE (t(141) = 1.98, p = 0.049) than those without MS. Serum concentration of cortisol was similar between groups. Concerning categorical variables, participants with MS were not significantly different from those without MS for distribution of smokers, education level, or marital status (p>0.05). With regard to socioeconomic status, 80.5% were employed, 7% unemployed, and 12.5% retired, without significant differences between groups. Even though there were no differences in age and BMI between groups, in subsequent analyses we statistically controlled for age and BMI, to be more rigorous.
Table II. Scores of participants with and without MS, using the IDF classification, on the main continuous and categorical study variables.
depicts a logistic multivariate regression, whereby age, BMI, serum cortisol concentration, and number of LE were independent variables and MS-status was the dependent variable. In this analysis, only number of LE significantly differentiated between participants with and without MS. We then tested whether LE correlated with components of the MS. Among the components of MS, waist circumference and hypertension were related to number of LE (r = 0.160, p = 0.05; r = 0.168, p = 0.04, respectively) and hypertension also significantly correlated with summed weight of LE (r = 0.174, p = 0.037). All other MS components were unrelated to LE (p>0.05). We then also tested relationships between cortisol and the MS components. Of all five components, serum cortisol concentration showed a significant positive correlation with glucose concentrations (r = 0.27, p = 0.001), but cortisol was unrelated to hypertension, HDL-cholesterol and triglyceride concentrations, or to waist circumference (all p>0.05).
Table III. Multivariate logistic regression, with main variables predicting MS.
We then tested whether LE interacted with cortisol in relation to MS, using a multiple logistic regression. We entered age, BMI, LE, cortisol, and the LE × cortisol interaction term. This interaction was not significant (p = 0.10), however, when analyzing men with serum cortisol values below the median (13.7 μg/dl), LE were unrelated to MS (p = 0.52, RR = 1.04, 95% confidence interval [CI] = 0.92–1.18). In contrast, in men with cortisol values above the median, number of LE was significantly related to MS (p = 0.03, RR = 1.16, 95% CI: 1.01–1.33; p = 0.03) (). In both cases, these findings were present after controlling for age and BMI. These analyses were repeated with further adjustment for PSAt concentration, to determine the reliability of these interactions beyond the effects of PSAt. When PSAt was included, the LE × cortisol interaction became closer to significant (p = 0.065). However, LE remained a significant predictor of MS in men with high (p = 0.05) cortisol concentration, but not in men with low cortisol (p = 0.834). Thus, the pattern of relations remained similar, when considering PSAt as well.
Figure 1. Relationship between MS, cortisol, and LE. Data are mean ± SD. Low cortisol: men with serum cortisol concentration < 13.7 μg/dl. High cortisol: men with cortisol ≥ 13.7 μg/dl. Multivariate analysis was done, for each level of cortisol separately, testing whether LE correlated with MS, independent of other factors related to MS: p = 0.03 in High cortisol group, MS vs. no-MS.
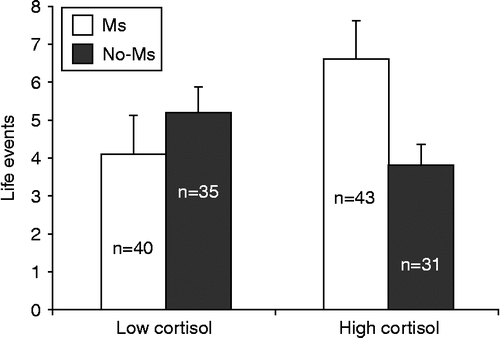
When the participants were classified according to ATP-III criteria, no differences in summed weight of LE or number of LE were observed between participants with and without MS.
Discussion
This study found that LE were significantly more prevalent in men with the than without MS, according to IDF criteria, independent of the effects of age and BMI. However, this relationship was subsequently found only in men with elevated serum cortisol concentrations. Hence, LE permissively interacted with cortisol in relation to MS. No other factor among the background or biometric factors (e.g. age and BMI) was associated with MS status. Furthermore, when examining the components of MS, number of LE was only related to hypertension and waist circumference, while summed weight of LE correlated with hypertension alone, but not with other MS components. Finally, serum cortisol concentration was related only to glucose concentration, and not to other MS components.
