Abstract
Allostatic load (AL) has been shown to be a useful marker of physiological strain during chronic stress and burnout in non-clinical working populations. The usability of the AL index for a clinical population with severe stress-related exhaustion was tested in this study. Thirteen biomarkers assembled as an AL index were analysed using blood samples from 90 patients with stress-related exhaustion (43 men and 47 women, age 31–61 years) and 90 healthy controls (46 men and 44 women, age 25–56 years). The AL scores did not differ between patients and controls. For men, some indication of higher cardiovascular risk was seen in the patient group: male patients had higher body mass index and waist–hip ratio and a poorer blood lipid status than male controls. We found lower plasma glucose concentrations in both female and male patients than those in controls. The male patients also showed increased fasting serum insulin concentrations. Further analysis using homeostasis model assessment for insulin resistance and β-cell function showed indications of insulin resistance in the patient group, particularly in the males, and an increased insulin secretion in both male and female patients. In conclusion, AL index does not seem to capture plausible physiological strain in patients diagnosed with stress-related exhaustion. The finding of lower plasma glucose concentrations, probably due to higher insulin secretion, in patients with severe stress-related exhaustion, needs to be further investigated, including mechanisms and the clinical relevance.
Introduction
The concept of allostatic load (AL) refers to the consequences of repeated activation of allostatic responses during stressful situations (McEwen Citation1998). The AL model could reflect a possible biological pathway explaining how chronic stress can lead to health impairments (McEwen Citation1998). The concept of AL focuses on the cumulative, overall risks that result from the combined effects of multiple factors across multiple physiological regulatory systems. This composite measure includes hypothalamus–pituitary–adrenal axis functioning, sympathetic nervous system activation and risk factors for cardiovascular disease and Type 2 diabetes, and has been suggested to be more sensitive for detecting stress-related dysregulation and prediction of future health risks than any single factor alone (Seeman et al. Citation2002).
The initial AL model proposes that parameters quantifying AL can be categorized into mediators such as cortisol, epinephrine, norepinephrine and dehydroepiandrosterone-sulphate (DHEA-S) causing primary effects, which in turn lead to six secondary outcomes, namely increased waist–hip ratio (WHR), glycated haemoglobin (HbA1c), systolic blood pressure (SBP), diastolic blood pressure (DBP), total serum cholesterol/HDL-ratio and decreased serum high-density lipoprotein (HDL), which ultimately result in tertiary outcomes representing actual disease (McEwen and Seeman Citation1999).
Several of the measures included in the AL index, namely blood pressure, lipid profiles and WHR, are also associated with the metabolic syndrome that has been shown to increase the risk of cardiovascular diseases. Besides the cluster of metabolic syndrome biomarkers, the AL model also includes a cluster of neuroendocrine biomarkers, i.e. levels of cortisol, epinephrine, norepinephrine and DHEA-S. These two clusters have been suggested to contribute independently to health risk (Juster et al. Citation2010). This implies that the AL model contributes additional information in explaining the biological load of chronic stress, compared to using only the components of the metabolic syndrome. Several papers have addressed the question of whether and how AL is related to work-related stress. Schnorpfeil et al. (Citation2003) investigated the association between AL and psychosocial work characteristics. They found a weak but significant positive association between an AL score based on 14 parameters and job demands in industrial workers. Similar associations between AL and work-related stress have been reported in other working populations (von Thiele et al. Citation2006; Li et al. Citation2007; Sun et al. Citation2007). Others have studied the relationship between AL and burnout, even though this is associated with some difficulties as the concept of burnout is not a unified one. Generally, burnout is defined as a consequence of high work-related stress exposure with exhaustion as a core component (Shirom and Melamed Citation2006). Recently, Bellingrath et al. (Citation2009) investigated AL in relation to work stress and exhaustion in female schoolteachers. They found a greater AL in teachers scoring high on exhaustion and chronic work-related stress (effort–reward imbalance). Another study found no association between burnout and a proxy measure of AL (not including primary mediators) in male managers (Langelaan et al. Citation2007). Furthermore, Juster et al. (Citation2011) found associations between increased AL and chronic stress/burnout symptoms in a healthy working population. Thus, in a working population, AL seems to be related to burnout, and this could be of central importance as AL measures can be used for preventive purposes in a working population. The question, however, of whether and how markers of AL are relevant to use for a clinical population with severe symptoms of stress-related exhaustion is still unanswered.
