Abstract
Reminders of an aversive event adversely impact retrieval of hippocampus-dependent memories and exacerbate stress-induced levels of anxiety. Interestingly, stress and anxiety shift control over learning away from the hippocampus and toward the striatum. The aims of the current study were to determine whether spatial memory and learning strategy are impacted by reminders of a stressor. Adult male Long-Evans rats (N = 47) were subjected to an inhibitory avoidance (IA) training trial in which 32 rats were exposed (3 s) to a single inescapable electrical footshock (0.6 mA). Prior to the retention trial of a Y-maze task and the probe trials of two different learning strategy tasks, some of the rats that were exposed to the footshock (n = 17) were reminded of the stressor on an IA retrieval trial. Both groups of rats exposed to the initial stressor exhibited hypoactivity, but no impairment in spatial memory, on the Y-maze task conducted 1 week after exposure to the footshock. One month after exposure to footshock, both groups of rats exposed to the initial stressor tended to prefer a striatum-dependent learning strategy on a water T-maze task. However, 2 months after exposure to footshock, only shocked rats that were reminded of the stressor exhibited a preference for a striatum-dependent learning strategy on a visible-platform water maze task, which corresponded with lower levels of activity in an open field. The results indicate that reminders of a stressor perpetuate the deleterious effects of stress on affective and cognitive processes.
Introduction
In rodents, heightened anxiety and impairments in hippocampus-dependent spatial learning and memory often arise after exposure to a single traumatic event. Following exposure to acute stressors, rodents typically exhibit increased levels of anxiety on a variety of tests that assess affective behaviors, including the open field (Van Dijken et al. Citation1992), the light-dark transition box (Adamec et al. Citation2007), the elevated plus-maze (Belda et al. Citation2008), the novelty-induced feeding task (Siegmund and Wotjak Citation2007), and the social interaction test (Haller and Bakos Citation2002; Mikics et al. Citation2008a,Citationb; Christianson et al. Citation2009). Correspondingly, exposure to acute stressors often results in poorer performance on tests of spatial cognition such as the Y-maze task (Conrad et al. Citation2004), the object location task (Howland and Cazakoff Citation2010), the radial-arm maze (Diamond et al. Citation1996), the water version of the radial-arm maze (Diamond et al. Citation1999; Woodson et al. Citation2003; Park et al. Citation2008), and the Morris water maze (de Quervain et al. Citation1998; Kim et al. Citation2005). Although the impact of exposure to a single traumatic event has been well documented, the effects of subsequent exposures to reminders of an acute stressor on anxiety and cognition remain largely unexplored.
Results from animal models of posttraumatic stress disorder (PTSD) indicate that reminders of a traumatic event, which are designed to simulate the intrusive thoughts that characterize the human disorder, compound the detrimental effects of the initial stressor on anxiety and spatial cognition (Korte et al. Citation1999; Zoladz et al. Citation2010). For example, rats that were reminded of electrical footshocks exhibited higher levels of anxiety on an elevated plus-maze (Korte et al. Citation1999) and made more errors in a water-based version of a radial-arm maze (Zoladz et al. Citation2010) compared with rats that were never exposed to the shock and with rats that experienced the shock but were never exposed to reminders of the experience. These results indicate that reminders of a stressor reactivate the aversive memory, which in turn potentiates the adverse effects on affective behaviors and hippocampus-dependent learning and memory processes.
Interestingly, an intimate link exists between affect and cognition such that exposure to stress (Kim et al. Citation2001; Schwabe et al. Citation2008; Ferragud et al. Citation2010) and heightened levels of anxiety (Packard and Wingard Citation2004; Elliott and Packard Citation2008; Hawley et al. Citation2011a) shift the way information is learned. When navigating a spatial environment, rodents are able to employ different types of learning strategies that are mediated by specific brain structures. A hippocampus-dependent place strategy relies on the relationships between cues in the extra-maze environment and a goal (Packard and McGaugh Citation1996; Packard Citation1999; Colombo et al. Citation2003; Gold Citation2004; Hawley et al. Citation2011a, Citation2012). Alternatively, striatum-based stimulus-response and response strategies rely either on a cue proximal to a goal that signals its location (Packard and McGaugh Citation1992; McDonald and White Citation1994; Devan and White Citation1999; Kim et al. Citation2001; Daniel and Lee Citation2004; Martel et al. Citation2007; Hawley et al. Citation2011a) or on proprioceptive cues that signal specific body movements toward a goal, respectively (Packard and McGaugh Citation1996; McIntyre et al. Citation2003; Korol et al. Citation2004; Pleil and Williams Citation2010; Hawley et al. Citation2012). Importantly, the brain structures that govern the two strategies work in a cooperative fashion such that when the integrity of the hippocampus is compromised, the striatum-based memory system emerges to modulate learning (McDonald and White Citation1994; Packard and McGaugh Citation1996). Notably, following exposure to a single episode of inescapable stress (Kim et al. Citation2001), or upon administration of anxiogenic agents (Packard and Wingard Citation2004; Elliott and Packard Citation2008), rodents eschew a place strategy and instead exhibit a preference for a striatum-dependent response learning strategy. Therefore, if exposure to a reminder of an aversive event heightens anxiety (Korte et al. Citation1999) and impairs retrieval of a recently formed spatial memory (Zoladz et al. Citation2010), then exposure to a reminder of an aversive event would also be expected to bias rats toward striatum-dependent learning strategies. Consistent with previous research (Zoladz et al. Citation2010), rats were subjected to an inhibitory avoidance (IA) retrieval trial, or remained in their home cage, 30 min prior to the retention trial of a Y-maze task, which assesses spatial memory (Conrad et al. Citation1996; Wright and Conrad Citation2005; Hawley et al. Citation2011a), and again 30 min prior to the probe trials of two different dual-solution learning strategy tasks, which assess learning strategy preference (McDonald and White Citation1994; Packard and Wingard Citation2004; Elliott and Packard Citation2008). Four days following the final learning strategy test, an open field test was conducted to confirm the effects of multiple reminders of an aversive event on activity and anxiety (Louvart et al. Citation2005; Hawley et al. Citation2011b). Relative to rats that were not shocked and to rats that were never reminded of the shock, we hypothesized that rats reminded of the footshock would exhibit an impairment in spatial memory, a bias toward striatum-dependent learning strategies, and elevated levels of anxiety.
Materials and methods
Animals
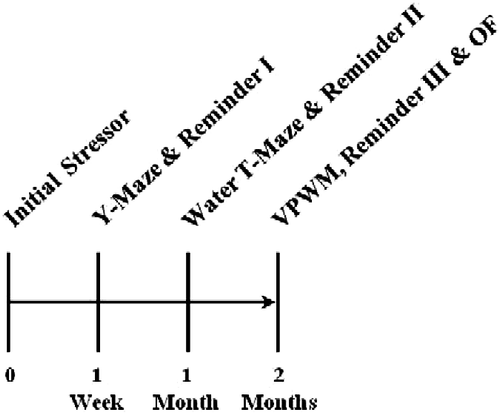
A total of 47 male Long-Evans rats, 55 days of age upon arrival, were purchased from Harlan, Inc. (Indianapolis, IN, USA). Rats were housed individually, provided free access to food and water, and maintained under a standard 12 h:12 h light-dark cycle (lights on at 07:00 h) in animal care facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All procedures were approved by the Tulane University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Citation1996). Rats were acclimated to the housing conditions for approximately 2 weeks prior to the onset of behavioral procedures. During that time, rats were handled for 1 min/day on four different days in order to habituate to the experimenters. Ambient temperature in both the vivarium and behavioral testing rooms was maintained in a range between 20°C and 22°C. All behavioral tests were conducted between 08:00 and 17:00 h. A timetable for the behavioral procedures is outlined in .
