Abstract
Extensive research has shown that psychosocial stress can induce cognitive impairment. However, few studies have explored impairment following acute stress exposure in individuals with central obesity. Central obesity co-occurs with glucocorticoid excess and can lead to elevated cortisol responses to stress. It is not clear whether centrally obese individuals exhibit greater cognitive impairment following acute stress. Cortisol responses to stress versus no-stress control were compared in 66 high- and low waist to hip ratio (WHR) middle-aged adults (mean age of 46 ± 7.17 years). Cognitive performance post exposure was assessed using Cambridge Automated Neuropsychological Test Battery. It was hypothesised that high WHR would exhibit greater cortisol in response to stress exposure and would show poorer cognitive performance. Males, particularly of high WHR, tended to secrete greater cortisol during stress exposure. Exposure to stress and increasing WHR were specifically associated with poorer performance on declarative memory tasks (spatial recognition memory and paired associates learning). These data tentatively suggest a reduction in cognitive performance in those with central obesity following exposure to acute stress. Further research is needed to elucidate the effects of stress on cognition in this population.
Introduction
Central obesity is defined as a waist to hip ratio (WHR) of greater than 0.85 in females and 0.90 in males (WHO Citation2000) and reflects the presence of central fat about the abdomen. Central obesity can occur independently or as a symptom of metabolic syndrome, a cluster of metabolic and cardiovascular risk factors (Isomaa Citation2003). The presence of central fat together with impaired glucose tolerance, insulin resistance and elevated blood lipids is associated with increased risk of type II diabetes and has been associated with cognitive impairment (e.g. Bent et al. Citation2000). Impairments in glucoregulation can result in cognitive impairment (e.g. Convit Citation2005), in particular, on tasks of working memory, declarative memory (Messier et al. Citation2003) and verbal memory (Lamport et al. Citation2009). Further, type II diabetes has been associated with atrophy of the hippocampus, a key brain structure in cognitive processing (Bruehl et al. Citation2009), and such atrophy may be an early indicator of onset of the diabetes (Gold et al. Citation2007).
Central obesity has also been associated with glucocorticoid excess (Bjorntorp and Rosmond Citation2000a and Citation2000b) based on studies of patients with Cushing's syndrome, a condition of elevated cortisol of which central obesity is a central feature. Previous research has documented elevated basal cortisol in those with central obesity (high WHR) (e.g. Rosmond et al. Citation2000) and further, elevated cortisol secretion in response to stress (Epel et al. Citation2000b). Glucocorticoid excess can lead to abdominal fat deposition by blocking the action of insulin, leading to further fat deposition and increased risk of impaired glucose tolerance and type II diabetes. Repeated exposure to psychosocial stress is thought to further exacerbate this process (Rebuffe-Scrive et al. Citation1992).
The effect of psychosocial stress on cognition has been shown to be both beneficial and detrimental. Acute stress may facilitate memory consolidation, particularly for emotional material, yet impair retrieval, specifically, declarative recall (Wolf Citation2009). Chronic stress has been shown to impair declarative memory, particularly in the elderly (Lupien et al. Citation2005). Concurrent with the effects of early onset type II diabetes, the effects of stress on cognition centre on the hippocampus, mediated by the effect of elevated cortisol on glucocorticoid receptors (Sapolsky et al. Citation1986). This can result, therefore, in enhanced risk of hippocampal atrophy and vulnerability to cognitive impairment.
Central obesity has been associated with impaired cognitive function due to a poor metabolic profile and elevated cortisol responses to stress. Further, both stress exposure and the onset of diabetes (of which central obesity is a common feature) have been associated with hippocampal atrophy. Few studies, however, have explored cognitive performance following acute stress exposure in high- and low-WHR individuals. Epel et al. (Citation2000a) conducted a limited assessment of cognitive performance using a stroop task following acute repeat stress exposure in high- and low-WHR females. Elevated cortisol responses to stress and poorer selective attention on an emotional stroop task were observed in high-WHR females. These findings are limited but highlight scope for a more detailed investigation.
The current study aimed to explore differences in the response to stress versus no stress in male and females with central obesity (high WHR) and those without (low WHR). Further, cognitive performance post-stress/no-stress exposure was assessed. It was hypothesised that high WHR would exhibit greater cortisol as a result of stress exposure and would further demonstrate poorer cognitive performance.
