Abstract
Obesity is highly co-morbid with anxiety and/or depression in children, conditions that may further worsen the metabolic and cardiovascular risks for obese individuals. Dysregulation of the hypothalamic–pituitary–adrenal axis is involved in the pathophysiology of anxiety disorders, depression, and obesity, and diverse cortisol concentrations may be found in obese children, depending on their degree of psychological distress. The aim of this study was to examine cortisol profiles among obese children with or without symptoms of anxiety and depression. A group of 128 children (53% females; mean age ± SD: 11.2 ± 2.2 years) derived from a pediatric obesity clinic were studied. Anxiety and depressive symptomatology were assessed with appropriate instruments. Morning serum and five diurnal salivary cortisol concentrations were measured. Obese children were 3.1/2.3 times more likely to report state and trait anxiety, respectively, and 3.6 times more likely to report depressive symptoms than children of the same age group, from a contemporary Greek sample. Trait anxiety and noon salivary cortisol concentrations were significantly positively correlated (p = 0.002). Overall, salivary cortisol concentrations were increased in children with anxiety or depression symptomatology compared to obese children without any affective morbidity (p = 0.02) and to those with anxiety and depression co-morbidity (p = 0.02). In conclusion, in obese children, emotional distress expressed by symptoms of anxiety and/or depression is associated with circadian cortisol profiles reflecting a potential pathway for further morbidity. Longitudinal studies may reveal a role of cortisol in linking obesity, anxiety, and depression to the development of further psychological and physical morbidity.
Introduction
Obesity, anxiety, and depression are highly co-morbid in adults (Wing et al. Citation1991; Rosmond et al. Citation1996) and youths (Mustillo et al. Citation2003; Reeves et al. Citation2008). Furthermore, similar to adults, children and adolescents in the highest quartiles for body mass index (BMI) have a higher prevalence of depression, meaning that associations between these two conditions are more likely to exist in individuals with more severe obesity (Onyike et al. Citation2003). The increasing prevalence of childhood obesity (Ogden et al. Citation2006) has been accompanied by a rising incidence of obesity-related somatic complications, especially metabolic syndrome manifestations (central adiposity, arterial hypertension, insulin resistance, and dyslipidemia) and diabetes mellitus type 2 (Weiss et al. Citation2004, Kassi et al. Citation2011).
Anxiety disorders and depression in adults have been linked to abdominal obesity, elevated arterial blood pressure, and metabolic abnormalities, such as dyslipidemia and insulin resistance (Rosmond et al. Citation1998), as well as to diabetes mellitus type 2 and cardiovascular disease (Vogelzangs et al. Citation2007). Relationships between anxiety and/or depression and adverse health outcomes may be mediated by unfavorable clinical and metabolic characteristics (such as obesity and obesity-related metabolic complications; Pervanidou and Chrousos Citation2007, Citation2011a). Importantly, morbidity related to anxiety and/or depression may not be seen in the early stages of development.
In interpreting the associations between obesity and anxiety/depression, the direction of causality is unclear. Although anxiety and depression are considered distinct disorders, they share a number of specific clinical symptoms related to stress (American Psychiatric Association Citation1994). Both behavioral and biological factors are involved in the pathophysiologic pathways linking obesity with anxiety/depression. In addition to the poor health habits, such as sedentary behaviors, lack of physical activity, unhealthy eating, and sleep problems, which generally characterize patients with anxiety and/or depression and obesity (American Psychiatric Association Citation1994; Kennard et al. Citation2006; Pettit et al. Citation2006; Schneider et al. Citation2007), several inherent biological mechanisms may be involved (Reeves et al. Citation2008).
The hypothalamic–pituitary–adrenal (HPA) axis is an obvious target of research linking anxiety/depression and obesity with adverse health outcomes as cortisol abnormalities characterize both emotional disorders, and hyper-cortisolism has been associated with the development of obesity and the metabolic syndrome (Chrousos Citation2009; Pervanidou and Chrousos Citation2011). Dysregulation of the HPA axis, most often manifested by cortisol hypersecretion, but also by cortisol hyposecretion, has been shown in a large number of studies of patients with stress-related disorders (Chrousos and Gold Citation1992; Chrousos Citation2009; Hillman et al. Citation2012; Pervanidou and Chrousos Citation2012). Children with anxiety disorders and depression, similar to adults, exhibit HPA axis and overall stress system dysregulation, as evidenced in most cases by elevations of peripheral levels of cortisol and catecholamines (Pervanidou Citation2008). Finally, a recent pediatric study showed that in girls, an association between depression and BMI was mediated by cortisol reactivity to a stress test (Dockray et al. Citation2009).