Figure 1. Timeline for behavioral testing. The initial stressor consisted of exposure to electrical footshock during an IA training trial. To re-activate the memory for the aversive event, reminders of the stressor (IA retrieval trials) were performed 30 min prior to the retention trial on the Y-maze and again 30 min prior to the probe trials on the learning strategy tasks. VPWM = visible-platform water maze; OF = open field test.
Stress and reactivation of an aversive memory: IA
The stress paradigm, which included exposure to reminders of the initial stressor, was adapted from a protocol developed to interfere with the retrieval of hippocampus-dependent memories (Zoladz et al. Citation2010). The initial stressor was a brief exposure to an inescapable electrical footshock, which was administered in an enclosed chamber (50 × 25 × 30 cm) that was divided into two equal-sized compartments separated by an automatic guillotine door (Coulborn Instruments, Whitehall, PA, USA). The illuminated compartment had walls lined externally with white posterboard and the dark compartment had walls lined externally with black posterboard. The apparatus, including the drop pan below the grid floor, was cleaned thoroughly with 70% ethanol between trials.
On the day of exposure to the initial stressor, which took place during the IA training trial, rats were placed into the illuminated compartment, and after 30 s, the guillotine door was automatically raised allowing access to the dark compartment. Photocells detected entry into the compartment, which signaled the guillotine door to close, thereby sequestering the rat. Three seconds after the door closed, a single electrical shock (0.6 mA) was delivered for 3 s through the grid floor. Rats were removed from the dark compartment 15 s after the shock was terminated. Control rats underwent an identical procedure, but did not receive footshock. Latency to enter the dark compartment prior to administration of the stressor was scored to account for pre-existing differences in anxiety-like behavior.
Rats were divided into the following groups to examine the effects of the initial stressor (IA training trial) and subsequent reminders (IA retrieval trial) on cognitive and affective measures: not shocked/reminded (n = 15), shocked/not reminded (n = 15), and shocked/reminded (n = 17). Rats in the not shocked/reminded and the shocked/reminded groups were re-exposed to the chamber (IA retrieval trial) 30 min prior to each of the retention and probe trials on the cognitive tasks described later, whereas rats in the shocked/not reminded group remained in their home cages (Zoladz et al. Citation2010). The procedures for the IA retrieval trials were identical to those of the IA training trial with the exception that entry into the dark compartment was not followed by footshock. Longer latencies to enter the dark compartment confirmed re-activation of the aversive memory (Zoladz et al. Citation2010). Failure to enter the dark compartment during the IA retrieval trial resulted in a maximum latency of 600 s.
Spatial recognition memory: Y-maze task
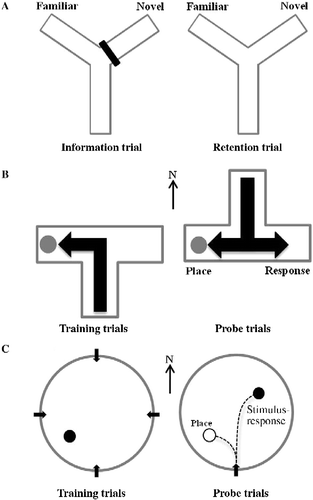
One week after exposure to the footshock stressor, rats were tested on a Y-maze task () to determine whether exposure to a reminder of the stressor impacted retrieval of a hippocampus-dependent memory in a non-aversive environment (Conrad et al. Citation1996). The Y-maze was constructed from opaque Plexiglas that formed three identical arms (50 × 10 × 20 cm; Stoelting ANY-maze, Wood Dale, IL, USA) and was surrounded by a variety of 2D and 3D extra-maze cues with different shapes, colors, and sizes. Rats were placed into the maze for a 15-min information trial and were allowed to freely explore the start arm and a second arm, but access to the third arm was blocked by an opaque plastic partition. Rats were returned to their home cages following the information trial for a delay interval of 4 h. After the delay, each rat was administered a 5-min retention trial in which they were returned to the same start arm and were allowed to freely explore all three arms. However, 30 min prior to the retention trial, the not shocked/reminded and the shocked/reminded groups were subjected to an IA retrieval trial (Re-exposure I). Retention trials on the Y-maze were video-recorded by an overhead camera for scoring in which an entry into an arm was defined as all four paws crossing into the arm proper. The maze was cleaned thoroughly with 70% ethanol and air-dried after each trial to remove olfactory cues. On the retention trial, within-group spatial memory was indicated by the percentage of entries into the novel arm relative to the familiar arm (Wright and Conrad Citation2005; Hawley et al. Citation2011a). The difference between the percentage of entries in the novel and the familiar arm was calculated for between-group comparisons of spatial memory (Conrad et al. Citation2004).
Learning strategy: water T-maze task
One month after administration of the footshock stressor, a dual-solution version of the water T-maze task () was used to determine whether a second exposure to a reminder of the stressor impacted learning strategy. The free-standing water T-maze was constructed from clear Plexiglas that formed three equal-sized arms (60 × 30 × 45 cm). The maze was surrounded by a variety of 3D spatial cues affixed to black curtains that hung from the ceiling to the floor. The maze filled with water to a level of 20 cm was made opaque by addition of white non-toxic paint and maintained at a temperature of approximately 25°C. One day prior to training, rats were placed in the water T-maze for a 1-min swim without an escape platform in order to habituate to the water. Then, rats received six training trials each day for 2 consecutive days followed by a single probe trial on the third day (Packard and Wingard Citation2004). For a given rat, a submerged escape platform was located in the west arm of the maze across all training trials and during the probe trial. During training, rats were placed into the arm of the maze located in the south position and were allowed 60 s to escape to the platform. Once the platform was mounted, rats were left undisturbed for an additional 15 s. Rats that failed to locate the platform within 60 s were guided to it by an experimenter. Training trials were separated by an inter-trial interval of 30 s. For the probe trial, the maze was rotated 180°, the platform was relocated in the same arm as during training, and the rat was placed into the maze in the start arm which was then located in the north position. However, 30 min prior to the probe trial, the not shocked/reminded and the shocked/reminded groups were subjected to an IA retrieval trial (Re-exposure II). The first full body entry into either the arm that contained the platform or the opposite arm was recorded for all trials. Learning was indicated by a greater percentage of entries into the arm that contained the platform as training progressed (Elliott and Packard Citation2008; Hawley et al. Citation2012). On the probe trial, rats that returned to the training arm were categorized as place learners and rats that entered the opposite arm were categorized as response learners.
Learning strategy: visible-platform water maze task
Two months after the initial stressor, a modified version of the visible-platform water maze (VPWM) task () was used to determine whether a third exposure to a reminder of the footshock stressor impacted performance on a dual-solution learning strategy task that has been previously shown to be sensitive to the effects of shock (McDonald and White Citation1994; Kim et al. Citation2001). A white circular pool, 180 cm in diameter, filled with water to a depth of 26 cm was made opaque by the addition of non-toxic white paint and maintained at a temperature of approximately 25°C. Several days prior to training, rats were placed in the water maze without an escape platform for a 1-min swim in order to habituate to the water. During training trials and the probe trial, a visible black platform measuring 9.5 cm in diameter and projecting 3 cm above the water surface was located 30 cm from the wall of the pool. Eight training trials were conducted from each of the four cardinal points in a pseudo-randomized order with the visible escape platform located in the southwest quadrant of the pool. Rats were allowed 60 s to locate the platform where they remained for an additional 30 s. Rats were guided to the platform if they did not locate it within 60 s. Training trials were separated by an inter-trial interval of 30 s (Kim et al. Citation2001). Twenty-four hours later, a probe trial was conducted in which the platform was moved to the opposite quadrant of the pool (northeast). Rats entered the pool on the probe trial from the south location, which was the position most distal from the relocated platform (Kanit et al. Citation1998, Citation2000). However, 30 min prior to the probe trial, the not shocked/reminded and the shocked/reminded groups were subjected to an IA retrieval trial (Re-exposure III). The path length to locate the escape platform during training was recorded by tracking software (HVS Image, Ltd, Buckingham, UK) interfaced to an overhead camera. During training, increasingly shorter escape path lengths as training progressed served as an indicator of learning. During the probe trial, rats that initially swam to within 5 cm of the training location of the visible platform were categorized as place learners and those that swam directly to the newly relocated platform were categorized as stimulus-response learners by experimenters blind to conditions (McDonald and White Citation1994).