Method
Participants
Seventy healthy adults (35 males and 35 females) aged between 35 and 65 (mean = 46 ± 7.36 years) were recruited. Thirty-nine participants were of low WHR (17 males and 22 females) and 31 of high WHR (14 males and 17 females). Participants were recruited on the basis of whether they self-classified as an ‘apple’ body shape (high WHR) or a ‘pear’ body shape (low WHR). Classification was then confirmed by measurement at screening. Four participants failed to provide sufficient saliva for cortisol analysis. The non-completers did not differ with respect to gender and age to the completers. Sixty-six participants completed the study (mean = 46 ± 7.17 years). The characteristics of the final sample are detailed in .
Table I. Characteristics of the final sample (mean ± SD).
Exclusion criteria included use of any over-the-counter or prescribed medication and smokers. Of the female participants, 58% of the sample was pre-menopausal, 31% post-menopausal and 11% reported experience of peri-menopausal symptoms. However, none of the females were currently using or had ever used any form of hormonal replacement therapy (HRT). Nine per cent of the females in the sample reported use of oral contraceptives. None of the participants were hypertensive. The study was granted ethical approval by the Institute of Psychological Sciences Ethics Committee and all participants provided full informed consent prior to participation.
Materials
Physiological measures
Participants were classified as high or low WHR based on The World Health Organisation guidelines (WHO Citation2000) defining central obesity as a WHR of greater than 0.85 in females and 0.90 in males. WHR was determined by taking separate measurements (cm) of waist (level midway between the lower rib margin and iliac crest) and hip circumference (maximum circumference over the buttocks) (Molarius et al. Citation1999; Visscher et al. Citation2001).
Psychological measures
The National Adult Reading Test (NART, Nelson Citation1982) was administered to provide estimate of IQ to be used as a predictor in the analysis of cognitive performance. The NART has been found to be reliable in estimating IQ (O'Carroll et al. Citation1992).
Stress induction
The Trier Social Stress Test (TSST) (Kirschbaum et al. Citation1993) combines public speaking with a mental arithmetic task performed before a panel of two judges. The procedure for the TSST in the current study conformed to previous applications (e.g. Kirschbaum et al. Citation1993). A panel of two judges (one male and one female) was used. Participants were allocated 10 min preparation time before completing the public speaking (5 min) and mental arithmetic (5 min) tasks. Saliva samples for cortisol assessment were collected at baseline and throughout the TSST procedure (six samples in total). Participants assigned to the no-stress control condition were asked to follow the same procedure as the stress condition, but were asked to read and informally discuss a job advertisement with the researcher and to complete a one page set of basic mathematical questions (adapted from Domes et al. Citation2002). Participants were randomly assigned to either the stress or no-stress condition.
Cognitive assessment
Cognitive performance was assessed using the Cambridge Automated Neuropsychological Test Battery (CANTAB; Robbins et al. Citation1994). The following tests were included: (i) delayed matching to sample (DMS; % correct all delays), (ii) paired associates learning (PAL; total correct), (iii) spatial recognition memory (SRM; % correct), (iv) pattern recognition memory (PRM % correct), (v) spatial working memory (SWM; number of errors) and (vi) stockings of Cambridge (Tower of London) (SOC; minimum number of moves to solve the problem and mean initial thinking time). These tests have been described in detail in the literature (e.g. Robbins et al. Citation1994). Participants were also required to complete the auditory verbal learning task (AVLT; Rey Citation1964). This included assessment of immediate (total number of words recalled over five trials) versus delayed (number of words recalled after 20 min) verbal recall. Cognitive tests were selected in order to measure declarative memory, spatial memory and aspects of working memory which are impaired by stress (Wolf Citation2009) and because they are associated with hippocampal function (e.g. PAL, SRM and AVLT) influenced by elevations in cortisol and impaired glucoregulation. Previous research has documented the sensitivity of such tests to cognitive ageing (Rabbitt and Lowe Citation2000), hydrocortisone administration (Young et al. Citation1999) and glucoregulation (Ryan et al. Citation2006). All tests were administered in the same order.
Procedure
Participants were informed that the session involved completion of a number of cognitively challenging psychological tests. All test sessions were conducted at the same time of the day with participants arriving at the unit at 13:30 h. Upon arrival, participants were provided with a standard lunch (white bread plain cheese sandwich with salted crisps and a glass of water, approximately 500 calories) and allowed to relax. Twenty minutes post arrival, (i) a baseline measure of salivary cortisol was obtained (time = 0 min) and participants were randomly exposed to either the stress (S) (TSST) or no-stressor (NS) task. Further, cortisol samples were obtained (ii) following the preparation phase (time = +20 min) (iii) following the (S)/(NS) public speaking phase (time = +30min) and (iv) following completion of the (S)/(NS) mental arithmetic phase (time = +40 min). Following completion of the TSST/control, participants began the cognitive test battery (CANTAB). The cognitive assessment procedure lasted approximately 55 min. Further, saliva samples were obtained (v) upon completion of the cognitive test battery (time = +110 min) and (vi) following 5 min of relaxation (time = +120 min).