There is evidence for alterations in peripheral glucocorticoid metabolism in obesity, without apparent changes in circulating cortisol concentrations. These alterations may be implicated in obesity, insulin resistance, and diabetes type 2, and include increased activity in adipose tissue of 11β-hydroxy-steroid dehydrogenase type 1 (11β-HSD1), which generates active cortisol from cortisone, and in liver of 5α-reductase (5αR), which inactivates cortisol (Seckl and Walker Citation2004; Morton and Seckl Citation2008; Tomlinson et al. Citation2008; Gathercole and Stewart Citation2010; Pereira et al. Citation2012).
Given that symptoms of anxiety and/or depression are highly prevalent in obese children (Mustillo et al. Citation2003), and that both obesity and anxiety/depression could be related to a dysregulated HPA axis (Weigensberg et al. Citation2008; Chrousos Citation2009), we hypothesized a hypercortisolemic state in obese children with symptoms of anxiety and/or depression. More precisely, we aimed to investigate: (1) the prevalence of symptoms of anxiety/depression in obese children; (2) potential associations between anxiety/depression, BMI, and cortisol concentrations; and (3) whether, among obese children, those with symptoms of anxiety and/or depression have different cortisol profiles (hyper- or hypo-cortisolism) compared to those without such symptoms.
Methods
Patient selection
The study was approved by the Ethics Committee of the “Aghia Sophia” Children's Hospital and written informed consent was obtained from the participants and their parents. The experimental group consisted of children and adolescents assessed at the Obesity Clinic of our Department of Pediatrics; the study was carried out according to the Helsinki Declaration (Williams Citation2008). Obesity was defined as a BMI greater than the 97th percentile for age and gender, according to the Greek BMI curves (Chiotis et al. Citation2004); all participants were obese. Exclusion criteria were: (1) underlying chronic illnesses, such as cardiac, hepatic, and renal diseases; (2) chronic use of medications; (3) syndromic obesity; (4) intellectual disabilities or a preexisting diagnosis of a psychiatric disorder; and (5) chromosomal disorders affecting puberty. Children with obesity-related alterations in their clinical and/or metabolic characteristics were not excluded from the study.
Data collection
BMI calculation
Children's BMI was calculated from the weight in kilograms divided by the square of the height in meters. BMI SDs and z-scores were calculated based on the contemporary Greek growth charts (Chiotis et al. Citation2004).
Pubertal assessment
Participants' pubertal development was determined by physical examination by a certified pediatrician based on the 5 Tanner Stages of pubic hair and genital development in boys and pubic hair and breast development in girls (Tanner Citation1962). Children with a Tanner Stage (for genital development in boys and breast development in girls) 2–4 were characterized as mid-pubertal, whereas those with stage 5 as post-pubertal.
Psychometric instruments
Anxiety and depressive symptomatology were assessed using the following instruments. The State-Trait Anxiety Inventory for Children (STAI-C) is a “how I feel questionnaire” that consists of two forms of 20 items each with a three-point response format (Spielberger et al. Citation1973). It assesses global anxiety that varies with situations (state anxiety) and anxiety that is stable across time and situations (trait anxiety). It has been used both in community and medical samples. The adaptation of STAI-C to the Greek population has shown an acceptable internal consistency [Cronbach's alpha (α) = 0.85 and 0.83 for the first and the second factor of S-Anxiety scale, respectively; α = 0.80 for T-Anxiety], and reliability. It was also found that the scales have a multifactorial structure and can be used among the Greek population to examine children's state and trait anxiety (Psychountaki et al. Citation2003). In this study, STAIC1 refers to State anxiety and STAIC2 refers to trait anxiety. The Children's Depression Inventory (CDI) is the most widely used scale for depressive symptoms in children (Kovacs Citation1992). It is a 27-item self-report measure that assesses core symptoms of depression. The child is asked to endorse one of the three statements which best applies to him or her during the last 2 weeks. Responses were scored 0, 1, or 2, with 2 representing the severe form of the symptom. When applied to the Greek population, internal consistency (α = 0.80) and test–retest reliability were satisfactory. As the Greek version of the CDI is restricted to children aged 8–12 years, we calculated z-scores for CDI of children older than 12 years based on the values of a 12-year-old child (Giannakopoulos et al. Citation2009).
All scores in the CDI and STAI-C questionnaires were standardized and reported as z-scores, according to age and gender, based on the Greek version of both instruments (Psychountaki et al. Citation2003; Giannakopoulos et al. Citation2009). The Greek versions of the two instruments are derived from contemporary representative samples of Greek children. The participants' symptoms of anxiety and/or depression were classified as normal if they had z-score ≤ 1.6 which corresponds to ≤ 95th percentile, or high if they had z-score >1.6 which corresponds to >95th percentile. The STAI-C and CDI were administered during the first visit at the Obesity Clinic.