Activity and anxiety: open field test
Four days after completion of the VPWM task, activity and anxiety were assessed for 5 min in an open field constructed of black Plexiglas (90 × 90 × 45 cm). To determine the cumulative effects of multiple reminders of an aversive event on activity and anxiety, rats were not subjected to an IA trial prior to testing. The floor and walls of the field were cleaned thoroughly with 70% ethanol and air-dried after each trial to remove olfactory cues. Trials were monitored by an overhead video camera interfaced with tracking software (HVS Image, Ltd) that recorded activity in the field, which was divided into 16 equal-sized center (n = 4) and peripheral squares. Activity was indicated by total path length and anxiety was indicated by the percentage of time spent in the center squares of the open field (Hawley et al. Citation2011a).
Statistical analyses
An analysis of variance (ANOVA) with a between-subjects effect of stress condition (not shocked/reminded, shocked/not reminded, shocked/reminded) was conducted on the latency to enter the dark compartment prior to administration of the footshock stressor during the IA training trial. Reactivation of the aversive memory, which was indicated by the latency to re-enter the dark compartment, was examined by ANOVA with a within-subjects effect of IA retrieval trial (Reminders I-III), subsequent orthogonal contrasts, and a between-subjects effect of experiencing the initial stressor (not shocked/reminded, shocked/reminded). Independent sample t-tests were conducted to confirm memory for the initial stressor during each IA retrieval trial. Between-group differences in activity and spatial recognition memory on the Y-maze task were analyzed by ANOVA. Paired sample t-tests were conducted to confirm within-group spatial memory. Learning on the training trials of the water T-maze and the VPWM, as well as swim speed on the VPWM, was analyzed by ANOVA with a between-subjects effect of stress condition and a within-subjects effect of trial block (1-6) and trial (1-8), respectively. Between-group differences and within-group learning strategy preference on the probe trial of the water T-maze and the VPWM task (place vs. response/stimulus-response) were determined by conducting χ2 analyses. Activity and anxiety on the open field test were analyzed by ANOVA. When warranted, post hoc analyses were conducted using Fisher's least significant difference (LSD) tests. Where appropriate, data are represented as either the group mean ± SEM or categorically. Statistical significance was indicated by p < 0.05.
Results
Reminder of the initial stressor reactivated the aversive memory
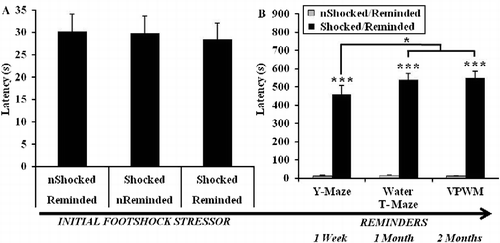
No differences in anxiety-like behavior between the stress conditions were found prior to administration of footshock (), which was indicated by the latency to enter the dark compartment during the IA training trial [F(2,44) = 0.07, p = 0.94]. For the IA retrieval trials, the results depicted in indicate an effect of footshock [F(1,30) = 182.79, p < 0.001], such that longer latencies were exhibited by rats exposed to footshock during all of the reminders, which took place just prior to the retention trial on the Y-maze [t(30) = ± 9.03, p < 0.001], and just prior to the probe trials on the water T-maze [t(30) = ± 13.31, p < 0.001] and the VPWM [t(30) = ± 13.55, p < 0.001]. Additionally, an interactive effect of stress condition by IA retrieval trial (reminders) was uncovered [F(2,60) = 3.29, p < 0.05], such that rats exposed to footshock exhibited longer latencies to enter the dark compartment during the reminders that preceded the probe trials of learning strategy tasks (Reminders II and III) relative to the reminder that preceded the retention trial of the Y-maze task (Reminder I; p < 0.05).
Figure 3. Results from an IA paradigm designed to induce the initial stressor (electrical footshock) and subsequently reactivate the aversive memory. (A) During the training phase of the IA task, there were no differences in the latency to enter the dark compartment of a shuttle box where a brief footshock was administered. (B) During the IA retrieval trials (reminders), which were conducted 1 week, 1 month, and 2 months after exposure to footshock (IA training trial), longer latencies to enter the dark compartment were exhibited by rats administered the initial footshock relative to rats that were not exposed to footshock during IA training (***p < 0.001). For rats exposed to the footshock and exposed to an IA retrieval trial, latencies to enter the dark compartment increased from the first reminder relative to the second and third reminders (*p < 0.05). “n” denotes rats that were not administered a footshock during the IA training trial or not reminded of the shock (IA retrieval trials). Group sizes were as follows: non-shocked/reminded (n = 15), shocked/non-reminded (n = 15), shocked/reminded (n = 17). Initial crossover latencies prior to administration of footshock on the IA training trial were examined by ANOVA. Subsequent crossover latencies on IA retrieval trials were examined by repeated measures ANOVA, within-group orthogonal contrasts, and independent sample t-tests. Data are represented as group mean ± SEM.
Exposure to the stressor resulted in lower levels of activity on the Y-maze task
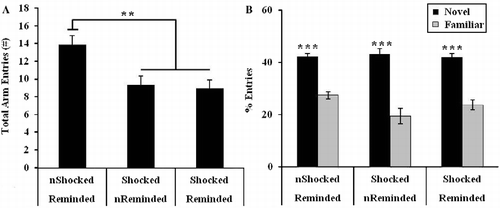
The results depicted in indicate that rats exposed to footshock during the IA training trial exhibited lower levels of activity on the Y-maze task [F(2,44) = 7.26, p < 0.01]. Post hoc analyses confirmed that the not shocked/reminded group made more arm entries than both the shocked/not reminded (p < 0.01) and the shocked/reminded (p < 0.01) groups during the retention trial. No difference in activity emerged between the two groups of rats exposed to the initial stressor (p = 0.78). However, three rats, all of which were in the shocked/reminded stress condition, did not move from the start arm on the retention trial and were therefore excluded from additional Y-maze analyses. The results illustrated in indicate that the not shocked/reminded [t(14) = ± 6.27, p < 0.001], the shocked/not reminded [t(14) = ± 4.96, p < 0.001], and the shocked/reminded [t(14) = ± 7.43, p < 0.001] groups exhibited a preference for the novel relative to the familiar arm. Additionally, no difference in spatial recognition memory between stress conditions was uncovered [F(2,41) = 1.77, p = 0.18].
Figure 4. (A) Activity and (B) spatial recognition memory exhibited during the retention trial of a Y-maze task. (A) Both groups of rats exposed to electrical footshock (IA training trial) made fewer arm entries on the retrieval trial of the Y-maze task than the group not exposed to footshock (**p < 0.01). (B) Neither footshock alone, nor reminder of the footshock, impacted spatial memory as all stress conditions exhibited recognition of the novel arm (***p < 0.001 novel vs. familiar arm). “n” denotes rats that were not administered a footshock during the IA training trial or not reminded of the shock (IA retrieval trial). Group sizes were as follows: non-shocked/reminded (n = 15), shocked/non-reminded (n = 15), shocked/reminded (n = 14). Group differences in activity and spatial memory were examined by ANOVA. Paired samples t-tests were conducted to examine within group spatial memory. Data are represented as mean ± SEM.
