Abstract
We investigated the effect of genetic selection for temperament on the way that stressors affect the behaviour and the adrenal and reproductive axes of sheep. We tested three hypotheses: (i) isolation would increase cortisol secretion and decrease luteinising hormone (LH) secretion more in nervous sheep than in calm sheep; (ii) isolation combined with simulated human presence would increase cortisol secretion and decrease LH secretion more in nervous sheep than in calm sheep and (iii) isolation combined with stressors that were not specific to the selection process (i.e. non-selection stressors) would increase cortisol secretion and decrease LH secretion equally in calm and nervous sheep. Isolation alone increased cortisol secretion and decreased LH secretion in nervous sheep but not in calm sheep. Compared to calm sheep, nervous sheep were more agitated during the first 2 h of isolation but not during the second 2 h of isolation. Exposure to non-selection stressors increased cortisol secretion, decreased LH pulse amplitude and the mean plasma concentrations of LH in both calm and nervous sheep. We conclude that genetic selection for temperament affects the behavioural expression of the stress response and the secretion of adrenal and reproductive hormones during isolation, but has less impact on their reactivity to non-selection stressors.
Introduction
Exposure to a stressor increases the secretion of stress hormones within the hypothalamic–pituitary–adrenal (HPA) axis. This system has two functions: first, to help the animal cope with the stressor and second, to restore homeostasis (Tilbrook et al. Citation2002; Tsigos and Chrousos Citation2002). Despite this mechanism, even brief exposure to a stressor can disrupt reproductive function (reviews; Dobson and Smith Citation2000; Tilbrook et al. Citation2000, Citation2002). In sheep, the deleterious effects of stress on reproduction are mediated through suppression of luteinising hormone (LH) pulse amplitude (Breen and Karsch Citation2006; Breen et al. Citation2007; Pierce et al. Citation2008) through direct inhibition of GnRH pulse amplitude at the level of the hypothalamus (Wagenmaker et al. Citation2009) and reduced pituitary responsiveness to GnRH (Breen et al. Citation2004, Citation2007). The magnitude of the cortisol response plays an important role in mediating the suppressive effects of stress on reproduction because the reduction in pituitary responsiveness to GnRH is mediated via the type II glucocorticoid receptor (Breen et al. Citation2007).
The magnitude of the cortisol response of individuals to a stressor can vary markedly among individuals of the same sex and species (Tilbrook and Clarke Citation2006). Temperament determines how an individual perceives and reacts to their environment (Boissy Citation1995) and thus may contribute to the variability observed among individuals in their physiological reactivity to stress. This trait has been used at the University of Western Australia to select sheep that are more reactive (nervous temperament) or less reactive (calm temperament) to isolation and human presence (Murphy et al. Citation1994; Bickell et al. Citation2009a,Citationb). The fear-related behaviours used to select these sheep (locomotor activity and vocalisations; Romeyer and Bouissou Citation1992) are highly repeatable (r = 0.40–0.76; Blache and Ferguson Citation2005), moderately heritable (h = 0.45; Blache and Ferguson Citation2005) and minimally affected by non-genetic factors (Bickell et al. Citation2009b). The robust and repeatable divergence in the behavioural responses of the two lines to their selection stressors thus make calm and nervous sheep a valuable model to study the effects of stress on reproduction.
Type of stressor can have a profound effect on the neuroendocrine expression of the stress response. For example, the cortisol response of sheep to shearing is greater than that observed in response to yarding and handling (Fulkerson and Jamieson Citation1982). This relationship is particularly pertinent to animal selected for extremes in anxiety or fear-related behaviours. For example, rats selected for a high expression of anxiety-related behaviours (HAB) secrete more corticosterone than rats selected for low expression of anxiety-related behaviours (LAB) when they are forced onto the open arms of the elevated plus maze (Landgraf et al. Citation1999). However, when faced with a social stressor such as an intruder male, LAB rats secrete more corticosterone than HAB rats (Frank et al. Citation2006). This observation indicates that the nature of the stressor used in the selection process of the HAB/LAB rats (i.e. non-social vs. social) directly influences the behavioural and neuroendocrine responses of the two lines to other stressors (Veenema and Neumann Citation2007).