The aim of this study was to investigate AL by using an index of 13 biomarkers in a clinical population of patients with stress-related exhaustion. In addition, the difference between patients and controls for each biomarker was explored. Standard procedures for this patient group involved measurements of body mass index (BMI), WHR, blood pressure, total circulating levels of cholesterol, HDL, low-density lipoprotein (LDL), triglycerides, glucose and HbA1c, and salivary cortisol concentration, primarily for differential diagnostic purposes. To more fully cover metabolic and inflammation aspects of AL, insulin and C-reactive protein (CRP) were measured using serum samples from 90 patients. Based on previous research on high job demands, chronic fatigue syndrome, burnout and exhaustion, we hypothesized that AL would be greater in patients with stress-related exhaustion than in healthy controls.
Methods
Participants
This study included 90 consecutive patients who were referred from primary care physicians to a specialized outpatient clinic because of symptoms of severe stress-related exhaustion. All patients fulfilled the diagnostic criteria for exhaustion disorder (ED) previously described by Jonsdottir et al. (Citation2009). The concept of clinical burnout can also be used to describe this patient population with 95% of the patients scoring above 4.0 on the Shirom–Melamed Burnout Questionnaire (SMBQ), clearly indicating burnout (). Fifty-seven patients reported 100% sick leave, 5 patients reported 75% sick leave, 11 patients reported 50% sick leave, 1 patient reported 25% sick leave and 16 patients reported no sick leave. Participants were screened for mood disorders including depression and/or anxiety by using the one-page Primary Care Evaluation of Mental Disorders questionnaire (Spitzer et al. Citation1999). This was followed up by a physician who used a structured interview form conforming with the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association Citation2000) criteria for diagnostic assessment of mood and anxiety disorder. Seventy-six per cent of the patients fulfilled the diagnostic criteria for depression and 48% for an anxiety disorder. Fifteen patients were diagnosed with mild depression (International Classification of Diseases and Related Health Problems, ICD-10, code F32.0) and 53 were diagnosed with moderate depression (ICD-10 code F32.1) (World Health Organization Citation1992). The self-reported duration of symptoms before seeking medical help was more than 5 years for 14% of the patients, 3–5 years for 20%, 1–2 years for 22% and less than 1 year for 45%. Ninety healthy controls were also included, originally recruited from a cohort study surveying psychosocial work environment and health, and through advertising in a local daily newspaper. The control group participated in other ongoing studies on stress-related health, and blood sample measures from these individuals served as reference controls in this study.
Table I. Demographic factors for patients with stress-related exhaustion and controls.
Characteristics of all participants included in the study are shown in . An exclusion criterion for both patients and healthy controls was systemic disease (such as thyroid disorder, hypertension and diabetes). This was ensured partly by asking the participants of known diseases, partly by measuring blood pressure and performing electrocardiographic registration. Venous blood samples were also taken, and erythrocyte sedimentation rate and concentrations of glucose, haemoglobin, HbA1c, free thyroxine (T4) and thyroid-stimulating hormone were measured. Individuals with psychiatric disease (except for depression, anxiety and exhaustion for the patient group) were excluded, and this was ensured by using the Comprehensive Psychopathological Rating Scale and Hospital Anxiety and Depression (HAD) scale (Zigmond and Snaith Citation1983). Other exclusion criteria were: present infection, pregnancy, breast-feeding, medication with substances having systemic effects (except for antidepressants for the patients), a BMI below 18.5 or over 30 kg/m2, vitamin B12 deficiency and over-consumption of alcohol measured by the Alcohol Use Disorders Identification Test (AUDIT) screening instrument (Babor et al. Citation2001).
Data on self-reported exercise habits were available for 62 controls (33 men and 29 women) and for all patients except for two females. Burnout symptoms were measured using the SMBQ (Melamed et al. Citation1992). Data from the HAD scale were used to describe self-reported symptoms of depression and anxiety. For information on validity and details of the Swedish versions of SMBQ and HAD, see Lisspers et al. (Citation1997) and Grossi et al. (Citation2005). The study was approved by the Regional Ethical Review Board in Gothenburg, Sweden, and was conducted in accordance with the Declaration of Helsinki. All participants in the study gave written informed consent.