Cortisol assay
Salivette samples were thawed and spun at 3500 rpm. Cortisol was determined by a non-commercial time-resolved fluorescence (DELFIA) immunoassay developed for research purposes at Unilever Science Park, Colworth. The amount of cortisol present in the saliva samples was determined using a competitive inhibition immunoassay utilising a Biogenesis polyclonal anti-cortisol antibody and fluorescently labelled cortisol. The in-house assay was adapted to AutoDELFIA (Perkin Elmer, Buckinghamshire, UK) for high throughput testing and was validated against the widely used Salimetrics Kit (Salimetrics LLC, Salimetrics Europe Ltd., Newmarket, Suffolk, UK). The precise assay range (standards n = 26, run on three separate occasions) where both within and between assay (coefficient of variation) CV% < 10 was determined to be between 2 and 30 nM/l with CV% only rising above 10% below 2 nM/l cortisol concentration. The sensitivity of the assay was defined as the value 3 standard deviations below the mean of the zero standard measurement value (mean value–3SD) (n = 26). The determined assay sensitivity was 0.273 nM/l comprising the mean value determined from three separate precision runs. Samples were routinely tested in duplicate and then the average result was used. The mean %CV was 1.99.
Statistical analysis
Data for cortisol were positively skewed and normalised using a logarithmic transformation. Cortisol obtained over the test session was analysed using a 2 × 2 × 2 × 6 repeated measures analysis of variance model with condition (stress/no stress), WHR (high/low) and gender (male/female) as between-subject factors and time as a within-subject factor. Age and menopausal status (pre vs. post) (females only) were included as covariates. All significant interactions were explored post hoc using Bonferroni corrected independent samples t-tests. Further, a linear multiple regression using a backwards enter model was used to explore the predictive value of these variables in terms of cortisol rise (delta measure of time 4—baseline (time 1)).
Scores generated from the CANTAB data handling facility were imported and analysed in PASW 17 (SPSS Inc. Chicago, IL, USA). Cognitive performance was analysed using a series of linear multiple regressions using a backwards enter model to explore predictors of performance. Age, predicted IQ and menopausal status (pre vs. post) (females only) were entered in the first block followed by condition (stress or no stress), WHR (raw score), gender (male or female) and net cortisol rise. The final block consisted of the interaction terms condition × WHR and condition × WHR × gender. As WHR is a continuous variable, it was centred prior to inclusion in the interaction term (Aiken and West Citation1991). Score on each of the cognitive tests was included as the outcome variable.
Results
Cortisol profile during the stress/no-stress task in high/low WHR
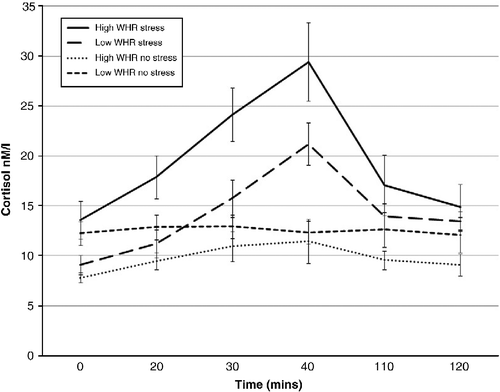
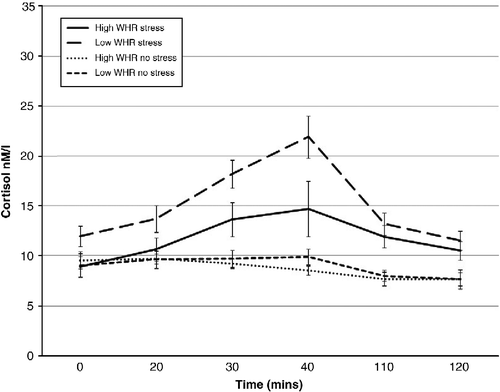
The pattern of cortisol response to the stress/no-stress task in high/low-WHR males and females is shown in , respectively.