In this study, we combined the unique genetics of the UWA temperament flock with two experimental paradigms to determine how temperament and type of stressor affect the behavioural and neuroendocrine expression of the stress response and the associated impact on LH secretion. Calm and nervous sheep were either subjected to 4 h of isolation or isolation combined with hourly imposition of stressors that were specific (simulated human presence) or non-specific (restraint and novel object) to the selection process. We used this approach to determine the impact of temperament on the capacity of stress to interfere with reproductive function by testing three specific hypotheses: (i) isolation of ‘nervous’ sheep will induce a greater increase in cortisol secretion and decrease in LH secretion than that observed in ‘calm’ sheep; (ii) isolation of ‘nervous’ sheep combined with another selection stressor (human presence simulated by a mannequin) will induce a greater increase in cortisol secretion and decrease in LH secretion than that observed in ‘calm’ sheep and (iii) isolation combined with stressors not involved in the selection index will induce a similar increase in cortisol secretion and decrease in LH secretion in both ‘calm’ and ‘nervous’ sheep.
Materials and methods
These experiments were carried out in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (Seventh Edition, 2004) and were approved by the Animal Ethics Committee of The University of Western Australia (RA3/100/947).
UWA temperament flock
The UWA temperament flock comprises two lines of Merino sheep that have been selected for 17 generations for extremes in behavioural reactivity to isolation from the flock in the presence or absence of humans (Murphy et al. Citation1994; Beausoleil et al. Citation2008). Behavioural reactivity to stress (locomotor activity and vocalisation frequency) is assessed at approximately 3–4 mo of age using two behavioural tests described previously (Murphy et al. Citation1994; Bickell et al. Citation2009a). Briefly, the novel arena test challenges the sheep with the conflict of having to pass a human to gain access to a pen of flock mates. The human quantifies locomotor's activity by counting the number of times each sheep crosses the lines on the floor of the arena. The second test challenges the sheep with isolation stress in a solid plywood box (1.5 m3) that prevents visual communication with flock mates. Locomotor's activity within the box is recorded by a digital agitation metre calibrated for low, medium and high levels of agitation prior to the test (Murphy et al. Citation1994; Blache and Ferguson Citation2005). An overall score for behavioural reactivity to stress is then calculated as described in detail by Beausoleil et al. (Citation2008) and sheep with low behavioural reactivity to stress are termed ‘calm’ whereas those with high behavioural reactivity to stress are termed ‘nervous’.
Experimental animals
Castrated male sheep from calm (n = 14) or nervous (n = 12) lines of the UWA temperament flock were allocated to one of three treatments, all balanced for age (14–16 mo) and live weight (): calm control (n = 5), nervous control (n = 4), calm isolation (n = 4), nervous isolation (n = 4), calm layered stressor (n = 5) and nervous layered stressor (n = 4). The sheep were selected based on their parental temperament (i.e. calm or nervous) and agitation score at weaning (). Castrated males were used for this study to avoid the confounding effects of gonadal hormones on cortisol and LH secretion (Tilbrook et al. Citation2000, Citation2002).
Table I. Mean ± SEM live weight and agitation score at weaning in calm and nervous sheep that were either maintained with flock mates (control) or subjected to isolation stress or the layered stressor paradigm.
Experimental procedure and treatments
On each day of the experiment, blood was sampled from one animal from each treatment (randomised for temperament) every 10 min for 6 h via a jugular cannula inserted the day prior to the experiment. During the first 2 h, all sheep remained in a group pen (approximately 1.5 m × 1.2 m) with three companion sheep that were used throughout the experiment. During the control period, an operator entered the pen and gently restrained each sheep to gain access to the jugular cannula that was secured under tape at the rear of the sheep's head. At the end of the control period, sheep in the isolation and layered stressor treatments were isolated in a solid plywood box (1.5 m3) for the remaining 4 h of the experiment. Among these animals, the jugular cannula was removed from the tape and suspended from the ceiling by a length of elastic that allowed the animal to move freely around the isolation box (). The operator was then able to collect the blood samples from outside of the box without making physical or visual contact with the sheep. The sheep may still have been able to hear the operator but conversation was not permitted around the isolation box. Sheep in the isolation treatment were not subject to any additional stressors other than isolation itself, whereas sheep in the layered stressor treatment were exposed to additional stressors as described below. Control sheep remained with the companion sheep for the duration of the experiment and blood was sampled throughout as described for the control period.