Biomarkers and biochemical analysis
A research nurse measured SBP and DBP as the mean of two measurements after the seated participants had rested for approximately 5 min, and measured height, weight, waist and hip circumference for WHR and BMI calculations. Waist circumference was measured at the narrowest point between the iliac crest and the umbilicus. Hip circumference was measured at the maximum buttocks. BMI was computed based on the ratio of the weight in kilograms to height in metres squared (kg/m2).
All subjects had blood drawn from an antecubital vein by a research nurse between 07:30 h and 10:00 h after fasting since 22:00 h the day before. Total blood volume was 69.5 ml, including blood samples for standard measurements not included in the AL index and for biobank storage. Five samples (total volume 26 ml) were drawn for plasma measurements: 3 ml was collected in a Li-heparin tube for differential diagnostics, 2 ml was drawn in a NaF/K oxalate tube for analysis of glucose and 21 ml was drawn in EDTA tubes and centrifuged at 4°C, 1835g for 15 min and stored at − 80°C in a biobank for other research purposes. Six samples (total volume 32 ml) were drawn for serum measurements: 12 ml for differential diagnostics only, 4 ml for both differential diagnostic purposes and analysis of triglycerides, HDL, total cholesterol (TC) and LDL and 16 ml were frozen at − 80°C in the biobank. All serum samples were centrifuged for 10 min at 1835g. Three samples of whole blood (total volume 11.5 ml) were drawn: 8.5 ml for differential diagnostic purposes and 3 ml for HbA1c analysis. For assessment of the individual cortisol response to awakening, saliva samples were collected at home immediately after waking up and 15 min thereafter using Salivette tubes (Sarstedt, Nümbrecht, Germany). The subjects were free to choose a typical weekday for saliva collection, and they were instructed not to brush their teeth, eat or drink anything 30 min before taking a sample. Compliance was monitored through notes of actual sampling time in a paper diary. Female subjects were instructed to perform the saliva collection on 1 day in the follicular phase of the menstrual cycle, i.e. within the period of 5–10 days from their first day of menses; five women in the patient group and two women in the control group were post-menopausal. The mean of the first and second cortisol sample was used in the statistical analyses. Insulin resistance and β-cell function were calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) = [fasting insulin (mU/l) × fasting plasma glucose (mM)]/22.5] and β-cell function (HOMA-β) = [20 × fasting insulin (mU/l)]/[fasting plasma glucose (mM) − 3.5] (Matthews et al. Citation1985).
All biochemical analyses were performed at the Laboratory for Clinical Chemistry, Sahlgrenska University Hospital. Blood samples were assayed for TC in serum, HDL in serum, LDL in serum, triglycerides in serum, CRP in serum, insulin in serum, glucose in plasma and HbA1c in blood using standard laboratory protocols. Saliva samples were stored at − 20°C until free cortisol concentrations in saliva were analysed using a competitive radioimmunoassay (Spectria Coated Tube Radioimmunoassay, Orion Diagnostica, Espoo, Finland). Detection limit (DL) and coefficient of variation (CV) for each analysis were: TC: DL = 0.08 mmol/l, CV < 3%; HDL: DL = 0.08 mmol/l, CV < 5%; LDL: DL = 0.08 mmol/l, CV < 4%; triglycerides: DL = 0.02 mmol/l, CV < 4%; CRP: DL = 0.11 mg/l CV < 4%; insulin: DL = 0.2 mU/l, CV < 10%; glucose: DL = 0.11 mmol/l, CV < 3%; HbA1c: DL = 0.4%, CV < 2%; cortisol: DL = 1 nmol/l, CV < 14%.
Allostatic load
For each of the 13 biomarkers included in the AL index, subjects were classified into quartiles based on the distribution of scores in the total sample of both patients and controls. AL was measured by summing the number of parameters for which the subject fell into the highest risk quartile (i.e. top quartile for all parameters except HDL for which membership in the lowest quartile corresponds to highest risk). AL scores could therefore range from 0 to 13. Use of the high-risk quartile criterion represents a data-driven approach to define contributions to higher AL from these various biological parameters. WHR, SBP, DBP, HDL, TC/HDL, cortisol and HbA1c were also included in the original operationalization of AL, as introduced by Seeman et al. (Citation2001). Additionally, and in line with Schnorpfeil et al. (Citation2003), we included BMI as an indicator of adverse nutritional intake and CRP as an indicator of inflammation. Furthermore, glucose and insulin were included as indicators of abnormal metabolic functioning. HDL, LDL and triglycerides were included as measures of lipid status, and HbA1c is an integrated measure of glucose metabolism over the past weeks. indicates the actual cut-off for each of the 13 biomarkers.