Patients with MS are at twice the risk of developing cardiovascular disease (CVD) over the next 5–10 years than individuals without the syndrome (Alberti et al. Citation2009). Stress at work has been linked to CVD in retrospective and prospective studies (Marmot et al. Citation1997; Rosengren et al. Citation2004; van Vegchel et al. Citation2005). The relationship between psychosocial stress and CVD has been identified as an important public health issue (Brotman et al. Citation2007) and although this association is well established, the intermediate mechanisms are yet to be fully elucidated. The INTERHEART Study (Rosengren et al. Citation2004) investigated the effects of chronic stressors on the incidence of myocardial infarction in a sample of 25,000 people from 52 countries. After adjusting for age, gender, geographic region, and smoking, those who reported “permanent stress” at work or at home had a 2.1-fold increased risk for developing a myocardial infarction. The impact of psychosocial factors on MS could be an intermediate step toward their effect on CVD, due to the role of MS in CVD risk. Researchers have attempted to understand the effects of psychosocial stress on physiological mediators and on components of MS (Van Dijk and Buwalda, Citation2008). MS was found to be more prevalent among individuals under psychosocial pressure or chronic stress. In a prospective study, Chandola et al (Citation2006) observed that men with chronic work stress were nearly twice as likely to develop MS as those with no exposure to work stress, and adjusting for health behaviors and obesity did not change the dose–response association. In a study on women, baseline stress predicted the development of MS over a 15-year follow-up (Räikkönen et al. Citation2007). In this study, we evaluated stress through the report of LE in the last 1–5 years; we observed that those individuals with MS presented a higher number and a higher summed weight of LE. No differences were observed in life-style or demographic factors (smoking habit, educational level, marital status, and socioeconomic status) between individuals with and without MS, and after controlling for age, BMI, and PSAt, our results concerning LE and MS remained significant. Among the components of MS, LE number correlated with waist circumference and hypertension, whereas summed weight of LE only correlated with hypertension.
Studies on monkeys have shown that early-life stress is associated with increased abdominal obesity and insulin resistance (Shively et al. Citation2009). Previous studies on men have shown that high job strain is a risk factor for increased abdominal obesity, independently of BMI (Ishizaki et al. Citation2008) and that psychosocial stress correlates with waist circumference (Anand et al. Citation2008). Furthermore, symptoms of anxiety and depression (Jonas et al. Citation1997), as well as hostility (Yan et al. Citation2003) and chronic stress (Sparrenberger et al. Citation2009) have been associated with incidence of hypertension in previously normotensive persons. Some studies have examined the effects of chronic stressors, such as war time, on blood pressure (Sparén et al. Citation2004). In addition, both low social support and low hedonistic response have been associated with elevated stress hormone reactivity in systemic hypertension (Wirtz et al. Citation2006), implying a relationship between chronic stress, stress hormones, and hypertension. These studies support those observed in the present one in which LE correlated with hypertension and waist circumference, which are two components of MS.
We evaluated the effects of cortisol on MS and its components. No differences were observed in serum cortisol concentration between men with and without MS; however, a significant association between serum cortisol and glucose concentrations was observed. Cortisol evidently plays a role in adiposity in MS, and an increase in urinary-free cortisol excretion has been reported in patients with MS (Anagnostis et al. Citation2009). The relationship between fasting hyperglycemia and cortisol reflects glucocorticoid effects on hepatic gluconeogenesis and insulin secretion (Anagnostis et al. Citation2009). Our results are in part in accordance with those of some researchers who reported no relationship between cortisol and waist circumference, TG or HDL-cholesterol levels (Weigensberg et al. Citation2008). These findings indicate that cortisol may be relevant to certain individual components of MS, but not to others, and that this relationship is not due to insulin resistance. Nevertheless, the link between obesity and cortisol levels remains a subject of debate. It must be taken into account that in clinical obesity there are alterations in cortisol metabolism, and enhanced local conversion of cortisone to cortisol via 11-β hydroxysteroid dehydrogenase type 1 in the adipose tissue may contribute to the development of MS (Sun et al. Citation2008). However, higher early morning plasma cortisol levels have been correlated with degree of coronary artery disease on angiograms in some studies (Koertge et al. Citation2002) but not others (Reynolds et al. Citation2010), with coronary artery disease being an important consequent of MS.