Exposure to the stressor biased rats toward a response learning strategy on the water T-maze task
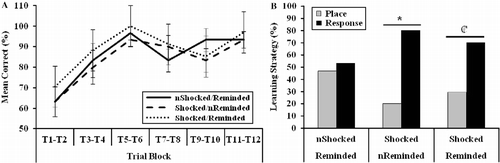
illustrates the results from the training trials on the dual-solution water T-maze task in which there was an effect of training trial block [F(5,220) = 10.69, p < 0.001], but no effect of stress condition [F(2,44) = 1.15, p = 0.33], or interactive effect of trial block and stress condition [F(10,220) = 0.35, p = 0.97] on learning. Therefore, as training progressed, arm choice accuracy increased independent of stress condition. The results from the probe trial depicted in indicate that the not shocked/reminded group did not exhibit a learning strategy preference [χ2(1) = 0.07, p = 0.80], but that both groups of rats exposed to the initial stressor tended to exhibit a bias rats toward a response learning strategy. While the shocked/not reminded group exhibited a significant preference for a response learning strategy [χ2(1) = 5.40, p < 0.05], the shocked/reminded group also tended to adopt this same strategy [χ2(1) = 2.88, p = 0.09]. No between-group difference in learning strategy preference was uncovered [χ2(2) = 2.53, p = 0.28].
Figure 5. Results from a dual-solution water T-maze task designed to examine (A) learning on training trials and (B) learning strategy on a probe trial. Rats exposed to electrical footshock (IA training trial), but not reminded of the stressor (IA retrieval trial), preferred a response strategy compared with a place strategy (*p < 0.05). Rats exposed to footshock (IA training trial) and subsequently reminded of the stressor (IA retrieval trial) 30 min prior to the probe trial also tended to prefer a response strategy compared with a place strategy (
![]() p = 0.09). “n” denotes rats that were not administered a footshock during the IA training trial or not reminded of the shock (IA retrieval trial). Group sizes were as follows: non-shocked/reminded (n = 15), shocked/non-reminded (n = 15), shocked/reminded (n = 17). Learning during training trials, which is represented as group mean ± SEM, was analyzed by repeated measures ANOVA. Learning strategy preference, which is represented categorically, was analyzed by χ2 analyses.
p = 0.09). “n” denotes rats that were not administered a footshock during the IA training trial or not reminded of the shock (IA retrieval trial). Group sizes were as follows: non-shocked/reminded (n = 15), shocked/non-reminded (n = 15), shocked/reminded (n = 17). Learning during training trials, which is represented as group mean ± SEM, was analyzed by repeated measures ANOVA. Learning strategy preference, which is represented categorically, was analyzed by χ2 analyses.
Reactivation of the aversive memory biased rats toward a stimulus-response learning strategy on the VPWM task
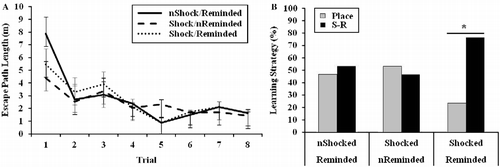
The results from the training trials on the VPWM illustrated in indicate that there was an effect of training trial [F(7,308) = 12.57, p < 0.001], but no effect of stress condition [F(2,44) = 0.24, p = 0.78], or interactive effect of trial and stress condition [F(14,308) = 1.06, p = 0.39] on learning. Therefore, escape path lengths decreased independent of stress condition as training progressed. The results of the probe trial, which are shown in , indicate that neither the not shocked/reminded [χ2(1) = 0.07, p = 0.80] nor the shocked/not reminded group [χ2(1) = 0.07, p = 0.80] exhibited a learning strategy bias. However, exposure to a reminder of the initial stressor during the IA retrieval trial biased the shocked/reminded group toward a stimulus-response strategy [χ2(1) = 4.77, p < 0.05]. No between-group difference in learning strategy preference was detected [χ2(2) = 3.30, p = 0.19]. Although there were no differences in swim speed on training trials [F(2,44) = 0.89, p = 0.42], there was a trend toward a significant difference in swim speed between stress conditions on the probe trial [F(2,44) = 3.20, p = 0.50]. The shocked/reminded group exhibited a propensity to swim somewhat faster than both the not shocked/reminded (p < 0.05) and the shocked/not reminded groups (p = 0.13). No differences in swim speed emerged between the not shocked/reminded and the shocked/not reminded groups (p = 0.35).
Figure 6. Results from a dual-solution VPWM task designed to examine (A) learning on training trials and (B) learning strategy on a probe trial. Rats exposed to electrical footshock (IA training trial) and reminded of the stressor (IA retrieval trial) 30 min prior to the probe trial preferred a stimulus-response (S-R) strategy compared with a place strategy (*p < 0.05). “n” denotes rats that were not administered a footshock during the IA training trial or not reminded of the shock (IA retrieval trial). Group sizes were as follows: non-shocked/reminded (n = 15), shocked/non-reminded (n = 15), shocked/reminded (n = 17). Learning during training trials, which is represented as group mean ± SEM, was analyzed by repeated measures ANOVA. Learning strategy preference, which is represented categorically, was analyzed by χ2 analyses.
Repeated reactivation of the aversive memory resulted in lower levels of activity on the open field test
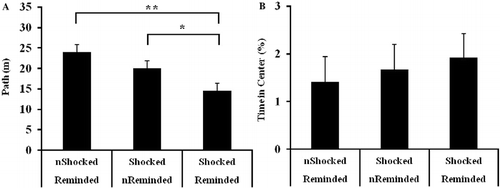
illustrates the results from the open field test in which there was an effect of stress condition on activity [A: F(2,44) = 7.11, p < 0.01] but not anxiety [B: F(2,44) = 0.25, p = 0.78]. Post hoc tests confirmed that the shocked/reminded group exhibited lower levels of activity than both the not shocked/reminded (p < 0.01) and the shocked/not reminded (p < 0.05) groups. The lower level of activity in the shocked/not reminded group than the not shocked/reminded group was not significant (p = 0.13).
Figure 7. (A) Activity and (B) anxiety exhibited during an open field test. Rats repeatedly reminded of the electrical footshock (IA retrieval trials) exhibited shorter path lengths relative to rats that were not exposed to footshock (**p < 0.01) and to rats that were never reminded of the footshock (*p < 0.05). Stress condition does not impact the percentage of time in the center of the open field. “n” denotes rats that were not administered a footshock during the IA training trial or not reminded of the shock (IA retrieval trial). Group sizes were as follows: non-shocked/reminded (n = 15), shocked/non-reminded (n = 15), shocked/reminded (n = 17). Group differences in activity and anxiety were examined by ANOVA and, when warranted, Fisher's LSD post hoc tests. Data are represented as group mean ± SEM.