Neuroendocrine evaluation
Salivary samples were collected by parents at home, on the nearest Sunday to the physical and psychological examination day, and returned to the investigators within 2 days. The samples were collected five times a day (08:00 h pre-breakfast, 12:00, 15:00, 18:00, and 21:00 h, pre-bed). The time of awakening was scheduled for 07:30 h. Detailed instructions were given to parents and children to collect the saliva samples correctly, using a Salivette device (Sarstedt, Nuembrecht, Germany). Participants were not receiving any medications and did not smoke. Children were told not to eat, drink, brush their teeth, or exercise for at least half-an-hour prior to sample collection. Each of the samples was collected by having the participant place a cotton swab in his or her mouth for 2 min, or chews it for 1 min. The cotton was then placed inside a plastic tube and kept in a refrigerator at 0–4°C. Within 2 days from collection, the samples were given to the investigator for further processing. Saliva was extracted from the cotton by centrifuging the plastic tubes and cotton at 1000g for 8 min to separate the saliva into the outer tube. The cotton was then removed and all samples were stored at − 85°C. Fasting morning blood samples (2–3 ml) were obtained from the median antecubital vein and placed into plastic tubes with no additives, for the measurement of cortisol. These samples were collected during a scheduled second visit at the Obesity Clinic, between 08:00 and 09:00 h. The blood samples were centrifuged and the serum obtained was stored at − 85°C. Samples were processed using the Elecsys Cortisol reagent kit produced by ROCHE Co. (Basel, Switzerland). Serum and salivary cortisol concentrations were measured using an electrochemiluminescence immunoassay (Roche Co., Basel, Switzerland). The intra- and inter-assay precision coefficients of variation for serum cortisol concentrations were 1.1–1.3% and < 8% and those for salivary cortisol concentrations ranged from 1.5–6.1% and 4.1–8%, respectively. The analytical sensitivity (lower detection limit) for salivary cortisol concentrations was < 0.036 μg/dl.
Statistical analyses
Continuous variables are presented as mean ± SD, while categorical variables are presented using absolute (n) and relative (%) frequencies. The level of significance was set to ≤ 0.05. Comparisons of means were performed by Student's t-test, whereas relationships between categorical variables were assessed by Fisher's exact test. In the latter case, the relative risk was quantified and expressed as odds ratio. Correlations between continuous variables were evaluated by Pearson's r. Whenever sample sizes were less than 30, non-parametric procedures were applied (Mann–Whitney test and Spearman's rho). In addition to the five single measurements of salivary cortisol, the area under the curve (AUC) was also calculated for each individual. For the correlation of cortisol with STAIC1, STAIC2, CDI, and BMI z-scores, the level of significance was adjusted to 0.05/7 = 0.007, according to Bonferroni, as the null hypothesis included seven simultaneous correlations (five salivary cortisol measurements, serum cortisol, and AUC). The analysis of the daily variation of salivary cortisol levels was carried out through a repeated measures analysis with correlated error terms using an unstructured correlation matrix. For the assessment of a probable mediation effect of cortisol (mediator) on the relationship between anxiety/depression symptoms (independent variable) and BMI z-scores (dependent variable), the Baron and Kenny (Citation1986) criteria were applied. According to these criteria, a variable (cortisol) functions as a mediator when it meets the following conditions: (a) variations in levels of the independent variables (STAIC1, STAIC2, and CDI) significantly account for variations in the presumed mediator (cortisol); (b) variations in the mediator (cortisol) significantly account for variations in the dependent variable (BMI z-score); and (c) when the former two relationships are controlled, a previously significant relationship between the independent (STAIC1, STAIC2, and CDI) and dependent variable (BMI z-score) is no longer significant. All statistical procedures were run in the Stata 11.0 statistical software (StataCorp, 4905 Lakeway Drive College Station, TX 77845, USA).
Results
Anxiety and depressive symptoms in obese children
A total of 128 obese children (53% females) were included in the analysis and their characteristics regarding age, BMI, and pubertal status are shown in . Only children with fully completed questionnaires were included in the ensuing steps of the analysis. The number of participants in each group is shown in . Children with missing data (excluded from the ensuing analysis) did not differ from the children finally included, in terms of BMI, cortisol values, and psychological status.
Table I. Characteristics of the study population (N = 128).
Table II. Distribution of the study population according to their emotional status, based on the CDI (depressive symptoms), STAIC1 (state anxiety), and STAIC2 (trait anxiety) questionnaires.
Table III. Characteristics of obese children with depressive symptoms (high CDI), symptoms of state anxiety (high STAIC1) and symptoms of trait anxiety (high STAIC2), presence of both anxiety and depression (co-morbidity), and low symptoms of anxiety/depression.