Table II. Quartile distributions of all biomarkers and AL scores in patients with stress-related exhaustion and controls.
As both increased and decreased cortisol secretions have been associated with stress-related illness (Heim et al. Citation2000), another AL index was constructed with salivary cortisol concentration in the lowest quartile corresponding to highest risk and all other biomarkers as described above.
Statistical analysis
All statistical analyses were conducted in SPSS version 19 for Windows (IBM SPSS Statistics). Data are generally presented as mean values and standard deviations (SDs). A p value < 0.05 (two-tailed) was considered statistically significant. Differences between patients and controls in AL scores were analysed with independent samples t-tests and analysis of covariance, controlling for age and antidepressant use. A multivariate analysis of variance (MANOVA) was performed to test for possible differences between patients and controls in each of the 13 biomarkers. Insulin and CRP concentrations were not normally distributed and therefore they were ln-transformed before the MANOVA. Age, sex and antidepressant use were applied as covariates in the MANOVA to control for these factors statistically. An additional MANOVA including physical activity as a covariate was performed for the subset of subjects with data on self-reported physical activity. These analyses were also subsequently performed separately for men and women. Further explorative multivariate analyses were made using a two-step cluster analysis. The cluster analysis aimed at revealing natural groupings within the combined study sample (patients and controls) based on all biomarkers included in the AL index. Correlations were calculated using Spearman rank correlation analysis.
Results
AL in patients with stress-related exhaustion
Ten subjects had a missing value for one of the biomarkers and the AL index could therefore be calculated in a total of 170 participants (87 patients and 83 controls). AL scores were not significantly different between the patient group and the control group (). A MANOVA of all biomarkers showed a significant main difference between patients and controls (F(13,156) = 6.4, p < 0.001, η2 = 0.348), which remained significant after controlling for age, sex and antidepressant use (F(13,153) = 4.3, p < 0.001, η2 = 0.269). The univariate analyses revealed that this main difference was due to significantly lower plasma glucose concentrations in the patient group, as well as higher serum insulin, serum triglycerides and serum TC/HDL concentrations (). Controlling also for physical activity level resulted in a significant main effect of group (F(13,125) = 2.6, p = 0.003, η2 = 0.215) due to significantly lower plasma glucose concentrations (p value < 0.001), higher serum insulin concentrations (p value = 0.037) and higher serum triglyceride concentrations (p value = 0.038) in the patient group. Separate analyses for men and women also showed similar total AL scores in patients and controls (). A MANOVA of all biomarkers, however, showed significant main differences between patients and controls for both men (F(13,67) = 2.5, p = 0.008, η2 = 0.325) and women (F(13,71) = 3.1, p = 0.001, η2 = 0.359). In men, the patients presented significantly lower plasma glucose and serum HDL concentrations and significantly higher BMI, WHR, serum concentrations of TC/HDL, LDL and insulin (). Among women, the only difference between patients and controls was a significantly lower fasting plasma glucose concentration in female patients (). Controlling also for physical activity level rendered essentially the same results as above for women. The multivariate group effect was, however, no longer significant for men. Univariately, plasma glucose concentration was still significantly lower, and serum insulin, serum TC/HDL and BMI were significantly higher in male patients than in male control subjects after controlling for physical activity. Excluding patients on antidepressant medication from the analyses did not significantly alter the results.
Table III. Results from the MANOVA performed to test differences between patients with stress-related exhaustion and controls for each of the 13 biomarkers.
Table IV. Separate analyses for men and women showing the results from the MANOVA performed to test differences between patients with stress-related exhaustion and controls for each of the 13 biomarkers.