Discussion
Collectively, the results from the current study indicate that (1) exposure to a remote acute stressor biases rats toward a striatum-dependent learning strategy and (2) after the effects of an acute stressor subside, reactivation of an aversive memory exacerbates the levels of hypoactivity and maintains the preference for striatum-dependent learning strategies. One week after exposure to an inescapable footshock, shocked rats exhibited lower levels of activity on the retention trial of a Y-maze task that was administered 30 min following the first reminder of the stressor. However, there was no effect of the initial stressor, or an additional reminder of the stressor, on spatial recognition memory on the Y-maze task. One month after exposure to footshock, rats that were shocked, but not reminded of the stressor, exhibited a bias toward a response learning strategy on a dual-solution water T-maze task. Likewise, shocked rats that were reminded of the stressor 30 min prior to the probe trial in the water T-maze task also tended to exhibit a preference for a response learning strategy. Two months after exposure to footshock, shocked rats that were reminded of the stressor 30 min prior to the probe trial in a dual-solution VPWM task exhibited a preference for a stimulus-response learning strategy. At this time point, rats exposed to the footshock, but never reminded of the stressor, as well as rats that were not shocked, did not demonstrate a bias for either learning strategy on the VPWM task. Four days following the probe trial of the VPWM, rats repeatedly reminded of the stressor exhibited significantly lower levels of activity than both the group of rats that were not shocked, as well as the group that were shocked but never reminded of the stressor.
A large body of literature has documented the detrimental effects of acute stress on spatial learning and memory in rodents (Cazakoff et al. Citation2010). Of particular relevance to the current findings, recent evidence indicates that exposure to a reminder of an acute stressor interferes with the retrieval of spatial memories (Zoladz et al. Citation2010). Specifically, exposure to a reminder of a footshock immediately prior to a retention trial on a water-based version of the radial-arm maze task effectively disrupted retrieval of a spatial memory. The discrepancy between results from the study by Zoladz et al. (Citation2010) and results from the Y-maze task in the current study is likely due to a combination of factors. First, the interval between the learning and the retrieval phase was 24 h in the study by Zoladz et al. (Citation2010), whereas in the current study the delay was only 4 h. When considered in light of the findings on the learning strategy tests in the current study, and the findings by Zoladz et al. (Citation2010), it is likely that a significantly longer delay on the Y-maze may have been necessary to detect differences in memory retrieval between stress conditions (McLaughlin et al. Citation2008). An additional difference between the paradigms to take into consideration is the nature of the environment in which memory was assessed. Conceivably, in the study by Zoladz et al. (Citation2010), the aversive nature of the water maze interacted with the reminder of the stressor to impact memory retrieval, an effect that was obscured by the relatively non-aversive nature of the Y-maze task in the current study.
On the retention trial of the Y-maze task, all rats exposed to the initial footshock 1 week earlier, regardless of exposure to an additional reminder of the stressor, exhibited lower levels of activity as indicated by a reduced number of total arm entries. Consistent with this result, previous research has established that exposure to inescapable footshock often results in a generalized state of anxiety, which is indicated by lower levels of activity in novel environments (van Dijken et al. Citation1992; Baldi et al. Citation2004; Diehl et al. Citation2007; Daviu et al. Citation2010; Hawley et al. Citation2011b; submitted). As indicated by the total number of entries on the retention trial of the Y-maze task, exposure to a reminder of the stressor did not increase levels of hypoactivity induced by the footshock beyond those displayed by rats that were only exposed to footshock. However, it is interesting to note that three of the rats in the shocked/reminded stress condition failed to move from the start arm on the retention trial of the Y-maze. Although spatial recognition memory on the Y-maze task was not affected by reminders of the aversive event, exposure to a reminder of the stressor may have impacted anxiety-and fear-like behaviors (Korte et al. Citation1999). This interpretation is consistent with previous findings, which indicate that higher levels of anxiety-like behavior are associated with poorer spatial recognition memory on the Y-maze task (Hawley et al. Citation2011b), poorer reference memory in the water maze task (Herrero et al. Citation2006), and poorer place learning on single-solution learning strategy tasks (Wingard and Packard Citation2008; Packard and Gabriele Citation2009). In line with this interpretation, heightened levels of emotionality, which follow from reminders of an aversive event (Korte et al. Citation1999), disrupt memory retrieval on some spatial tasks (Zoladz et al. Citation2010).
Rats subjected to footshock 1 month earlier exhibited a propensity to adopt a response learning strategy on a dual-solution water T-maze task regardless of additional exposure to a reminder of the stressor. Although not necessarily consistent with our original prediction, the preference for a response learning strategy exhibited by rats exposed to inescapable stress is a finding that dovetails with previous research. Specifically, when administered either immediately prior to training trials (Kim et al. Citation2005) or immediately prior to a retrieval trial (de Quervain et al. Citation1998), exposure to inescapable shock results in memory impairments in the probe trial on hippocampus-dependent versions of the water maze. In accordance with the current findings, exposure to shock prior to training on a water-based version of a dual-solution learning strategy task shifted control over learning away from the hippocampus and toward the striatum-dependent memory system (Kim et al. Citation2001). Rats exposed to inescapable shock exhibited a greater preference for a stimulus-response learning strategy on a probe trial conducted 24 h after training when compared with rats that were not subjected to inescapable shock (Kim et al. Citation2001). Similar to previous findings, exposure to inescapable shock did not impact learning during training trials on either learning strategy task. Therefore, the deleterious effects of inescapable shock on spatial cognition manifest as an inability to retrieve a particular memory (de Quervain et al. Citation1998; Kim et al. Citation2005), which subsequently biased rats toward using the striatum-dependent memory system (Kim et al. Citation2001). The results from the current study extend earlier findings (Kim et al. Citation2001) as they suggest that exposure to inescapable shock prior to training on a dual-solution learning task results in a preference for a response learning strategy that extends well beyond 24 h.
Notably, exposure to a single episode of inescapable stress exerts rather long-lasting effects on affective behaviors (Adamec et al. Citation2007; Belda et al. Citation2008; Mikics et al. Citation2008a,Citationb). Of particular importance to the current findings on the water T-maze task, previous research indicates that the deleterious effects of footshock on emotionality were most pronounced 28 days after exposure to the stressor (Siegmund and Wotjak Citation2007; Mikics et al. Citation2008a,Citationb). For example, mice subjected to a single episode of inescapable footshock exhibited maximal signs of generalized fear 28 days later, which was characterized by higher levels of freezing to a neutral tone that did not predict footshock, when compared with mice tested 1 day after the initial stressor. Given the relationship between emotionality and learning strategy (Packard Citation2009), the bias for a response learning strategy expressed by rats exposed to footshock, but not subjected to subsequent reminders of the stressor, may have resulted from an extended period of fear incubation (Houston et al. Citation1999; Golub et al. Citation2009; Pickens et al. Citation2009a; Pamplona et al. Citation2010). Consistent with this interpretation, memory for the footshock strengthened from the reminder (Reminder I) that took place just prior to the retrieval trial on the Y-maze, relative to the reminders that took place prior to the probe trials on the learning strategy tasks (Reminders II and III). It is worth noting that in the current study the intensity (0.6 mA) and duration (3 s) of the footshock were mild relative to those employed in previous studies, which have demonstrated a long-lasting effect of footshock on emotionality (Siegmund and Wotjak Citation2007; Mikics et al. Citation2008a,Citationb). Perhaps, as the results of the current study and others indicate (de Quervain et al. Citation1998), when cognitive testing occurs in an aversive environment, exposure to relatively milder forms of shock may be sufficient to impact hippocampus-dependent learning and memory, even when testing occurs at a much later time point. Interestingly, footshock stressors of greater intensity (Baldi et al. Citation2004; Mikics et al. Citation2008b) and longer duration (Pickens et al. Citation2009b, Citation2010) increase the expression of fear-like behavior, which can last for upward of 2 months after cessation of stress (Pickens et al. Citation2009b, Citation2010). Conceivably, in the current study, rats exposed to inescapable footshock, but not subjected to subsequent reminders of the stressor, would have also exhibited a preference for a striatum-dependent learning strategy on the VPWM, if the intensity of the stressor was either greater or of longer duration.