Mean z-scores for STAIC1 [state anxiety] (mean = 0.18, SD = 1.18) and STAIC2 [trait anxiety] (mean = − 0.22, SD = 1.37) did not differ significantly from those expected (mean = 0, SD = 1, Student's t-test, p-values: 0.14 and 0.11, respectively). Mean z-scores for CDI [depressive symptoms] (mean = 0.33, SD = 1.32) were significantly higher in obese children than expected (Student's t-test, p = 0.007). The distribution of obese children according to their status on the three scales is shown in . The proportion above the upper normal limit (>95th percentile) was significantly higher than that expected (Student's t-test, state anxiety: p < 0.001, trait anxiety: p = 0.006, depressive symptoms: p < 0.001). Obese children were 3.1 times (odds ratio = 3.1) more likely to report state anxiety symptoms and 2.3 (odds ratio = 2.3) times more likely to report trait anxiety symptoms compared to the general population. Furthermore, obese children were 3.6 times (odds ratio = 3.6) more likely to report depression-related symptoms than the general population.
BMI z-scores were not related to STAIC1, STAIC2, and CDI scores. The characteristics of obese children with symptoms of state anxiety, trait anxiety and depression >95th percentile, presence of both anxiety and depressive symptoms (co-morbidity), and absence of symptoms (low symptoms) are presented in . The anxiety/depression status in obese children was not related to gender (results not shown), while pre-pubertal obese children were five times more likely to have high state anxiety scores than mid- and post-pubertal obese children (Fisher's exact test, p = 0.044, odds ratio = 5.0). No relationship between pubertal status and STAIC2 or CDI was found.
As expected, in our population, anxiety and depressive symptoms were strongly and positively related: Depressive Symptoms-State Anxiety: Pearson's r = 0.56, p < 0.001; Depressive Symptoms-Trait Anxiety: Pearson's r = 0.55, p < 0.001; and State Anxiety-Trait Anxiety: Pearson's r = 0.61, p < 0.001.
Circadian cortisol profiles, anxiety, and depression in obese children
When correlations of the five single salivary cortisol measurements, serum cortisol, and AUC with STAIC1, STAIC2, and CDI (z-scores) were evaluated, only noon (12:00 h) salivary cortisol concentration was significantly correlated with trait anxiety (Pearson's r = 0.34, p = 0.002). Furthermore, cortisol concentrations did not differ significantly between children with normal and high scores on the three scales. In addition, single cortisol values were not associated with co-morbidity or with low symptoms of depression and anxiety, but BMI z-scores were significantly higher in children with depression or anxiety than those with low anxiety/depressive symptomatology (Mann–Whitney test, 3.44 ± 1.37 vs. 2.87 ± 1.31, respectively, p = 0.027). Single salivary cortisol measurements, serum cortisol, and AUC were not associated with gender (results not shown). With respect to pubertal status, mid- or post-pubertal obese children had higher 15:00 h salivary cortisol values than pre-pubertal obese children (Student's t-test, 0.089 ± 0.078 vs. 0.032 ± 0.025, respectively, p < 0.001) and higher 21:00 h salivary cortisol values than pre-pubertal obese children (Student's t-test, 0.048 ± 0.058 vs. 0.022 ± 0.011, respectively, p = 0.005). BMI was not significantly related to cortisol values (results not shown).
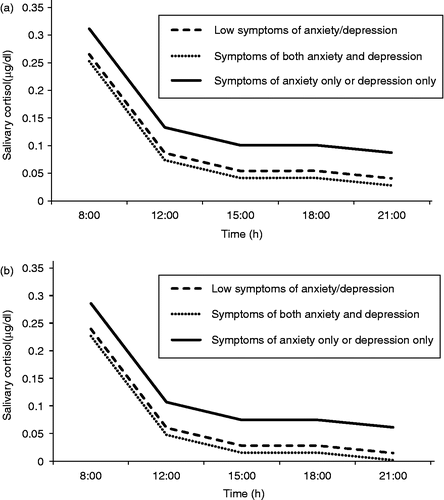
Finally, we carried out a repeated measures analysis in order to identify factors that significantly affect the variation of daily salivary cortisol concentrations in obese children. As a first step, we implemented three different models including time, gender, and age as common confounding variables and STAIC1 (model 1), STAIC2 (model 2), and CDI (model 3) as the variable under investigation. No significant effect of STAIC1 (model 1), STAIC2 (model 2), or CDI (model 3) on the cortisol concentrations was found. BMI z-scores were also not associated with cortisol concentrations. However, as shown above, the three scales are highly related in obese children, so we re-analyzed our data including STAIC1, STAIC2, and CDI in the same model. The results are shown in and clearly indicate that due to the observed correlation between STAIC1, STAIC2, and CDI, the three variables interact with respect to their effect on salivary cortisol values and needed to be considered simultaneously in the model. Therefore, we applied a new model including the co-morbidity variable (equals 1 if STAIC1>95th percentile and CDI>95th percentile, or if STAIC2>95th percentile and CDI>95th percentile, and equals 0 in all other cases) and the absence-of-morbidity variable (equals 0 if STAIC1 < 95th percentile and STAIC2 < 95th percentile and CDI < 95th percentile, and equals 1 in all other cases). Time, gender, and age remained significant confounding variables. According to this model, obese children with co-morbidity had lower average values of salivary cortisol concentration than those without (average difference 0.059 μg/dl, p = 0.029), whereas those with no morbidity also had lower average values of salivary cortisol than those who scored positively in at least one of the three scales (average difference 0.026 μg/dl, p = 0.028). To explore whether there is a continuum in the relationship between the degree of anxiety/depression symptomatology and cortisol levels in obese children, we combined the co-morbidity and the absence-of-morbidity variables in a factor with three levels: equal to 0 if STAIC1 < 95th percentile and CDI < 95th percentile and STAIC2 < 95th percentile (this level corresponds to absence of morbidity), equal to 2 if STAIC2>95th percentile and CDI>95th percentile or if STAIC1>95th percentile and CDI>95th percentile (this level corresponds to co-morbidity), and equal to 1 in all other cases (which corresponds to CDI positive or STAIC1/STAIC2 positive). Results are shown in . The extremes in this morbidity scale (children with no symptoms and those with combined anxiety and depression) had lower values of salivary cortisol concentration than those who scored abnormally for either anxiety or depression. The daily variations of mean salivary cortisol values for 10-year-old boys and girls, as predicted by the repeated measurements analysis, are shown in .