Cluster analysis
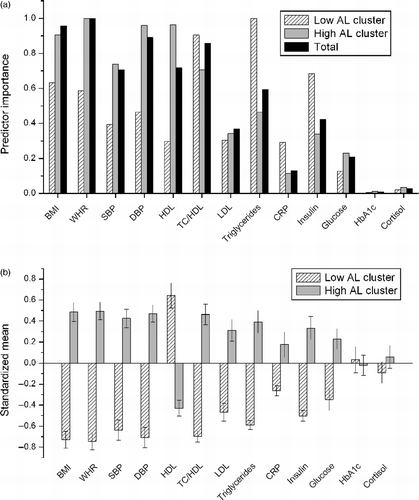
The explorative cluster analysis resulted in three clusters, as determined by the Bayesian information criterion. Two of the clusters exhibited similar characteristics and, therefore, a second analysis limited to two clusters was performed. Cluster 1 comprised 68 (40%) participants and was characterised by (sorted by importance according to ): low triglycerides, low TC/HDL, low insulin, low BMI, low WHR, low DBP, low SBP, low LDL, high HDL, low CRP and low glucose. Cluster 2 comprised 102 (60%) participants and was characterised by high WHR, low HDL, high DBP, high BMI, high SBP, high TC/HDL, high triglycerides, high LDL, high insulin, high glucose and high CRP. HbA1c and salivary cortisol concentrations were similar in the two clusters. Cluster 2 was named high AL as all biomarkers except HbA1c and cortisol were in the direction of high risk as defined by the AL index (). Consequently, cluster 1 was named low AL. The low AL cluster comprised 43% patients and 57% controls and the high AL cluster comprised 57% patients and 43% controls.
Figure 1. Two clusters were identified by multivariate analysis, including all 13 AL markers and 87 patients with stress-related exhaustion and 83 controls. (a) Relative importance of each AL biomarker for the two clusters and for the total model. (b) Characteristics of the two clusters illustrated by cluster mean values of standardized AL biomarker data; each biomarker has mean 0 and SD 1. Low AL (Cluster 1), n = 68 participants (29 patients; 39 controls); High AL (Cluster 2), n = 102 participants (58 patients; 44 controls). Note, total n = 170 participants as there was a missing Biomarker value for 10 of the original 180 participants. AL markers: BMI, body mass index; WHR, waist–hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, serum high-density lipoprotein; TC, serum total cholesterol; LDL, serum low-density lipoprotein; triglycerides, serum concentration; CRP, serum C-reactive protein; insulin, serum concentration; glucose, plasma concentration; HbA1c, blood glycated haemoglobin; cortisol, mean morning salivary concentration.
Further analyses of glucose and insulin findings
Within the patient group, the correlation between plasma glucose and serum insulin concentrations was ρ = 0.48 (p < 0.01), and between plasma glucose and blood HbA1c the correlation was ρ = 0.15 (p = 0.171). Comparisons of glucose and insulin between antidepressant users and non-users within the patient group were made. There were no significant differences between antidepressant users and non-users in plasma glucose concentrations (t = 0.1, p = 0.909) or serum insulin concentrations (t = –0.1, p = 0.955). Separate analyses for men and women showed significantly lower plasma glucose concentrations in female non-users than in female antidepressant users (t = –2.7, p = 0.011). Glucose concentrations did not differ significantly between males using antidepressants and non-users and there were no significant differences in insulin when splitting for sex (data not shown).
Patients and controls differ regarding insulin resistance and β-cell function
Lower plasma glucose concentrations in both male and female patients and higher serum insulin concentration among male patients rendered further analysis, and HOMA-IR and HOMA-β were thus determined. The results show that for the whole group (n = 180; 90 patients, 90 controls), HOMA-IR was significantly higher in the patients [1.6 (SD 0.98)] than in controls [1.3 (SD 0.76), p = 0.032], indicating insulin resistance. Similarly, HOMA-β was significantly higher in patients [123.3 (SD 58.9)] than in controls [75.8 (SD 40.2), p < 0.0001], indicating increased insulin secretion. Splitting by sex, both HOMA-IR and HOMA-β were higher in the male patients than in healthy males, while only HOMA-β was higher in the female patients (data not shown).