Given that the HPA axis may react at times with hyper- or hypoactivity, we divided the participants at the median of our observed cortisol levels (median cortisol = 13.7 μg/dl), which resembles means of samples in past studies of serum cortisol (Tomei et al. Citation2003; Gidron et al. Citation2011). As mentioned above, in this study, cortisol alone was not different between men with and without MS. It is possible that both environmental factors (e.g. LE) and biological vulnerability (e.g. HPA hyper-/hypoactivity) may be needed to induce metabolic alterations. In addition, it is possible that cortisol is related to MS risk in a more complex manner than initially considered. Our results indeed support a permissive interaction between LE and cortisol in relation to MS: hence, only in men with cortisol values above the median, LE predicted MS status, while this was not seen in men with cortisol values below the median. These results point to the possibility that the HPA axis may moderate the relationship between LE and health outcomes, similar to findings of Gidron et al. (Citation2011) concerning PSA. We did not have any evidence for cortisol to mediate the LE–MS link as cortisol was unrelated to MS. Thus, our results indicate that cortisol moderates the LE–MS relationship, which is limited to and more pronounced in men with higher cortisol levels.
One of the conflicting points of our results is that LE correlated with MS according to the IDF definition alone. Despite the increasing prevalence of MS worldwide, there is still a lack of uniformly accepted diagnostic criteria. Although the most widely applied definition is based on the NCEP-ATP-III criteria (Adult Treatment Panel III 2001), in this study we also used the definition of the IDF (Alberti et al. Citation2006) with more strict criteria to identify individuals at high risk and with the sine qua non condition of increased waist circumference, even though the IDF recently agreed that central obesity should not be an obligatory component (Alberti et al. Citation2009). The observation that the IDF, but not the NCEP-ATP-III definition of MS, was associated with LE may be explained by the fact that only the IDF definition has abdominal obesity as an obligatory MS criterion, and the lower cutoffs used by the IDF result in more people meeting the MS criteria. This may have provided greater statistical power to detect significant LE–MS relationships. In addition, the observation that LE were related to waist circumference, which is pivotal to the IDF definition of MS, may also partly explain why LE correlated with IDF-based MS and not with the ATP-based MS diagnosis. Supporting this observation, Elovainio et al. (Citation2011) have recently found associations between hostility and MS among women using the IDF definition, but not with others.
Another limitation of this study is its cross-sectional design, which prohibits any conclusions concerning the direction of relationships between LE and MS, unlike a longitudinal design. In addition, the use of the Holmes and Rahe questionnaire did not enable us to examine the subjective appraisal of LE by the participants, which could have potentially added predictive value beyond the mere number of LE or population-based weight of LE. Finally, only one single measure of morning serum cortisol concentration was obtained, which is not the optimal way to evaluate links between HPA activity and MS. Nevertheless, it is a valid and feasible approach for screening studies and has been previously applied (Weigensberg et al. Citation2008). Should these results be replicated in future studies, they call for allocating stress management interventions to people who have both high LE and cortisol, to examine whether such interventions prevent or ameliorate MS. This can be the next stage of research in future studies.
Finally, we conclude that LE were significantly more prevalent in men with MS than without MS, according to IDF criteria, independent of the effects of age and BMI, particularly and only in men with increased serum cortisol levels.
Acknowledgements
This study was supported by Research Grants from the University of Buenos Aires, (Argentina) UBACYT (01/2103; 2010-2012); and Roemmers Foundation 2010–2011.