A significant condition × WHR group × gender interaction (F (1, 57) = 10.957; p < 0.01) was observed. High-WHR males in the stress condition exhibited greater mean cortisol than high-WHR males in the no-stress condition (1.26 ± 0.06 and 0.97 ± 0.05 LOGnM/l, respectively; p = 0.004). Further, a trend for high-WHR males to secrete more cortisol across the session than low-WHR males in the same condition was observed (1.26 ± 0.06 and 1.12 ± 0.04 LOGnM/l, respectively; p = 0.073). High-WHR males in the stress condition secreted more cortisol than high-WHR females in the same condition (1.26 ± 0.06 and 1.02 ± 0.06 LOGnM/l, respectively; p = 0.025) and further, low-WHR males in the no-stress condition secreted more cortisol than low-WHR females in the same condition (1.08 ± 0.03 and 0.93 ± 0.03 LOGnM/l, respectively; p = 0.008). Finally, low-WHR females in the stress condition secreted greater mean cortisol than low-WHR females in the no-stress condition (1.15 ± 0.03 and 0.93 ± 0.03 LOGnM/l, respectively; p = 0.000).
A significant time × condition interaction (F (5,260) = 13.973; p < 0.01) revealed significantly greater cortisol responses at times 2, 3, 4, 5 and 6 (biggest p = 0.001) in the stress condition than in the no-stress exposure. Baseline cortisol did not differ significantly between conditions (p = 0.329). Males exhibited greater mean cortisol across the session than females (F (1, 57) = 12.012; p < 0.01; 1.11 ± 0.22 and 1.00 ± 0.19 LOGnM/l, respectively). Finally, significantly greater mean cortisol was observed in the stress condition than in no-stress condition (F (1, 57) = 28.829; p < 0.01; 1.13 ± 0.02 and 0.976 ± 0.02 LOGnM/l, respectively).
The rise in cortisol in response to the stress/no-stress task was analysed using a linear multiple regression using a backwards enter model to explore predictors of the response. Age was entered in the first block followed by condition (stress or no stress), WHR (centred raw score) and gender (male or female). The final block consisted of the interaction terms, condition × WHR and condition × WHR × gender. The final model for net cortisol rise consisted of condition, age and gender accounting for 53% of the variance in the model. The model significantly predicted cortisol rise (F (3, 62) = 25.644; p < 0.000). Condition, as expected, was identified as the biggest significant predictor (β = − 0.698; p < 0.000). However, age (p = 0.023) and gender (p = 0.014) were also significant predictors.
Cognitive performance
Cognitive performance was analysed using a series of linear multiple regressions using a backwards enter model to explore predictors of performance. Age and predicted IQ were entered first followed by condition (stress or no stress), WHR (raw score) and gender (male or female). The final block consisted of the interaction terms condition × WHR and condition × WHR × gender. Score on each of the cognitive tests was the outcome variable. The final models for each cognitive test are displayed in . Beta values are included to reflect the degree of correlation with each index of cognitive performance.
Table II. Final models in cognitive assessment.
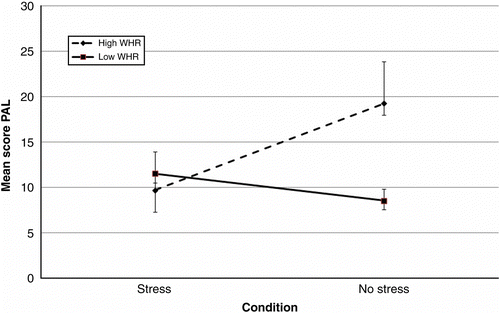
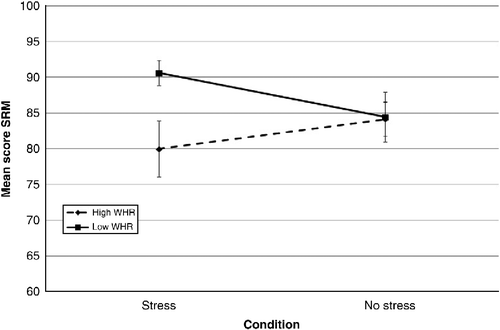
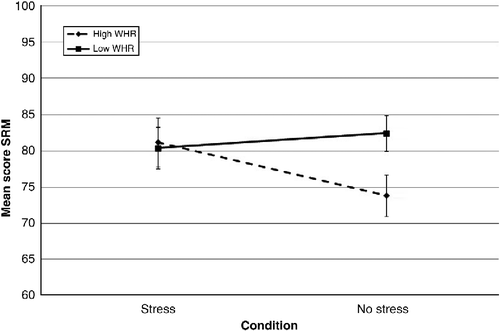
As shows, performance on the PAL task was associated with age, WHR and condition × WHR. All were significant predictors with condition × WHR, the biggest predictor of performance. Increasing WHR was associated with improved performance on the task in the no-stress condition, yet poorer performance in those exposed to stress (). Condition, WHR and gender were also prominent predictors of performance on the SRM task. An increase in WHR was concurrent with a reduction in performance on the SRM task for males in the stress condition (). For females in the stress condition and males in the no-stress condition, performance did not vary with increasing WHR (). Further, females in the no-stress condition demonstrated poorer performance with increasing WHR. In general, males outperformed females on this task (85.36 ± 1.58 and 79.61 ± 1.49, respectively).