Table IV. Effect of state anxiety (STAIC1), trait anxiety (STAIC2), depressive symptoms (CDI), age, gender, and time on daily variation of salivary cortisol concentration as assessed by repeated measurements analysis.*
Table V. The effect of psychological morbidity on the variation of cortisol measurements as assessed by repeated measures analysis.*
Figure 1. Mean daily variation of salivary cortisol concentration, as predicted by repeated measures analysis (repeated measures analysis with correlated error terms using an unstructured correlation matrix; details are given in ), for girls (1a) and boys (1b) at the age of 10 years. Anxiety and depression symptoms were determined by the State and Trait Anxiety Inventory for Children (STAIC1 and STAIC2), and the CDI, respectively.
Discussion
Our study examined cross-sectional relationships between anxiety/depression, weight status, and cortisol profiles in a clinical population of obese children. We found (a) an increased prevalence of anxiety and depressive symptoms, (b) a significant association between noon (12:00 h) salivary cortisol concentration and trait anxiety, and (c) that the psychological status (anxiety and/or depression) is associated with the overall circadian salivary cortisol concentrations among obese children.
Emotional problems in obese children
An increased prevalence of self-reported anxiety and depressive symptoms was found in obese children, compared to the general population. Furthermore, mean values for depressive z-scores were significantly higher in obese children than the general population, while the percentage of obese children above the upper normal limit was significantly higher than that expected in the general population in all scales. Lastly, among obese children, those with normal values in all three psychometric scales had lower BMI z-scores (although above the upper normal limit) than those who scored high on even one scale.
These findings are consistent with studies showing high incidence of internalizing symptoms and disorders in obese individuals (Mustillo et al. Citation2003; Onyike et al. Citation2003; Goldbacher and Matthews Citation2007). Furthermore, studies in children have shown that treatment seekers (clinical samples) were more likely to have disturbed eating behaviors (loss of control and/or emotional eating) than overweight youngsters not seeking weight loss treatment (Goossens et al. Citation2009). Disturbed eating behaviors, though not examined in our population, are often component symptoms of anxiety and/or depression and might link these conditions to obesity. In addition, several longitudinal studies have shown that anxiety disorders and depression are associated with a higher BMI z-score, especially in females (Mustillo et al. Citation2003; Anderson et al. Citation2006; Noll et al. Citation2007; Sanderson et al. Citation2011). Interestingly, in our cohort, anxiety and/or depression status in obese children were not related to gender, whereas pre-pubertal obese children were five times more likely to have state anxiety than mid- and post-pubertal obese children, as shown in the high state anxiety scores. No relationship between STAIC2 or CDI and pubertal status was found. This finding was not expected, especially for depressive symptoms, because it is widely accepted that female gender and age increase the prevalence of the diagnosis of depression in the general population. The young age of our participants, the fact that all children were obese, and the lack of a full clinical diagnosis may explain these findings. In addition, the fact that z-scores in CDI, for children older than 12, were calculated based on values of 12-year-old children might have influenced the results.
Associations between anxiety, depression, BMI, and cortisol
Though testing cortisol as a mediator between obesity and anxiety/depression has significant limitations in a cross-sectional setting, we attempted to examine such a hypothesis in our sample. Our results do not support a mediation effect of cortisol on the relationship between anxiety/depression symptoms, since two of the Baron and Kenny criteria were not satisfied: first, there was no effect to mediate, as the STAIC1, STAIC2, and CDI were not associated with BMI z-scores; second, the mediator (cortisol concentration) was not related to the dependent variable (BMI z-scores) and this was assessed both by individual correlations and repeated measures.