Figure 1. Schema showing the structure of the layered stressor paradigm (1a) and the set-up (1f) used to expose the animals to the selection stressors (isolation and exposure to a mannequin placed at B) and the non-selection stressors (blower placed at A and animal restrained inside isolation box). 1b and 1c show an animal confined to the isolation box and the method of blood collection from outside the isolation box. Figure 1d and 1e show the placement of the blower outside the isolation box with the back door open and the mannequin used to simulate human presence.
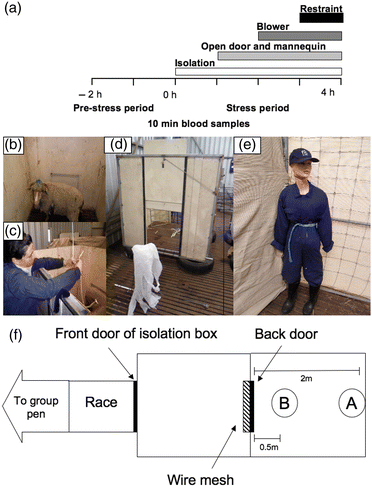
Layered stressor paradigm
The layered stressor paradigm developed by Breen et al. (Citation2007) which stimulated a robust and sustained increase in cortisol secretion consisted of hourly imposition of stressors in a sequence, beginning with isolation followed by restraint, blindfold and finally exposure to a barking dog. In this experiment, we modified the paradigm to include stressors that were either specific (isolation and human presence simulated by a mannequin) or non-specific to the selection tests of the UWA temperament flock (). The sheep were initially isolated for the first 1 h of the paradigm in a solid plywood box identical to that used to assess their behavioural reactivity to isolation at weaning (). After 1 h, the rear door of the isolation box was opened so the animal could see out through metal mesh over the back of the door. Human presence was simulated using a mannequin dressed in overalls which was tied to the fence approximately 2 m from the open door of the box in the eye line of the animal (). The door remained open and the mannequin was in place for the last 3 h of the paradigm. It should be noted that the animal was able to move around the isolation box and could choose to face away from the open door, but we found that once the door was opened the sheep spent most of their time facing the open door. The second half of the layered stressor paradigm consisted of hourly imposition of two proven stressors of sheep; white flapping plastic (Romeyer and Bouissou Citation1992; Bickell et al. Citation2011) referred to herein as the ‘blower’ and restraint (Tilbrook et al. Citation1999) layered at hourly intervals on top of the selection stressors (). The animal was restrained for the last 2 h of the paradigm using a nylon harness that was fitted to the sheep as described by Watson and Radford (Citation1960) by one of the experimental team. The harness was then tied to the floor of the isolation box using baler twine. The animal was still able to move their feet and legs but was unable to move away from the open door facing the mannequin. The ‘blower’ was placed approximately 0.5 m from the open door of the box as shown in and remained switched on for the final hour of the layered stressor paradigm.
Quantification of anxiety-related behaviour
Locomotor activity and vocalisations within the isolation box were recorded using an agitation metre calibrated for low, medium and high levels of agitation as described above (Blache and Ferguson Citation2005). Cumulative agitation scores were recorded every 10 min over the 4-h isolation period in the isolation and layered stressor treatments.
Blood processing and immunoassay
Blood samples were centrifuged almost immediately after collection for 10 min at 3000 rpm. Plasma was then harvested and frozen in plastic tubes at 20°C until immunoassay for cortisol and LH. Plasma concentrations of cortisol were quantified in duplicate using commercial radioimmunoassay kits (Diasorin Australia Ltd, North Ryde, NSW) modified and validated for sheep as described by Beausoleil et al. (Citation2008). The sensitivity of the assay was 1.1 ng/mL. Quality control samples (13.5 and 32.2 ng/mL) were used to calculate inter-assay (5.0% and 5.6%) and intra-assay (8.2% and 7.9%) variation. Plasma LH concentrations were determined in duplicate using a previously validated double-antibody radioimmunoassay using ovine LH for iodination and standards (Martin et al. Citation1980). The sensitivity of the assay was 0.1 ng/mL. All samples were included in one assay. Quality control samples (0.5, 1.1 and 1.9 ng/mL) were used to calculate intra-assay variation (9.6%, 3.4% and 5.3%).