Age was the biggest predictor of performance on the AVLT task (both total words recalled and delayed recall of list A) in which performance decreased with increasing age. Gender was identified as the biggest predictor of performance on the PRM task with males outperforming females (95.09 ± 1.09 and 89.36 ± 1.53, respectively). Gender was also the biggest predictor of performance on the SOC task (minimum number of moves to solve the problem). However, on this task, females solved the problem in fewer moves than males (7.97 ± 0.31 and 10.11 ± 0.31, respectively). However, in terms of the mean initial thinking time (for a five-move problem) on the SOC task, predicted IQ was the biggest predictor of performance, whereby increases in predicted IQ were associated with more initial thinking time. IQ was also a prominent predictor of performance on the SWM task (percentage correct, between errors). An increase in predicted IQ was associated with improved performance on this task. Finally, IQ was also the biggest predictor of performance on the DMS task (% correct all delays). Performance, again, improved with increasing predicted IQ.
supplementary analyses of cognitive performance were performed with menopausal status (pre vs. post) included as a predictor (in females only). Menopausal status was predictive of performance on the PRM, delayed AVLT and DMS tasks, whereby performance was better in post-menopausal females. Menopausal status was not found to be predictive of performance on the PAL and SRM tasks or any of the other cognitive tasks administered.
Discussion
The TSST was effective in inducing a stress response. Greater cortisol was observed during exposure to the TSST than with the no-stress condition, consistent with previous research (e.g. Schommer et al. Citation2003). Condition (stress/no stress) was also the biggest predictor of the increase in cortisol from baseline to peak response. Further, a trend was observed for WHR as a predictor of cortisol response. Within the stress condition, males secreted more cortisol during stress exposure than females. Further, gender and WHR interacted to produce a cortisol response. High-WHR males tended to secrete greater cortisol across the session than high-WHR males in the no-stress condition and low-WHR males and females in the stress condition.
Individuals with central obesity can exhibit a more pronounced cortisol response to a psychosocial stressor (Marin et al. Citation1992; Moyer et al. Citation1994; Epel et al. Citation2000b). However, these studies considered only females. In the current study, high-WHR males were found to be more responsive to stress and high-WHR females less responsive than their high-WHR counterparts. Males being more responsive to stress is consistent with previous research (Kirschbaum et al. Citation1995; Wolf et al. Citation2001). Kudielka et al. (Citation2004) observed elevated free salivary cortisol responses to the TSST in elderly males compared to females. In the current study, it is therefore plausible that these findings reflect a gender difference and not a sole effect of WHR as both high- and low-WHR males demonstrated more elevated cortisol than high- and low-WHR females.
The assessment of cognitive performance post-stress exposure in those with central obesity to date is under researched. Epel et al. (Citation2000a) conducted a limited assessment of cognitive performance following repeated exposure to acute stress in high- and low-WHR females. Elevated cortisol responses to stress were observed in high-WHR individuals (females only) and poor selective attention on an emotional stroop task. Yet, no effects of stress and WHR on memory were observed. In the current study, subtle effects of stress exposure and central obesity on cognitive performance were observed (accounting for a small percentage of the variance). The interaction of WHR, condition and gender was associated with performance on declarative and spatial memory tasks (PAL, SRM, DMS and PRM), which arguably centre on hippocampal function, known to be sensitive to elevations in stress (Wolf Citation2009). Both WHR and the interaction between condition and WHR were predictive of performance on the PAL task, in which increasing WHR was associated with poorer performance on the PAL task under stress. This supports the hypothesis that stress and a high WHR may lead to reduced cognitive performance on hippocampal-related tasks. However, in the no-stress condition, increasing WHR was associated with better performance on the PAL task. These findings may be explained using the inverted ‘U’ hypothesis (Luine et al. Citation1993). An increase in WHR was concurrent with an increase in basal cortisol (due to an apparent glucocorticoid excess in central obesity (Bjorntorp and Rosmond Citation2000a and Citation2000b)) and facilitated performance in the absence of stress. The introduction of stress elevated cortisol levels to a point of impairment. Performance on the SRM task was also associated with WHR and the interaction between condition and WHR. Spatial memory is also associated with hippocampal function and also sensitive to elevations in stress. Increasing WHR and stress were associated with poorer performance in males. In general, increasing WHR was associated with poor performance on this task, suggesting that the combination of elevated cortisol and WHR may act specifically on the hippocampus and reduce performance on certain hippocampal-related tasks. It remains to be elucidated, however, whether these deficits in performance are due to the interaction between stress and WHR or a poor metabolic profile, which can also lead to cognitive impairment (Messier et al. Citation2003; Convit Citation2005).