In our study, a significant positive association was found between the degree of trait anxiety and noon (12:00 h) salivary cortisol. This is particularly important as a pronounced noon salivary “lunch-related cortisol” is one of the features of stressed adults (Chrousos and Gold Citation1998; Rosmond et al. Citation1998). Trait anxiety was the only measure associated with cortisol values, compared to measures of concurrent stress, such as state anxiety and current depressive symptoms. These findings indicate that trait anxiety may be more relevant to stress-related cortisol elevations. Trait anxiety represents a more fundamental component of stress perception. Anxiety theory, formulated by Spielberger (Citation1966), suggests that individuals with high trait anxiety levels are more anxiety-prone because they perceive situations as more dangerous or threatening than individuals with low trait anxiety. Consequently, they are more vulnerable to stress and tend to respond with more intense state anxiety reactions than do individuals who are low in trait anxiety. Thus, although it is not clear whether cortisol disturbances in stress-related disorders are related to trait and/or state conditions, trait anxiety may represent a better self-reported indicator of an inherent vulnerability to stress.
Daily cortisol profiles among obese children with or without anxiety/depression
The examination of salivary cortisol concentrations with repeated measures analysis in obese children with or without symptoms of anxiety/depression revealed some interesting and unexpected results: a significant difference was found in overall salivary cortisol concentrations between those children with high anxiety or high depression and those with low such symptoms. Interestingly, the group with both anxiety and depression had significantly lower cortisol concentrations than those with only anxiety or only depressive symptoms and similar, albeit slightly lower, to those with low affective symptoms (). This finding implies the importance of the clinical assessment in interpreting neuroendocrine findings, but also the importance of taking into account co-morbidity. Obese children with symptoms of both anxiety and depression (T scores>95th percentile with both instruments) may thus constitute a distinct clinical entity with a different neuroendocrine profile compared to those with only anxiety or only depressive symptoms. Since a clinical diagnosis was not made, symptoms of both anxiety and depression may belong to a separate diagnostic entity, such as post-traumatic stress disorder or atypical depression (Gold and Chrousos Citation2002; Pervanidou Citation2008). Only a clinical diagnostic procedure and a longitudinal examination of these children can clarify the clinical entity of these children and its further role in obesity- and stress-related morbidity.
Other studies in children with full psychiatric diagnoses in the entire BMI range have shown inconsistent results: studies in adolescents with clinical depression present either a flattened diurnal cortisol profile with elevated evening cortisol levels (Shirtcliff and Essex Citation2008), or elevated evening peri-sleep onset cortisol (Dahl et al. Citation1991; Forbes et al. Citation2006). Regarding anxiety disorders, either hyper- or hypo-cortisolism is noted inconsistently (Pervanidou et al. Citation2007a, Citation2007b; Pervanidou Citation2008). Age, developmental history, previous experiences, and time since these experiences occurred, as well as co-morbidity may all influence the neuroendocrine profile of an anxiety disorder (Pervanidou and Chrousos Citation2010a, Citation2010b). A recent, similar study of 58 post-pubertal adolescents (Van den Bergh and Van Calster Citation2009) showed that adolescents with a high degree of depressive symptomatology in the CDI had a high, flattened cortisol profile compared to those with a lower degree of symptoms.
In our study, obesity serves as an unfavorable background for both behavioral–emotional and metabolic complications. Practically, our findings indicate that the emotional status of an obese child (as indicated by self-completed questionnaires) may influence diurnal cortisol level variation, even if a clinical diagnosis of anxiety or depression is not made. As our study did not include a control group of normal weight children, we are not able to examine the same hypothesis in children with a lower BMI.
Although it is not possible to prove the contribution of cortisol abnormalities in the health status of a child at this young age, cortisol abnormalities in obese individuals may serve as an additional pathway leading to further psychological, endocrine, and metabolic complications. They may also play a role in worsening the vicious cycle between obesity and emotional problems. Clinical observations and data from experimental studies agree that chronic excess of cortisol is associated with increased collection of visceral fat (Chrousos and Gold Citation1998; Rosmond et al. Citation1998; Björntorp Citation2001). Clinical examples of patients treated with corticosteroids, having endogenous Cushing syndrome, or suffering from depression have shown an increased appetite and food intake leading to obesity, especially central obesity (Björntorp Citation2001; Chrousos Citation2009).
Strengths and weaknesses
The novelty of this study is that instruments examining symptoms of anxiety/depression and cortisol measurements were applied in a clinical population of obese children, seeking medical help. These findings may extend the existing knowledge on HPA axis dysregulation in children with anxiety disorders and depression to individuals with no apparent psychiatric disorders. Limitations of this study are the lack of a control group, the fact that salivary cortisol concentrations were measured on only one day, specifically, a day of leisure and of presumably lower stress than week days, and the z-scores in CDI of children older than 12 years were calculated based on the values of a 12-year-old child, as the Greek version of the CDI is restricted to children 8–12 years old.
From a clinical perspective, our results support recognizing and treating anxiety and depression as part of a comprehensive obesity prevention program.