Two months after exposure to the initial stressor, the preference for a striatum-based learning strategy seemed to have dissipated in shocked rats that were never reminded of the stressor. Accordingly, on the VPWM task, only shocked rats that were reminded of the aversive event exhibited a preference for a stimulus-response learning strategy. Rats that were not exposed to footshock, as well as rats that were exposed to the footshock but were not reminded of the stressor, did not exhibit a learning strategy preference. When considered in light of the findings from previous studies (Zoladz et al. Citation2010), poorer performance on hippocampus-dependent tasks, which follows from reactivation of an aversive memory, corresponds with the expression of a stimulus-response learning strategy.
Arguably, as the results of the open field test in the current study indicate, reminders of the aversive event were necessary to heighten emotionality (Korte et al. Citation1999) to levels that bias rats toward use of the striatum-dependent memory system (Packard Citation2009) on the learning strategy test that was conducted 2 months after the stressor. Although, no difference emerged between the stress conditions on traditional indicators of anxiety in the open field, the lower levels of activity exhibited by the shocked/reminded group relative to both the not shocked/reminded and the shocked/not reminded groups indicate that repeated reactivation of the aversive memory heightened emotionality (van Dijken et al. Citation1992). Consistent with this interpretation, previous research indicates that rodents reminded of an aversive event exhibited heightened levels of anxiety as indicated by decreases in the time spent on the open arms of an elevated plus-maze (Korte et al. Citation1999; Louvart et al. Citation2005), a heightened startle reflex (Pynoos et al. Citation1996), prolonged latencies to begin feeding on a novelty-suppressed feeding task (Hawley et al. Citation2011b, submitted), decreases in social interaction (Louvart et al. Citation2005; Siegmund and Wotjak Citation2007), and alterations in sexual behaviors (Hawley et al. Citation2011b, submitted). Additionally, it is especially interesting to note that heightened levels of emotionality, which impair learning, can be maintained for an indefinite period of time by subsequent reminders of the initial stressor (Maier Citation2001). Moreover, in the current study, because memory for footshock did not diminish with the passage of time (McGaugh Citation1966), it is tempting to speculate just how long after the initial stressor reactivation of the aversive memory would have biased rats toward using the striatum-dependent memory system to navigate. Given that retrieval of a spatial memory is vulnerable to disruption by reactivation of a remote aversive memory (Zoladz et al. Citation2010), it is likely that a striatum-dependent learning strategy would dictate learning in rats reminded of the initial stressor for as long as the memory for the footshock persisted.
Admittedly, in the current study, because rats were subjected to three contextual reminders of the initial stressor it is uncertain whether the stimulus-response learning strategy exhibited by rats on the VPWM task was due entirely to the reminder that preceded the probe trial or to a cumulative effect of multiple re-exposures. Additionally, the current results do not discern whether the preference for a stimulus-response strategy exhibited by rats reminded of the aversive event was caused by increased emotionality alone or by heightened levels of arousal (Woodson et al. Citation2003). Considering that the shocked/reminded group tended to swim somewhat faster on the probe trial of the VPWM, in addition to exhibiting lower levels of activity in the open field, it may be that the increased emotionality expressed by repeated reactivation of the aversive memory was a manifestation of a heightened state of vigilance, which consequently predisposed rats toward adopting a striatum-dependent learning strategy.
From a neurobiological perspective, it is particularly intriguing to note that the amygdala is positioned to regulate both the consolidation of an aversive memory as well as out-of-context learning and memory processes that are impacted by stress. Specifically, lesions of the amygdala impaired the recall of fear-associated stimuli (Maren et al. Citation1996; Wilensky et al. Citation1999, Citation2000) and eliminated stress-induced impairments in spatial memory (de Quervain et al. Citation1998; Kim et al. Citation2001, Citation2005). Interestingly, with regard to learning strategy, the amygdala modulates the shift from the hippocampus to the striatum-based memory system when anxiety is elevated. Administration of an anxiogenic drug directly within the amygdala either prior to training (Packard and Wingard Citation2004) or prior to a probe trial (Elliott and Packard Citation2008) results in a preference for a response learning strategy. Furthermore, amygdala inactivation neutralizes both the impairing and enhancing effects of anxiety on hippocampus- and striatum-dependent tasks, respectively (Packard and Gabrielle Citation2009). Therefore, impairments induced by stress and anxiety on hippocampus-dependent learning and memory (de Quervain et al. Citation1998; Kim et al. Citation2005; Sadowski et al. Citation2009; Schwabe et al. Citation2010), which are modulated by the amygdala (Kim et al. Citation2005; Wingard and Packard Citation2008; Packard and Gabriele Citation2009), essentially redirect control over learning to the striatum-based memory system (Kim et al. Citation2001; Elliott and Packard Citation2008; Hawley et al. Citation2011b). Consistent with this interpretation, recent evidence indicates that stress predisposes rats toward using non-spatial strategies even in learning situations that demand use of hippocampus-based strategies (VanElzakker et al. Citation2011). Specifically, exposure to an acute stressor was associated with increased c-fos activity within the dorsolateral striatum of rats that exhibited superior memory on a radial-arm water maze, supporting the notion that under stress, some rats shift toward using a strategy that relies on a discrete cue that signals the location of a goal (Kim et al. Citation2001). Conceivably, in the current study, amygdala activation brought about by exposure to a reminder of the stressor resulted in a dysregulation in the functioning of the hippocampus that subsequently allowed the striatum-dependent memory system to emerge and assume control over learning.
In addition to the role of the amygdala, impairments in spatial memory retrieval following exposure to acute stressors are associated with elevated levels of corticosterone (Woodson et al. Citation2003; Park et al. Citation2008; Tronche et al. Citation2010; Li et al. Citation2012). Furthermore, while systemic administration of corticosterone is sufficient to block the retrieval of a recently formed spatial memory (Couburn-Litvak et al. Citation2003; de Quervain et al. Citation2003; Roozendaal et al. Citation2004a; Khaksari et al. Citation2007; Ferguson and Sapolsky Citation2008; Dorey et al. Citation2011), attenuation of corticosterone synthesis following exposure to either acute (de Quervain et al. Citation1998; Dorey et al. Citation2011) or chronic stressors (Wright et al. Citation2006) rescues stress-induced deficits in learning and memory. Moreover, the stress-induced rise in corticosterone subsequently results in altered synaptic plasticity in the hippocampus (Diamond et al. Citation1996; Mesches et al. Citation1999; Kim et al. Citation2006; Wong et al. Citation2007; Cazakoff et al. Citation2010), an effect that is likely modulated by the actions of corticosterone and other various neurotransmitters within the amygdala (Akirav and Richter-Levin Citation2002; Roozendaal et al. Citation2004b; Vouimba et al. Citation2006; Vouimba et al. Citation2007). Much like the effects of acute stressors, exposure to a reminder of a footshock induces both a rise in corticosterone (Hagewoud et al. Citation2011) and altered synaptic plasticity in the hippocampus (Li et al. Citation2005). Arguably, the actions of corticosterone may have contributed to the spatial memory impairments observed in previous studies (Zoladz et al. Citation2010) and the greater reliance on striatum-dependent learning strategies exhibited by rats in the current study that were reminded of an aversive event.