Oestrogen may be protective against the negative effects of stress (Galea et al. Citation1997; Wolf et al. Citation2001) and can dampen cortisol responses when exogenously administered (Kudielka et al. Citation1999). This could account for the current findings; however, oestrogen was not assessed in the current study. A small proportion of the females within the study may have been perimenopausal and this may account for lower cortisol responses in those females. However, Saab et al. (Citation1989) observed heightened neuroendocrine responses in menopausal females versus pre-menopausal females (and also post-menopausal females (Kudielka and Kirschbaum Citation2005)) which conflicts with the current findings. Further, menopausal status was not identified as a significant covariate of cortisol response in the current data. Menopausal status can also influence cognition due to reduced oestrogen, leading to a reduction in performance (e.g. Halbriech et al. Citation1995). Menopausal status was predictive of performance on some cognitive tests, namely the pattern recognition (PRM), delayed AVLT and DMS task. Improved performance was observed in post-menopausal women. It is possible that this accounts to differential cognitive effects in males and females in the current study as the current sample comprised both pre and post-menopausal females. However, menopause was associated with improved performance which conflicts with previous findings. Improved performance post-menopause has been observed in females taking HRT (e.g. oestrogen) (LeBlanc et al. Citation2001); however, none of the participants in the current study reported HRT use. Further, menopausal status was not found to be predictive of performance on the tests of interest i.e. PAL and SRM. It is therefore unlikely that menopausal status alone accounts for the current findings.
Menstrual cycle phase may also influence cortisol responses to stress (e.g. Hlavacova et al. Citation2008). Previous research conflicts as to the influence of menstrual cycle phase on cortisol responses to stress and few studies have explored this using the TSST. Many observe a lack of influence of phase on cortisol response (Pico-Alfonso et al. Citation2007), whereas others have observed an elevated cortisol response in the luteal phase (Kirschbaum et al. Citation1999) and a blunted follicular phase cortisol response (Childs et al. Citation2010). A blunting effect of menstrual cycle phase may account for the current findings; however, only half (and not all) the female sample was pre-menopausal and the menstrual cycle phase of these women at the time of testing is not known. Future research should carefully screen female participants to prevent this possible confound. Further, a dampened response in high-WHR females may be due to the increased circulating leptin. Prior animal research has shown leptin to attenuate the neuroendocrine response to stress (Huang et al. Citation1998), reducing cortisol release (Wilson et al. Citation2005). Leptin is, conversely, often reduced in males (Menendez et al. Citation2000). Leptin, however, was not assessed in the current study.
Some limitations must be addressed. A between-subject design was adopted in which the order of the tests was unchanged for each participant. This may be viewed as problematic due to a cortisol decrease post exposure to the stress/no-stress condition. demonstrate cortisol elevation at the time of test administration before starting to decline. Further, tasks on which impairment was observed were not those initially completed in the battery. Future research should consider a counterbalanced method of test administration. Although a standard meal administration is acceptable in most experimental paradigms, previous research suggests that the time lag between meal consumption and assessment of saliva for cortisol analysis should be longer than that implemented in the current study. Consumption of food may prompt a cortisol response lasting for up to 90 min. As such samples should not be taken within this window of time. As all participants received the same meal at the same time, it is unlikely to have affected the results in the current study. However, future research should take such factors into consideration to ensure accuracy of the cortisol response to the stressor. Inclusion of a measure of sympathetic arousal should be considered. This would permit a more comprehensive assessment of the stress response and would permit further assessment of the relationship between stress and cognition as epinephrine, for example, can enhance memory (Cahill and Alkire Citation2003). Finally, care should be taken to carefully screen female participants for menopausal status and to control for menstrual cycle phase. The findings of this study have shown subtle influences of menopausal status on cognitive performance in females and future research should take this into consideration.