Declaration of interest : The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- American Psychiatric Association. 1994. Diagnostic and statistical manual of mental disorders-DSM-IV. Washington, DC: American Psychiatric Press.
- Anderson SE, Cohen P, Naumova EN, Must A. 2006. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch Pediatr Adolesc Med. 160 3: 285–291.
- Baron RM, Kenny DA. 1986. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 51 6: 1173–1182.
- Björntorp P. 2001. Do stress reactions cause abdominal obesity and comorbidities?. Obes Rev. 2 2: 73–86.
- Chiotis D, Krikos X, Tsiftis G, Hatzisymeaon M, Maniati-Christidi M, Dacou-Voutetakis C. 2004. Body mass index and prevalence of obesity in subjects of Hellenic origin aged 0-18 years, living in the Athens area. Ann Clin Paediatr Univ Atheniensis. 51 2: 139–154.
- Chrousos GP. 2009. Stress and disorders of the stress system. Nat Rev Endocrinol. 5 7: 374–381.
- Chrousos GP, Gold PW. 1992. The concepts of stress and stress system disorders. JAMA. 267:1244–1252.
- Chrousos GP, Gold PW. 1998. A healthy body in a healthy mind—and vice versa—the damaging power of “uncontrollable” stress. J Clin Endocrinol Metab. 83 6: 1842–1845.
- Dahl R, Ryan ND, Puig-Antich J, Nguyen NA, Al-Shabbout M, Meyer VA, Perel J. 1991. 24-Hour cortisol measures in adolescents with major depression: A controlled study. Biol Psychiatry. 30 1: 25–36.
- Dockray S, Susman EJ, Dorn LD. 2009. Depression, cortisol reactivity, and obesity in childhood and adolescence. J Adolesc Health. 45 4: 344–350.
- Forbes EE, Williamson D, Ryan DN, Birmaher B, Axelson DA, Dahl RE. 2006. Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biol Psychiatry. 59:24–30.
- Gathercole LL, Stewart PM. 2010. Targeting the pre-receptor metabolism of cortisol as a novel therapy in obesity and diabetes. J Steroid Biochem Mol Biol. 122 1-3: 21–27.
- Giannakopoulos G, Kazantzi M, Dimitrakaki C, Tsiantis J, Kolaitis G, Tountas Y. 2009. Screening for children's depression symptoms in Greece: The use of the Children's Depression Inventory in a nation-wide school-based sample. Eur Child Adolesc Psychiatry. 18 8: 485–492.
- Gold PW, Chrousos GP. 2002. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol Psychiatry. 7 3: 254–275.
- Goldbacher EM, Matthews KA. 2007. Are psychological characteristics related to risk of the metabolic syndrome? A review of the literature. Ann Behav Med. 34 3: 240–252.
- Goossens L, Braet C, Van Vlierberghe L, Mels S. 2009. Loss of control over eating in overweight youngsters: The role of anxiety, depression and emotional eating. Eur Eat Disord Rev. 17 1: 68–78.
- Hillman JB, Dorn LD, Loucks TL, Berga SL. 2012. Obesity and the hypothalamic-pituitary-adrenal axis in adolescent girls. Metabolism. 61 3: 341–348.
- Kassi E, Pervanidou P, Kaltsas G, Chrousos G. 2011. Metabolic syndrome: Definitions and controversies. BMC Med. 9 May 5: 48.
- Kennard B, Silva S, Vitiello B, Curry J, Kratochvil C, Simons A, Hughes J, Feeny N, Weller E, Sweeney M, Reinecke M, Pathak S, Ginsburg G, Emslie G, March J, TADS Team. 2006. Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry. 45 12: 1404–1411.
- Kovacs M. 1992. Manual for the Children's Depression Inventory (CDI). Psychopharmacol Bull. 21:995–998.
- Morton NM, Seckl JR. 2008. 11Beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res. 36:146–164.
- Mustillo S, Worthman C, Erkanli A, Keeler G, Angold A, Costello EJ. 2003. Obesity and psychiatric disorder: Developmental trajectories. Pediatrics. 111:851–859.
- Noll JG, Zeller MH, Trickett PK, Putnam FW. 2007. Obesity risk for female victims of childhood sexual abuse: A prospective study. Pediatrics. 120 1: e61–e67.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. 2006. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 295 13: 1549–1555.
- Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. 2003. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 158 12: 1139–1147.
- Pereira CD, Azevedo I, Monteiro R, Martins MJ. 11β-Hydroxysteroid dehydrogenase type 1: Relevance of its modulation in the pathophysiology of obesity, the metabolic syndrome and type 2 diabetes mellitus. Diabetes Obes Metab. 2012 Feb 9. doi: 10.1111/j.1463-1326.2012.01582.
- Pervanidou P. 2008. Biology of posttraumatic stress disorder in childhood and adolescence. J Neuroendocrinol. 20 5: 632–638.