Discussion
This is the first study to investigate AL in a clinical population with stress-related exhaustion. In contrast to previous research on burnout and exhaustion in non-clinical populations (Bellingrath et al. Citation2009; Juster et al. Citation2011), we found no association between stress-related exhaustion and AL index in this study. The literature on AL in stress-related conditions, including both burnout and stress-related exhaustion is, however, lacking consensus (Langelaan et al., Citation2007). Furthermore, the related condition of chronic fatigue syndrome (CFS) has been associated with high AL (Maloney et al. Citation2009). Although stress-related exhaustion and CFS share many symptoms, there are some important differences between the diseases, mainly regarding the aetiology, but also several symptoms, such as tender lymph nodes and post-exertional malaise, are not present in the patients included in this study. The original purpose of measuring the biomarkers used in this study was for differential diagnostics, and the measurements were thus not specifically designed to evaluate AL. Consequently, our AL index was based on available parameters. The original AL score is based on 10 parameters, namely measurements of cortisol, epinephrine and norepinephrine, DHEA-S, WHR, HbA1c, HDL, TC/HDL ratio, SBP and DBP (Seeman et al. Citation2001), but has later been modified or extended to include, e.g. LDL, BMI, CRP, fasting glucose level, IL-6 and other biomarkers to additionally account for immunological and metabolic processes (Juster et al. Citation2010). There is currently no consensus in the literature of which parameters should be included in the AL index, and the biomarkers chosen in this study may thus not represent the best AL indicators. However, the explorative cluster analysis indicated that a high AL cluster and a low AL cluster could be identified using the selected biomarkers. The patients were distributed over both the high AL cluster and low AL cluster, this further indicates that AL does not discriminate patients with stress-related exhaustion from healthy controls.
The classical AL index is based on the assumption that AL results from chronically high activation of stress response systems. However, according to McEwen (Citation2000), AL can also be the result of inadequate responses to acute stress, and an AL index based on high-risk quartiles will not capture this dysfunctional state. AL measures, or at least not the markers used in this study, do not seem to capture the possible dysfunctional state related to AL in a clinical patient population, particularly not in women. For men, after adjusting for age and physical activity, some indication of higher cardiovascular risk was seen, but the AL index did not differ significantly between patients and controls. It is important to bear in mind that for both patients and controls, individuals with pathological values indicating, for example, hypertension or diabetes were excluded from this study. Thus, we cannot conclude whether the prevalence regarding cardiovascular diseases, metabolic disorders or obesity differs between the groups; but at least within an essentially normal range, the AL does not seem to differ between patients and controls. Neuroendocrine- and cardiovascular-related dysfunctions could plausibly be present in this clinical population of patients with stress-related exhaustion but not revealed by using AL measures. This would be in contrast with the usability of the AL measure as a marker of health impairment due to chronic stress in a non-clinical working population (Bellingrath et al. Citation2009; Juster et al. Citation2011).
Interestingly and somewhat unexpectedly, we found lower plasma glucose concentrations in the patients than in controls. When the data were split for gender, fasting glucose concentration was lower in both female and male patients, whereas HbA1c was not, suggesting an optimized interplay between the pancreas and liver to keep fasting plasma glucose lower in the patients (DeFronzo Citation2009). The mechanism behind the lower glucose concentration within the ‘normal range’ in the patients with stress-related exhaustion is unclear. In diabetic patients with poor glycaemic control, there is a strong correlation between HbA1c and fasting plasma glucose concentration, whereas in well-controlled patients and in non-diabetic subjects, the contribution from post-prandial glucose becomes relatively more important (Monnier and Colette Citation2006). Therefore, it could be speculated that our patient group displayed a reduction in fasting but not in post-prandial glucose levels, resulting in no consistent difference in HbA1c compared with the control group. In future work, it would be of interest to specifically address also post-prandial glucose control in patients with stress-related exhaustion. The male patients also showed increased fasting serum insulin concentrations. This does not seem to explain the lower glucose concentrations in the female patients because the insulin concentrations were similar in female patients and healthy controls. Importantly, the glucose and insulin findings remained after controlling for BMI, WHR, age and sex, leaving us with a limited number of confounding factors explaining the suppressed glucose concentrations in the patients. To elucidate the mechanisms behind the lower glucose and higher insulin concentrations, more sophisticated techniques for analyses of insulin sensitivity, such as clamp techniques, are suggested for further investigations.