In conclusion, the findings of the current study suggest that subtle interactions between stress and central obesity may exist. The findings demonstrate a tendency for greater cortisol responses to stress in males with central obesity and for poorer performance on declarative memory tasks. However, it is unclear as to whether these effects are a consequence of stress exposure or are due to a poor metabolic profile. Further research is needed to fully elucidate the mechanisms involved and to clarify the basis of the effects observed.
Acknowledgements
This research was supported with funding from the Medical Research Council (MRC, UK) and Unilever Discover, Colworth Science Park, UK.
Declaration of interest : The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aiken LS, West SG. 1991. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage.
- Bent N, Rabbitt P, Metcalfe D. 2000. Diabetes mellitus and the rate of cognitive aging. Br J Clin Pharmacol. 39:349–362.
- Bjorntorp P, Rosmond R. 2000. Neuroendocrine abnormalities in visceral obesity. Int J Obes Relat Metab Disord. 24:S90–S85.
- Bjorntorp P, Rosmond R. 2000. Obesity and cortisol. Nutrition. 16:924–936.
- Bruehl H, Wolf OT, Convit A. 2009. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 34:815–821.
- Cahill L, Alkire MT. 2003. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol Learn Mem. 79:194–198.
- Childs E, Dlugos A, De Wit H. 2010. Cardiovascular, hormonal and emotional responses to the TSST in relation to sex and menstrual cycle. Psychophysiology. 47:550–559.
- Convit A. 2005. Links between cognitive impairment in insulin resistance an explanatory model. Neurobiol Aging. 26:S31–S35.
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. 2002. Hypothalamic–pituitary–adrenal axis reactivity to psychological stress and memory in middle aged women: High responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology. 27:843–853.
- Epel E, McEwen B, Lupien S. Cortisol reactivity to repeated stress as a function of fat distribution: Effects on cognition. Psychoneuroendocrinology. 2000a; 25 suppl. 1: s32.
- Epel ES, McEwen B, Seeman TE, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics J. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000b; 62:623–632.
- Galea LAM, McEwen B, Tanapat P, Deak T, Spencer RL, Dhabhar FS. 1997. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 81:689–697.
- Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. 2007. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 50:711–719.
- Halbriech U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH. 1995. Possible acceleration of age effects on cognition following menopause. J Psychiat Res. 29 3: 153–163.
- Hlavacova N, Wawruch M, Tisonova J, Jezova D. 2008. Neuroendrocrine activation during combined mental and physical stress in women depends on trait anxiety and the phase of the menstrual cycle. Ann N Y Acad Sci. 1148:520–525.
- Huang Q, Rivest R, Richard D. 1998. Effects of leptin on corticotrophin releasing factor (CRF) synthesis and CRF neuron activation in the paraventricular hypothalamic nucleus of obese (ob/ob) mice. Endocrinology. 139:1524–1532.
- Isomaa B. 2003. A major health hazard: The metabolic syndrome. Life Sci. 73:2395–2411.
- Kirschbaum C, Pirke CM, Hellhammer DH. 1993. The “Trier Social Stress Test”—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 28:76–81.
- Kirschbaum C, Prüssner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. 1995. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Am Psychosom Soc. 57 5: 468–474.
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. 1999. Impact of gender, menstrual cycle phase and oral contraceptives on the activity of the hypothalamic–pituitary–adrenal axis. Psychosom Med. 61:154–162.
- Kudielka BM, Kirschbaum C. 2005. Sex differences in HPA axis responses to stress: A review. Biol Psychol. 69:113–132.
- Kudielka BM, Kirschbaum AB, Hellhammer DH, Kirschbaum C. 2004. HPA axis responses to laboratory psychosocial stress in health elderly adults, younger adults and children: Impact of age and gender. Psychoneuroendocrinology. 29:83–98.
- Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. 1999. Psychological and endocrine responses to psychological stress and dexamethasone/corticotrophin-releasing hormone in healthy postmenopausal women and young controls: The impact of age and a two week estradiol treatment. Neuroendocrinology. 70:422–430.
- Lamport DL, Lawton CL, Mansfield MW, Dye L. 2009. Impairments in glucose tolerance can have a negative impact on cognitive function: A systematic research review. Neurosci Biobehav Rev. 33:394–413.