Declaration of interest : The authors report no conflicts of interest, and have nothing to declare. The authors alone are responsible for the content and writing of the paper.
References
- Alberti KG, Zimmet P, Shaw J. 2006. Metabolic syndrome - A new world-wide definition: A Consensus Statement from the International Diabetes Federation. Diabet Med. 23:469–480.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SCJr. 2009. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120:1640–1645.
- Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. 2009. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome. A hypothesis. J Clin Endocrinol Metab. 94:2692–2701.
- Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S, INTERHEART Investigators. 2008. Risk factors for myocardial infarction in women and men: Insights from the INTERHEART study. Eur Heart J. 29:932–940.
- Bellingrath S, Weigl T, Kudielka BM. 2008. Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort-reward-imbalance. Biol Psychol. 78:104–113.
- Björntorp P, Rosmond R. 2000. Neuroendocrine abnormalities in visceral obesity. Int J Obes Relat Metab Disord. 24:S80–S85.
- Brotman DJ, Golden SH, Wittstein IS. 2007. The cardiovascular toll of stress. Lancet. 370:1089–1100.
- Brunner EJ, Marmot MG, Nanchahal K, Shipley MJ, Stansfeld SA, Juneja M, Alberti KG. 1997. Social inequality in coronary risk: Central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia. 40:1341–1349.
- Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. 2002. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: Nested case-control study. Circulation. 106:2659–2665.
- Chandola T, Brunner E, Marmot M. 2006. Chronic stress at work and the metabolic syndrome: Prospective study. BMJ. 332:521–525.
- Elovainio M, Merjonen P, Pulkki-Råback L, Kivimäki M, Jokela M, Mattson N, Koskinen T, Viikari JS, Raitakari OT, Keltikangas-Järvinen L. 2011. Hostility, metabolic syndrome, inflammation and cardiac control in young adults: The Young Finns Study. Biol Psychol. 87:234–240.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP). 2001. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (Adult Treatment Panel III). JAMA. 285:2486–2497.
- Gidron Y, Fabre B, Grosman H, Nolazco C, Mesch V, Mazza O, Berg G. 2011. Life events, cortisol and levels of prostate specific antigen: A story of synergism. Psychoneuroendocrinology. 36:874–880.
- Grant I, Sweetwood H, Gerst MS, Yager J. 1978. Scaling procedures in life events research. J Psychosom Res. 22:525–530.
- Grosman H, Fabre B, Mesch V, Lopez MA, Schreier L, Mazza O, Berg G. 2010. Lipoproteins, sex hormones and inflammatory markers in association with prostate cancer. Aging Male. 13:87–92.
- Holmes TH, Rahe RH. 1967. The social readjustment rating scale. J Psychosom Res. 11:213–218.
- Ishizaki M, Nakagawa H, Morikawa Y, Honda R, Yamada Y, Kawakami N. 2008. Japan Work Stress and Health Cohort Study Group. Influence of job strain on changes in body mass index and waist circumference—6-year longitudinal study. Scand J Work Environ Health. 34:288–296.
- Jonas BS, Franks P, Ingram DD. 1997. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch Fam Med. 6:43–49.
- Koertge J, Al-Khalili F, Ahnve S, Janszky I, Svane B, Schenck-Gustafsson K. 2002. Cortisol and vital exhaustion in relation to significant coronary artery stenosis in middle-aged women with acute coronary syndrome. Psychoneuroendocrinology. 27:893–906.
- Marmot MG, Bosma H, Hemingway H, Brunner E, Stansfeld S. 1997. Contribution of job control and other risk factors to social variations in coronary heart disease incidence. Lancet. 350:235–239.
- Räikkönen K, Matthews KA, Kuller LH. 2007. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: A comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 30:872–877.
- Reynolds RM, Walker BR, Haw S, Newby DE, Mackay DF, Cobbe SM, Pell AC, Fischbacher C, Pringle S, Murdoch D, Dunn F, Oldroyd K, Macintyre P, O'Rourke B, Pell JP. 2010. Low serum cortisol predicts early death after acute myocardial infarction. Crit Care Med. 38:973–975.