It is notable that HOMA-β was increased in patients of both genders, indicating a compensatory mechanism in response to stress-related exhaustion. One possibility is that both insulin resistance and increased insulin secretion in male patients are due to elevated levels of free fatty acids (FFAs) due to stimulation of adipose tissue lipolysis by sympathoadrenergic over-activity. Chronically elevated levels of FFA in the circulation drive basal insulin secretion but impair the glucose-stimulated insulin response (Boden Citation1997). This will also lead to insulin resistance via alterations in insulin signalling and glucose transport. Speculatively, the link between stress-related exhaustion and increased insulin secretion in patients may also be due to epinephrine-mediated insulin release via activation of adrenergic receptors on the β cells (Hiatt et al. Citation1978). Furthermore, male ED patients were slightly overweight in contrast to controls. This implicates different dietary habits and/or physical activity habits of putative significance for the observed hyperinsulinaemia. We lack data on heredity for Type 2 diabetes, but several facets of the insulin resistance syndrome including WHR, HDL-C and triglyceride levels support that male ED patients were insulin resistant, albeit with lower fasting glucose concentrations than the controls. This metabolic pattern was not observed in women. Future studies including monitoring of peripheral blood flow (Murdolo et al. Citation2008) and circulating glucagon-like peptide-1 (GLP-1) concentrations (Johansson et al. Citation2002) during oral glucose tolerance tests may shed new light on the lower glucose concentrations observed in males and females with stress-related exhaustion.
There are several limitations to this study. Complete information on socio-economic status (SES) is available for only the patients (educational level). Both the healthy controls and the patients represent a population with relatively high education and considering the recruitment procedures, we have no reason to believe that SES differed greatly between the patients and controls, but we cannot rule out that SES could explain some of the results. Moreover, we cannot completely rule out the possibility that the lower plasma glucose concentration and higher serum insulin concentration in the exhausted group may be due to residual confounding, i.e. a result of other unknown factors not included in this study. Another limitation is that the sampling protocol was not originally designed to measure AL index, and thus several measures, such as available data on cortisol secretion, are limited. In this study, we used awakening salivary cortisol concentrations. An integrated measure of cortisol over several hours could have better reflected whether changes in cortisol level are present in this patient group. Also, circulating norepinephrine, cytokines, FFA and GLP-1 measures were not available, and we have no valid information on alcohol consumption in the groups. Importantly, however, all individuals with an overconsumption of alcohol according to the AUDIT questionnaire were excluded. We used a crude measurement of physical activity and data on physical activity were missing for several participants. However, the measure of exercise habits has previously been shown to discriminate between sedentary and active counterparts regarding maximal oxygen uptake (Saltin Citation1977) and has been validated against biological measures (Aires et al. Citation2003). A limitation of the AL index is the pre-defined direction of high risk, i.e. either the top quartile or the bottom quartile is considered high risk for each variable included in the index. This is problematic for parameters in which there is no consensus of whether high or low values are associated with AL. The rough categorizations of different markers included in the AL index could result in important information being missed. An example of this is seen in this study as plasma glucose concentrations did discriminate between groups in an unexpected way. A further limitation is the cross-sectional design, which precludes the assessment of a potential temporal relationship between AL and stress-related exhaustion.
We conclude that our AL index does not seem useful to capture chronic stress load in a clinical population of patients with stress-related exhaustion, as has been observed in a highly stressed working population. Lower plasma glucose concentrations are evidently present in patients with stress-related exhaustion, which most probably is due to increased insulin secretion. The mechanisms behind this need to be further studied.
Acknowledgements
We gratefully acknowledge the valuable help of Karin Nygren, Marie-Louise Norberg and Anna Palmgren for conducting the blood sampling and supervising the inclusion procedures. We also thank Emina Hadzibajramovic for statistical guidance. This study was supported by a grant from the Swedish government.
Declaration of interest : The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. JWE is employed at AstraZeneca.
References
- Aires N, Selmer R, Thelle D. 2003. The validity of self-reported leisure time physical activity, and its relationship to serum cholesterol, blood pressure and body mass index. A population based study of 332,182 men and women aged 40-42 years. Eur J Epidemiol. 18 6: 479–485.
- American Psychiatric Association. 2000. Diagnostic and statistical manual of mental disordersWashington, DC: American Psychiatric Association.
- Babor TF, Biddle-Higgins JC, Saunders JB, Monteiro MG. 2001. AUDIT: The alcohol use disorders identification test: Guidelines for use in primary health care. Geneva: World Health Organization.
- Bellingrath S, Weigl T, Kudielka BM. 2009. Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers. Stress. 12 1: 37–48.
- Boden G. 1997. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 46 1: 3–10.
- DeFronzo RA. 2009. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 58 4: 773–795.
- Grossi G, Perski A, Ekstedt M, Johansson T, Lindström M, Holm K. 2005. The morning salivary cortisol response in burnout. J Psychosom Res. 59 2: 103–111.
- Heim C, Ehlert U, Hellhammer DH. 2000. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 25 1: 1–35.