- LeBlanc ES, Janowsky J, Chan BK, Nelson HD. 2001. Hormone-replacement therapy and cognition: Systematic review and meta-analysis. JAMA. 285:1489–1499.
- Luine V, Spencer RL, McEwen B. 1993. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotinergic function. Brain Res. 616:65–70.
- Lupien S, Fiocco A, Wan N, Maheu FS, Lord C, Schramek T, Thanh Tu M. 2005. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 30:225–242.
- Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. 1992. Cortisol secretion in relation to body fat distribution in obese pre-menopausal women. Metabolism. 41 8: 882–886.
- Menendez C, Baldelli R, Lage M, Casabiell X, Pinero V, Solar J, Dieguez C, Casaneuva F. 2000. The in vitro secretion of human leptin is gender dependent but independent of body mass index of the donors. Eur J Endocrinol. 143:711–714.
- Messier C, Tsiakas M, Gagnon M, Desrochers A, Awad N. 2003. Effect of age and gluco-regulation on cognitive performance. Neurobiol Aging. 24:985–1003.
- Molarius A, Seidell JC, Sans S, Tuomilehto J, Kuulasmaa K. 1999. Waist and hip circumferences, and waist hip ratio in 19 populations of the WHO MONICA project. Int J Obes Relat Metab Disord. 23:116–125.
- Moyer A, Rodin J, Grilo C, Cummings N, Larson L, Rebuffe-Scriffe M. 1994. Stress-induced cortisol response and fat distribution in women. Obes Res. 2:255–262.
- Nelson J. 1982. National Adult Reading Test (NART): Test manualWindsor, UK: Nfer-Nelson.
- O'Carroll R, Walker M, Dunan J, Murray C, Blackwood D, Ebmeier KP, Goodwin GM. 1992. Selecting controls for schizophrenia research studies: The use of the national adult reading test (NART) is a measure of pre-morbid ability. Schizophr Res. 8:137–141.
- Pico-Alfonso MA, Mastorci F, Ceresini G, Ceda GP, Manghi M, Pino O, Troisi A, Sgoifo A. 2007. Acute psychosocial challenge and cardiac autonomic response in women: The role of estrogens, corticosteroids, and behavioural coping styles. Psychoneuroendocrinology. 32:451–463.
- Rabbitt P, Lowe C. 2000. Patterns of cognitive ageing. Psychol Res. 63:308–316.
- Rebuffe-Scrive M, Walsh UA, McEwen B, Rodin J. 1992. Effect of chronic stress and exogenous glucocorticoid administration on regional fat distribution and metabolism. Physiol Behav. 52:583–590.
- Rey A. 1964. L'Examen Clinique en Psychologie. Paris: Press Universitaire de France.
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. 1994. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 5:266–281.
- Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, Carlsson B, Bouchard C, Bjorntorp P. 2000. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic–pituitary–adrenal axis. Obes Res. 8 3: 211–218.
- Ryan CM, Freed MI, Rood JA, Cobitz AR, Waterhouse BR, Strachan MWJ. 2006. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 29 2: 345–351.
- Saab PG, Matthews KA, Stoney CM, McDonald RH. 1989. Premenopausal and postmenopausal women differ in their cardiovascular and neuroendocrine responses to behavioural stressor. Psychophysiology. 26:270–280.
- Sapolsky R, Krey L, McEwen B. 1986. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr Rev. 7:284–301.
- Schommer NC, Hellhammer DH, Kirschbaum C. 2003. Dissociation between reactivity of the HPA-axis and the sympathetic adrenal medullary system to repeated psychosocial stress. Psychosom Med. 65:450–460.
- Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. 2001. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: The Rotterdam study. Int J Obes Relat Metab Disord. 11:1730–1735.
- WHO. 2000. Asia-pacific perspective: Redefining obesity and its treatment. Geneva: World Health Organisation.
- Wilson ME, Fisher J, Brown J. 2005. Chronic subcutaneous leptin infusion diminishes the responsiveness of the hypothalamic–pituitary–adrenal (HPA) axis in female rhesus monkeys. Physiol Behav. 84:449–458.
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. 2001. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 26:711–720.
- Wolf OT. 2009. Stress and memory in humans: 12 years of progress?. Brain Res. 1293:142–154.
- Young AH, Sahakian BJ, Robbins TW, Cowen PJ. 1999. The effects of chronic administration of hydrocortisone on cognitive function in normal male volunteers. Psychopharmacology. 145:260–266.