Data analysis
Prior to analysis, endocrine data were divided into three periods: Period 1 (control period; 0–2 h); Period 2 (2–4 h) and Period 3 (4–6 h). The 4 h of exposure to the stressor(s) were grouped into 2-h periods corresponding with the 2 h of selection stressors (isolation+simulated human presence with mannequin; Period 2) and 2 h of non-selection stressors (blower and restraint; Period 3). The isolation data were also grouped in this way to show changes in reactivity to the stressors over time. The agitation scores were similarly grouped into the first 2 h in the isolation box (Period 1) and the second 2 h in the isolation box (Period 2) for the isolation and layered stressor treatments. No pre-stressor or control period was possible for the agitation data because it is only possible to measure this variable in the isolation box.
Exclusion criteria
The mean concentration of cortisol was calculated for each animal during the control, selection stressor and non-selection stressor periods. If the concentration of cortisol was consistently elevated above 40 ng/mL during the control period, we deemed the animal to be stressed (Barnett and Hemsworth Citation1990; Wagenmaker et al. Citation2009) and excluded them from further analysis. Similarly, any sheep with no detectable pulses of LH during the control period were excluded from further analysis. Using these selection criteria, one calm sheep from the control treatment and one calm sheep from the layered stressor treatment were excluded. Consequently, the final number of animals per group was as follows: calm control (n = 4), nervous control (n = 4), calm isolation (n = 4), nervous isolation (n = 4), calm layered stressor (n = 4) and nervous layered stressor (n = 4).
Statistical analysis
The distributions of all data were initially assessed using the Anderson–Darling test for normality (Minitab 14.1). Where data were not from a normal distribution, data were transformed (see below) and the test repeated to ensure the validity of the data for parametric analysis. Log10 transformation was required to overcome skewness in the data for the mean plasma concentrations of cortisol and LH, and the amplitude of LH and cortisol pulses. The cumulative agitation scores were subjected to square root transformation followed by log10 transformation to overcome skewness in the data. Non-transformed data are presented for ease of interpretation. Pulses of LH and cortisol were detected using Munro, a modified version of the Pulsar algorithm (Merriam and Wachter Citation1982). Changes in cortisol pulse frequency and pulse amplitude were included in this study because they have been shown in rodents and humans to affect the magnitude of the adrenocortical response of an individual to a stressor (review: Lightman and Conway-Campbell Citation2010). The basal concentration of LH was calculated as the mean of the lowest points during a given period as described elsewhere (Martin et al. Citation1983). The same approach was taken to calculate the basal concentration of cortisol. Data for pulse frequency, pulse amplitude, mean and basal concentrations of cortisol and LH were subject to repeated measures ANOVA in Genstat 5 (Second Edition, Lawes Agricultural Trust, Rothamsted Experimental Station, Harpenden, Hertfordshire, UK) to assess the effect of temperament, treatment, time relative to stressor exposure and any interactions between these factors. Post hoc comparisons were conducted using Scheffe's multiple comparison procedure. Where a significant effect of time, treatment, temperament or interaction between the factors was detected, data were compared within treatment by Paired t-test (e.g. Period 1 vs. Period 1 in calm isolation treatment; Genstat 5) or between treatments by Student's t-test (e.g. Period 1 in calm control vs. Period 1 in calm isolation; Genstat 5).
Results
Cortisol secretion
There were independent effects of time and treatment and an interaction between time and treatment on the mean plasma concentrations of cortisol (time effect: F(2,36) = 17.5, P < 0.001; treatment effect: F(2,18) = 5.38, P < 0.05; time × treatment effect: F(4,36) = 3.43, P < 0.05; ). Similarly, there were independent effects of time and treatment on basal plasma concentrations of cortisol (time effect: basal: F(2,36) = 13.8, P < 0.001; treatment effect: basal: F(2,18) = 4.47, P < 0.001). There were no further significant interactions (P>0.1) between any of the factors on either the basal or mean plasma concentrations of cortisol.
Table II. Mean ( ± SEM) parameters of cortisol secretion in calm and nervous sheep.
There was an independent effect of time and interactions between time and treatment and time, temperament and treatment on cortisol pulse amplitude (time effect: F(2,36) = 15.3, P < 0.001; time × treatment effect: F(4,36) = 2.62, P < 0.05; time × treatment × temperament effect: F(4,36) = 3.38, P < 0.05) but no independent effects of treatment or temperament (P>0.1). Similarly, there were no independent effects of time, treatment or temperament, or any interaction between these variables, on cortisol pulse frequency (P>0.1).
Representative profiles of cortisol secretion in sheep from each combination of temperament and treatment are shown in .