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S, INTERHEART investigators. 2004. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet. 364:953–962.
- Rosmond R, Björntorp P. 2000. Quality of life, overweight, and body fat distribution in middle-aged men. J Behav Med. 26:90–94.
- Schoorlemmer RM, Peeters GM, van Schoor NM, Lips P. 2009. Relationships between cortisol level, mortality and chronic diseases in older persons. Clin Endocrinol (Oxf). 71:779–786.
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. 1997. Price of adaptation–allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med. 157:2259–2268.
- Shively CA, Register TC, Clarkson TB. 2009. Social stress, visceral obesity, and coronary artery atherosclerosis: Product of a primate adaptation. Am J Primatol. 71:742–751.
- Sparén P, Vagerö D, Shestov DB, Plavinskaja S, Parfenova N, Hoptiar V, Paturot D, Galanti MR. 2004. Long term mortality after severe starvation during the siege of Leningrad: Prospective cohort study. BMJ. 328:11.
- Sparrenberger F, Cichelero FT, Ascoli AM, Fonseca FP, Weiss G, Berwanger O, Fuchs SC, Moreira LB, Fuchs FD. 2009. Does psychosocial stress cause hypertension? A systematic review of observational studies. J Hum Hypertens. 23:12–19.
- Stratakis CA, Chrousos GP. 1995. Neuroendocrinology and pathophysiology of the stress system. Ann N Y Acad Sci. 771:1–18.
- Sun L, Stenken JA, Brunner JE, Michel KB, Adelsberger JK, Yang AY, Zhao JJ, Musson DG. 2008. An in vivo microdialysis coupled with liquid chromatography/tandem mass spectrometry study of cortisol metabolism in monkey adipose tissue. Anal Biochem. 381:214–223.
- Tafet GE, Smolovich J. 2004. Psychoneuroendocrinological studies on chronic stress and depression. Ann N Y Acad Sci. 1032:276–278.
- Tomei F, Rosati MV, Ciarrocca M, Baccolo TP, Gaballo M, Caciari T, Tomao E. 2003. Plasma cortisol levels and workers exposed to urban pollutants. Ind Health. 41:320–326.
- Troxel WM, Matthews KA, Gallo LC, Kuller LH. 2005. Marital quality and occurrence of the metabolic syndrome in women. Arch Intern Med. 165:1022–1027.
- Van Dijk G, Buwalda B. 2008. Neurobiology of the metabolic syndrome: An allostatic perspective. Eur J Pharmacol. 585:137–146.
- van Vegchel N, de Jonge J, Bosma H, Schaufeli W. 2005. Reviewing the effort-reward imbalance model: Drawing up the balance of 45 empirical studies. Socl Sci Med. 60:1117–1131.
- Vogelzangs N, Suthers K, Ferrucci L, Simonsick EM, Ble A, Schrager M, Bandinelli S, Lauretani F, Giannelli SV, Penninx BW. 2007. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology. 32:151–159.
- Walker BR. 2006. Cortisol cause and cure for metabolic syndrome?. Diabet Med. 23:1281–1288.
- Weigensberg MJ, Toledo-Corral CM, Goran MI. 2008. Association between the metabolic syndrome and serum cortisol in overweight Latino youth. J Clin Endocrinol Metab. 93:1372–1378.
- Wirtz PH, von Känel R, Mohiyeddini C, Emini L, Ruedisueli K, Groessbauer S, Ehlert U. 2006. Low social support and poor emotional regulation are associated with increased stress hormone reactivity to mental stress in systemic hypertension. J Clin Endocrinol Metab. 91:3857–3865.
- Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. 2003. Psychosocial factors and risk of hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA. 290:2138–2148.
- Zhang J, Niaura R, Dyer JR, Shen BJ, Todaro JF, McCaffery JM, Spiro A3rd, Ward KD. 2006. Hostility and urine norepinephrine interact to predict insulin resistance: The VA Normative Aging Study. Psychosom Med. 68:718–726.