From a clinical standpoint, the results of the current study have important implications for our understanding of how stress-induced pathologies like PTSD impact memory systems. PTSD is an anxiety disorder characterized by the unwanted re-experiencing of thoughts related to the initial trauma and by profound alterations in emotionality, which include a perpetual state of hyperarousal, hypervigilance, and heightened levels of anxiety (CitationAmerican Psychiatric Association 1996). In some instances, the hallmark re-experiencing of intrusive thoughts has been directly linked to heightened levels of emotionality such that a greater frequency of intrusive thoughts corresponds with an increase in distress (Dougall et al. Citation1999; Schooler et al. Citation1999). Moreover, for those with PTSD, the frequency of intrusive thoughts also negatively impacts cognitive functioning (Wessel et al. Citation2002). Together with the results of the current study, these findings suggest that intrusive thoughts are associated with a heightened state of emotionality, which can strengthen with time to adversely impact cognitive processes. However, whether the onset of intrusive thoughts in people with PTSD is sufficient to heighten anxiety, and in turn, redirect control over learning toward the striatum-dependent memory system remains to be determined.
Acknowledgements
This research was supported by the Louisiana Board of Regents, Flowerree Summer Research Fellowship, the Tulane University Department of Psychology and Tulane University Program in Neuroscience. The authors gratefully acknowledge the technical assistance of Paul Tran. The expert supervision of animal care by Kimberly Scamardo is greatly appreciated.
Declaration of interest : The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adamec R, Muir C, Grimes M, Pearcey K. 2007. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behav Brain Res. 179:192–207.
- Akirav I, Richter-Levin G. 2002. Mechanisms of amygdala modulation of hippocampal plasticity. J Neurosci. 22:9912–9921.
- American Psychiatric Association. 1994. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Press.
- Baldi E, Lorenzini CA, Bucherelli C. 2004. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem. 81:162–166.
- Belda X, Fuentes S, Nadal R, Armario A. 2008. A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Horm Behav. 54:654–661.
- Cazakoff BN, Johnson KJ, Howland JG. 2010. Converging effects of acute stress on spatial and recognition memory in rodents: A review of recent behavioural and pharmacological findings. Prog Neuropsychopharmacol Biol Psychiatry. 34:733–741.
- Christianson JP, Thompson BM, Watkins LR, Maier SF. 2009. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 12:445–450.
- Colombo PJ, Brightwell JJ, Countryman RA. 2003. Cognitive strategy-specific increases in phosphorylated cAMP response element-binding protein and c-Fos in the hippocampus and dorsal striatum. J Neurosci. 23:3547–3554.
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. 1996. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 110:1321–1334.
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. 2004. Acute stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacol Biochem Behav. 78:569–579.
- Couburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. 2003. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 80:11–23.
- Daniel JM, Lee CD. 2004. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 82:142–149.
- Daviu N, Fuentes S, Nadal R, Armario A. 2010. A single footshock causes long-lasting hypoactivity in unknown environments that is dependent on the development of contextual fear conditioning. Neurobiol Learn Mem. 94:183–190.
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. 2003. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 17:1296–1302.
- de Quervain DJ, Roozendaal B, McGaugh JL. 1998. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 394:787–790.
- Devan BD, White NM. 1999. Parallel information processing in the dorsal striatum: Relation to hippocampal function. J Neurosci. 19:2789–2798.
- Diamond DM, Fleshner M, Ingersoll N, Rose GM. 1996. Psychological stress impairs spatial working memory: Relevance to electrophysiological studies of the hippocampus. Behav Neurosci. 130:661–672.
- Diamond DM, Park CR, Heman KL, Rose GM. 1999. Exposing rats to a predator impairs spatial working memory in the radial arm maze. Hippocampus. 9:542–552.
- Diehl LA, Silveira PP, Leite MC, Crema LM, Portella AK, Billodre MN, Nunes E, Henriques TP, Fideliz-da-Silva LB, Heis MD, Gonçalves CA, Quillfeldt JA, Dalmaz C. 2007. Long lasting sex-specific effects upon behavior and S100b levels after maternal separation and exposure to a model of post-traumatic stress disorder in rats. Brain Res. 1144:107–116.
- Dorey R, Piérard C, Shinkaruk S, Tronche C, Chauveau F, Baudonnat M, Béracochéa D. 2011. Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology. 36:2639–2649.
- Dougall AL, Craig KJ, Baum A. 1999. Assessment of characteristics of intrusive thoughts and their impact on distress among victims of traumatic events. Psychosom Med. 61:38–48.
- Elliott AE, Packard MG. 2008. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol Learn Mem. 90:616–623.
- Ferguson D, Sapolsky R. 2008. Overexpression of mineralocorticoid and transdominant glucocorticoid receptor blocks the impairing effects of glucocorticoids on memory. Hippocampus. 18:1103–1111.
- Ferragud A, Haro A, Sylvain A, Velazquez-Sanchez C, Hernandez-Rabaza V, Canales JJ. 2010. Enhanced habit-based learning and decreased neurogenesis in the adult hippocampus in a murine model of chronic social stress. Behav Brain Res. 210:134–139.
- Gold PE. 2004. Coordination of multiple memory systems. Neurobiol Learn Mem. 82:230–242.
- Golub Y, Mauch CP, Dahlhoff M, Wotjak CT. 2009. Consequences of extinction training on associative and non-associative fear in a mouse model of posttraumatic stress disorder (PTSD). Behav Brain Res. 205:544–549.
- Hagewoud R, Bultsma LJ, Barf RP, Koolhaas JP, Meerlo P. 2011. Sleep deprivation impairs contextual fear conditioning and attenuates subsequent behavioural, endocrine and neuronal responses. J Sleep Res. 20:259–266.
- Haller J, Bakos N. 2002. Stress-induced social avoidance: A new model of stress-induced anxiety?. Physiol Behav. 77:327–332.
- Hawley WR, Grissom EM, Barratt HE, Conrad TS, Dohanich GP. 2012. The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol Behav. 105:1014–1020.
- Hawley WR, Grissom EM, Dohanich GP. The relationships between trait anxiety, place recognition memory, and learning strategy. Behav Brain Res. 2011a; 216:525–530.
- Hawley W, Grissom E, Keskitalo L, Hastings T, Dohanich G. Sexual motivation and anxiety-like behaviors of male rats after exposure to a trauma followed by situational reminders. Physiol Behav. 2011b; 102:181–187.
- Herrero AI, Sandi C, Venero C. 2006. Individual differences in anxiety trait are related to spatial learning abilities and hippocampal expression of mineralocorticoid receptors. Neurobiol Learn Mem. 86:150–159.
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. 1999. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 6:111–119.
- Howland JG, Cazakoff BN. 2010. Effects of acute stress and GluN2B-containing NMDA receptor antagonism an object and object-place recogition memory. Neurobiol Learn Mem. 93:261–267.
- Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council. 1996. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press.
- Kanit L, Taskiran D, Furedy JJ, Kulali B, McDonald R, Pogun S. 1998. Nicotine interacts with sex in affecting rat choice between “look-out” and “navigational” cognitive styles in the Morris water maze place learning task. Brain Res Bull. 46:441–445.
- Kanit L, Yilmaz OA, Taskiran D, Kulali B, Furedy JJ, Demirgören S, Pögün S. 2000. Sexually dimorphic cognitive style, female sex hormones, and cortical nitric oxide. Physiol Behav. 71:277–287.
- Khaksari M, Rashidy-Pour A, Vafaei AA. 2007. Central mineralocorticoid receptors are indispensable for corticosterone-induced impairment of memory retrieval in rats. Neuroscience. 149:729–738.
- Kim JJ, Koo JW, Lee HJ, Han JS. 2005. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 25:1532–1539.
- Kim JJ, Lee HJ, Han JS, Packard MG. 2001. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 21:5222–5228.
- Kim JJ, Song EY, Kosten TA. 2006. Stress effects in the hippocampus: Synaptic plasticity and memory. Stress. 9:1–11.
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. 2004. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 45:330–338.
- Korte SM, De Boer SF, Bohus B. 1999. Fear-potentiation in the elevated plus-maze test depends on stressor controllability and fear conditioning. Stress. 3:27–40.