- Pervanidou P, Chrousos GP. 2007. Post-traumatic stress disorder in children and adolescents: From Sigmund Freud's “trauma” to psychopathology and the (Dys)metabolic syndrome. Horm Metab Res. 39 6: 413–419.
- Pervanidou P, Chrousos GP. Neuroendocrinology of posttraumatic stress disorder. Prog Brain Res. 2010a; 182:149–160.
- Pervanidou P, Chrousos GPDevelopmental neuroendocrinology of posttraumatic stress disorder: The biological effects of childhood stress and trauma. In: Penkava NS, Haight LR. editors. Neuroendocrinology research developments. New York: Nova Science Publishers, Inc2010b105–117.
- Pervanidou P, Chrousos GP. 2011. Stress and obesity/metabolic syndrome in childhood and adolescence. Int J Pediatr Obes. 6 Suppl. 1: 21–28.
- Pervanidou P, Chrousos GP. 2012. Metabolic consequences of stress during childhood and adolescence. Metabolism. 61 5: 611–619.
- Pervanidou P, Kolaitis G, Charitaki S, Lazaropoulou Ch, Hindmarsh P, Bakoula Ch, Papassotiriou I, Tsiantis J, Chrousos GP. The natural history of neuroendocrine changes in pediatric posttraumatic stress disorder after motor vehicle accidents: Progressive divergence of noradrenaline and cortisol concentrations over time. Biol Psychiatry. 2007a; 62 10: 1095–1110.
- Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP. Elevated morning serum IL-6 or evening salivary cortisol predict PTSD in children and adolescents 6 months after a motor vehicle accident. Psychoneuroendocrinology. 2007b; 32 8–10: 991–999.
- Pettit JW, Lewinsohn PM, Joiner TEJr. 2006. Propagation of major depressive disorder: Relationship between first episode symptoms and recurrence. Psychiatry Res. 141 3: 271–278.
- Psychountaki M, Zervas Y, Karteroliotis K, Spielberger C. 2003. Reliability and validity of the Greek version of the STAIC. Eur J Psychol Assess. 19 2: 124–130.
- Reeves GM, Postolache TT, Snitker S. 2008. Childhood obesity and depression: Connection between these growing problems in growing children. Int J Child Health Hum Dev. 1 2: 103–114.
- Rosmond R, Dallman MF, Björntorp P. 1998. Stress-related cortisol secretion in men: Relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 83 6: 1853–1859.
- Rosmond R, Lapidus L, Marin P, Bjorntorp P. 1996. Mental distress, obesity and body fat distribution in middle-aged men. Obes Res. 4:245–252.
- Sanderson K, Patton GC, McKercher C, Dwyer T, Venn AJ. 2011. Overweight and obesity in childhood and risk of mental disorder: A 20-year cohort study. Aust N Z J Psychiatry. 45 5: 84–92.
- Schneider M, Dunton GF, Cooper DM. 2007. Media use and obesity in adolescent females. Obesity (Silver Spring). 15 9: 2328–2335.
- Seckl JR, Walker BR. 2004. 11Beta-hydroxysteroid dehydrogenase type 1 as a modulator of glucocorticoid action: From metabolism to memory. Trends Endocrinol Metab. 15 9: 418–424.
- Shirtcliff EA, Essex MJ. 2008. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 50 7: 690–703.
- Spielberger CD. 1966. Theory and research on anxiety. In: Spielberger CD. editors. Anxiety and behavior. New York: Academic Press3–20.
- Spielberger CD, Edwards CD, Lushene RE, Montuori J, Platzek D. 1973. Preliminary manual for the State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press.
- Tanner JM. 1962. Growth at adolescence. Oxford: Blackwell Scientific Publications.
- Tomlinson JW, Finney J, Gay C, Hughes BA, Hughes SV, Stewart PM. 2008. Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes. 57 10: 2652–2660.
- Van den Bergh BR, Van Calster B. 2009. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children's Depression Inventory. Psychoneuroendocrinology. 34 5: 791–794.
- Vogelzangs N, Suthers K, Ferrucci L, Simonsick EM, Ble A, Schrager M, Bandinelli S, Lauretani F, Giannelli SV, Penninx BW. 2007. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology. 32 2: 151–159.
- Weigensberg MJ, Toledo-Corral CM, Goran MI. 2008. Association between the metabolic syndrome and serum cortisol in overweigh Latino youth. J Clin Endocrinol Metab. 93 4: 1372–1378.
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali S, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin R, Caprio S. 2004. Obesity and metabolic syndrome in children and adolescents. N Engl J Med. 350:2362–2374.
- Williams JR. 2008. The declaration of Helsinki and public health. Bull World Health Organ. 86:650–651.
- Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga P. 1991. Waist to hip ratio in middle-aged women. Association with behavioral and psychological factors and with changes in cardiovascular risk factors. Arterioscler Thromb. 11:1250–1257.