- Hiatt N, Davidson MB, Chapman LW, Sheinkopf JA. 1978. Epinephrine enhancement of potassium-stimulated immunoreactive insulin secretion. Role of beta-adrenergic receptors. Diabetes. 27 5: 550–553.
- Johansson A, Olsson T, Cederquist K, Forsberg H, Holst JJ, Seckl JR, Ahren B. 2002. Abnormal release of incretins and cortisol after oral glucose in subjects with insulin-resistant myotonic dystrophy. Eur J Endocrinol. 146 3: 397–405.
- Jonsdottir IH, Hägg DA, Glise K, Ekman R. 2009. Monocyte chemotactic protein-1 (MCP-1) and growth factors called into question as markers of prolonged psychosocial stress. PLoS ONE. 4 11e7659.
- Juster R-P, McEwen BS, Lupien SJ. 2010. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 35 1: 2–16.
- Juster RP, Sindi S, Marin MF, Perna A, Hashemi A, Pruessner JC, Lupien SJ. 2011. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology. 36 6: 797–805.
- Langelaan S, Schaufeli W, van Doornen L, Bakker A, van Rhenen W. 2007. Is burnout related to allostatic load?. Int J Behav Med. 14 4: 213–221.
- Li W, Zhang JQ, Sun J, Ke JH, Dong ZY, Wang S. 2007. Job stress related to glyco-lipid allostatic load, adiponectin and visfatin. Stress Health. 23 4: 257–266.
- Lisspers J, Nygren A, Söderman E. 1997. Hospital anxiety and depression scale (HAD): Some psychometric data for a Swedish sample. Acta Psychiatr Scand. 96 4: 281–286.
- Maloney EM, Boneva R, Nater UM, Reeves WC. 2009. Chronic fatigue syndrome and high allostatic load: Results from a population-based case–control study in Georgia. Psychosom Med. 71 5: 549–556.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28 7: 412–419.
- McEwen BS. 1998. Stress, adaptation, and disease: Allostasis and allostatic load. Ann N Y Acad Sci. 840 1: 33–44.
- McEwen BS. 2000. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacol. 22 2: 108–124.
- McEwen BS, Seeman T. 1999. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 896 1: 30–47.
- Melamed S, Kushnir T, Shirom A. 1992. Burnout and risk factors for cardiovascular diseases. Behav Med. 18 2: 53–60.
- Monnier L, Colette C. 2006. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 12 Suppl. 1: 42–46.
- Murdolo G, Sjostrand M, Strindberg L, Gudbjornsdottir S, Lind L, Lonnroth P, Jansson PA. 2008. Effects of Intrabrachial metacholine infusion on muscle capillary recruitment and forearm glucose uptake during physiological hyperinsulinemia in obese, insulin-resistant individuals. J Clin Endocrinol Metab. 93 7: 2764–2773.
- Saltin B. 1977. Physiological effects of physical conditioning. In: Hansen AT, Schnohr P, Rose G. editors. Ischaemic heart disease: The strategy of postponement. Chicago, IL: Year Book Medical Publishers104–115.
- Schnorpfeil P, Noll A, Schulze R, Ehlert U, Frey K, Fischer JE. 2003. Allostatic load and work conditions. Soc Sci Med. 57 4: 647–656.
- Seeman TE, McEwen BS, Rowe JW, Singer BH. 2001. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 98 8: 4770–4775.
- Seeman TE, Singer BH, Ryff CD, Dienberg Love G, Levy-Storms L. 2002. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 64 3: 395–406.
- Shirom A, Melamed S. 2006. A comparison of the construct validity of two burnout measures in two groups of professionals. Int J Stress Manag. 13 2: 176–200.
- Spitzer RL, Kroenke K, Williams JB. 1999. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 282 18: 1737–1744.
- Sun J, Wang S, Zhang J-Q, Li W. 2007. Assessing the cumulative effects of stress: The association between job stress and allostatic load in a large sample of Chinese employees. Work Stress. 21 4: 333–347.
- von Thiele U, Lindfors P, Lundberg U. 2006. Self-rated recovery from work stress and allostatic load in women. J Psychosom Res. 61 2: 237–242.
- World Health Organization. 1992. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva: World Health Organization.
- Zigmond AS, Snaith RP. 1983. The hospital anxiety and depression scale. Acta Psychiatr Scand. 67 6: 361–370.