- Li S, Fan YX, Wang W, Tang YY. 2012. Effects of acute restraint stress on different components of memory as assessed by object-recognition and object-location tasks in mice. Behav Brain Res. 227:199–207.
- Li Z, Zhou Q, Li L, Mao R, Wang M, Peng W, Dong Z, Zu L, Cao J. 2005. Effects of unconditioned and conditioned aversive stimuli in an intense fear conditioning paradigm on synaptic plasticity in the hippocampal CA1 area in vivo. Hippocampus. 15:815–824.
- Louvart H, Maccari S, Ducrocq F, Thomas P, Darnaudery M. 2005. Long-term behavioural alterations in female rats after a single intense footshock followed by situational reminders. Psychoneuroendocrinology. 30:316–324.
- Maier SF. 2001. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol Psychiatry. 49:763–773.
- Maren S, Aharonov G, Stote DL, Fanselow MS. 1996. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 110:1365–1374.
- Martel G, Blanchard J, Mons N, Gastambide F, Micheau J, Guillou JL. 2007. Dynamic interplays between memory systems depend on practice: The hippocampus is not always the first to provide solution. Neuroscience. 150:743–753.
- McDonald RJ, White NM. 1994. Parallel information processing in the water maze: Evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 61:260–270.
- McGaugh JL. 1966. Time dependent processes in memory storage. Science. 153:1351–1358.
- McIntyre CK, Marriott LK, Gold PE. 2003. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem. 79:177–183.
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD. 2008. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm Behav. 54:386–395.
- Mesches MH, Fleshner M, Herman KL, Rose GM, Diamond DM. 1999. Exposing rats to a predator blocks primed burst potentiation in the hippocampus in vitro. J Neurosci. 19:2–5.
- Mikics E, Baranyi J, Haller J. Rats exposed to traumatic stress bury unfamiliar objects - a novel measure of hyper-vigilance in PTSD models?. Physiol Behav. 2008a; 94:341–348.
- Mikics E, Toth M, Varju P, Gereben B, Liposits Z, Ashaber M, Halász J, Barna I, Farkas I, Haller J. Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology. 2008b; 33:1198–1210.
- Packard MG. 1999. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci USA. 96:12881–12886.
- Packard MG. 2009. Anxiety, cognition, and habit: A multiple memory systems perspective. Brain Res. 1293:121–128.
- Packard MG, Gabrielle A. 2009. Peripheral anxiogenic drug injections differentially affect cognitive and habit memory: Role of basolateral amygdala. Neuroscience. 164:457–462.
- Packard MG, McGaugh JL. 1996. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place response learning. Neurobiol Learn Mem. 65:65–72.
- Packard MG, McGaugh JL. 1992. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: Further evidence for multiple memory systems. Behav Neurosci. 106:439–446.
- Packard MG, Wingard JC. 2004. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem. 82:243–252.
- Pamplona FA, Henes K, Micale V, Mauch CP, Takahashi RN, Wotjak CT. 2010. Prolonged fear incubation leads to generalized avoidance behavior in mice. J Psychiatr Res. 45:354–360.
- Park CR, Zoladz PR, Conrad CD, Fleshner M, Diamond DM. 2008. Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn Mem. 15:271–280.
- Pickens CL, Adams-Deutsch T, Nair SG, Navarre BM, Heilig M, Shaham Y. Effect of pharmacological manipulations of neuropeptide Y and corticotropin-releasing factor neurotransmission on incubation of conditioned fear. Neuroscience. 2009a; 164:1398–1406.
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry. 2009b; 65:881–886.
- Pickens CL, Navarre BM, Nair SG. 2010. Incubation of conditioned fear in the conditioned suppression model in rats: Role of food-restriction conditions, length of conditioned stimulus, and generality to conditioned freezing. Neuroscience. 169:1501–1510.
- Pleil KE, Williams CL. 2010. The development and stability of estrogen-modulated spatial navigation strategies in female rats. Horm Behav. 57:360–367.
- Pynoos RS, Ritzmann RF, Steinberg AM, Goenjian A, Prisecaru I. 1996. A behavioral animal model of posttraumatic stress disorder featuring repeated exposure to situational reminders. Biol Psychiatry. 39:129–134.
- Roozendaal B, de Quervain D, Schelling G, McGaugh JL. A systemically adminisered β-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem. 2004a; 81:150–154.
- Roozendaal B, Hahn EL, Nathan SV, de Quervain D, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci. 2004b; 24:8161–8169.
- Sadowski RN, Jackson GR, Wieczorek L, Gold PE. 2009. Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behav Brain Res. 205:19–25.
- Schooler TY, Dougall AL, Baum A. 1999. Cues, frequency, and the disturbing nature of intrusive thoughts: Patterns seen in rescue workers after the crash of Flight 427. J Trauma Stress. 12:571–585.
- Schwabe L, Dalm S, Schachinger H, Oitzl MS. 2008. Chronic stress modulates the use of spatial and stimulus-response learning strategies in mice and man. Neurobiol Learn Mem. 90:495–503.
- Schwabe L, Schachinger H, de Kloet ER, Oitzl MS. 2010. Stress impairs spatial but not early stimulus-response learning. Behav Brain Res. 213:50–55.
- Siegmund A, Wotjak CT. 2007. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res. 41:848–860.
- Tronche C, Piérard C, Coutan M, Chauveau F, Liscia P, Béracochéa D. 2010. Increased stress-induced intra-hippocampus corticosterone rise associated with memory impairments in middle-aged mice. Neurobiol Learn Mem. 93:343–351.
- Van Dijken HH, Mos J, van der Heyden JA, Tilders FJ. 1992. Characterization of stress-induced long-term behavioural changes in rats: Evidence in favor of anxiety. Physiol Behav. 52:945–951.
- VanElzakker MB, Zoladz PR, Thompson VM, Park CR, Halonen JD, Spencer RL, Diamond DM. 2011. Influence of pre-training predator stress on the expression of c-fos mRNA in the hippocampus, amygdala, and striatum following long-term spatial memory retrieval. Front Behav Neurosci. 5:1–13.
- Vouimba RM, Muñoz C, Diamond DM. 2006. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 9:29–40.
- Vouimba RM, Yaniv D, Richter-Levin G. 2007. Glucocorticoid receptors and β-adrenoceptors in basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1. Neuropharmacology. 52:244–252.
- Wessel I, Merckelbach H, Dekkers T. 2002. Autobiographical memory specificity, intrusive memory, and general memory skills in Dutch-Indonesian survivors of the World War II era. J Trauma Stress. 15:227–234.
- Wilensky AE, Schafe GE, LeDoux JE. 1999. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 19:1–5.
- Wilensky AE, Schafe GE, LeDoux JE. 2000. The amygdala modulates memory consolidation of fear-motivated inhibitory avoidance learning but not classical fear conditioning. J Neurosci. 20:7059–7066.
- Wingard JC, Packard MG. 2008. The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res. 193:126–131.
- Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, Brebner K, Liu L, Weinberg J, Christie BR, Phillips AG, Wang YT. 2007. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci USA. 104:11471–11476.
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. 2003. Emotion-induced amnesia in rats: Working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem. 10:326–336.
- Wright RL, Conrad CD. 2005. Chronic stress leaves novelty-seeking behavior intact while impairing spatial recognition memory in the Y-maze. Stress. 8:141–154.
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. 2006. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur J Neurosci. 24:595–605.
- Zoladz PR, Woodson JC, Hayes VF, Diamond DM. 2010. Activation of a remote (1-year old) emotional memory interferes with the retrieval of a newly formed hippocampus-dependent memory in rats. Stress. 13:36–52.