Figure 2. Representative profiles of the plasma concentration of cortisol in calm (left panels; black diamond) and nervous (right panels; grey square) sheep maintained with companion animals (top panels; control), subjected to isolation stress (middle panels) or the layered stressor paradigm (bottom panels). Isolation (I) is indicated by the pale grey shaded area. The onset of each of stressors in the layered stressor paradigm is indicated by the first letter of the stressor (i.e. M for mannequin, B for blower and R for restraint). Pulses of cortisol are indicated by the placement of an arrow at the peak of the pulse.
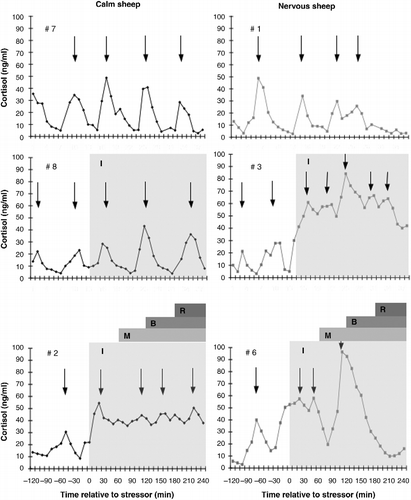
Control animals
The mean and basal plasma concentrations of cortisol did not change over time or differ between calm or nervous sheep throughout the experiment (P>0.1). Cortisol pulse amplitude increased between Periods 1 and 2 in calm sheep (P < 0.05) but not in nervous sheep (P>0.1). Cortisol pulse amplitude decreased between Periods 2 and 3 in both calm (P < 0.05) and nervous sheep (P < 0.01), but only differed between Periods 1 and 3 in nervous sheep (nervous, P < 0.05; calm, P>0.1). Cortisol pulse amplitude only differed between calm and nervous sheep during Period 3 (P < 0.05).
Isolation
Among nervous sheep, isolation increased the mean and basal plasma concentrations of cortisol above that observed during the control period (Period 1 vs. Period 3; P < 0.05), control sheep of the same temperament (Period 3; P < 0.05) and calm sheep exposed to the same stressor (Period 3; P < 0.05). The increase in cortisol secretion in nervous sheep was associated with a reduction in cortisol pulse amplitude compared to the control period (Period 1 vs. Period 3; P < 0.05). Among calm sheep, neither the mean and basal plasma concentrations of cortisol, nor cortisol pulse amplitude differed from the control period or control sheep of the same temperament throughout the experiment (P>0.1).
Layered stressor
Exposure of calm sheep to the selection stressors increased the mean and basal plasma concentrations of cortisol and cortisol pulse amplitude compared to the control period (at least P < 0.05) and control sheep of the same temperament (P < 0.05). Among nervous sheep, exposure to the selection stressors increased the mean plasma concentration of cortisol (P < 0.05) but not the basal plasma concentration of cortisol (P>0.1) compared to the control period and control sheep of the same temperament. Among calm sheep, the mean and basal plasma concentrations of cortisol during exposure to the non-selection stressors remained higher than the control period (P < 0.05) and control sheep of the same temperament (P < 0.05). However, cortisol pulse amplitude in calm sheep was lower during this period than during exposure to the selection stressors (Period 2 vs. Period 3; P < 0.05). Among nervous sheep, the mean plasma concentration of cortisol during exposure to the non-selection stressors did not differ from the control period (P>0.1) and was only associated with a numerical decrease in cortisol pulse amplitude (P < 0.1). However, cortisol pulse amplitude during this time period was greater than that observed in the control sheep of the same temperament (P < 0.05). There were no differences in mean and basal plasma concentrations of cortisol or cortisol pulse amplitude between calm and nervous sheep throughout the experiment (P>0.1).
LH secretion
There were independent effects of treatment and time and an interaction between time and treatment on LH pulse frequency (treatment effect: F(2,18) = 4.17, P < 0.05; time effect: F(2,36) = 9.61, P < 0.001; time × treatment effect: F(4,36) = 2.57, P < 0.05; ). There was no independent effect of temperament on this variable (P>0.1).
Table III. Mean ( ± SEM) parameters of LH secretion in calm and nervous sheep.
There was an independent effect of time on LH pulse amplitude and interactions between time and temperament, time and treatment and time, temperament and treatment (time effect: F(2,36) = 14.3, P < 0.001; time × treatment effect: F(4,36) = 4.80, P < 0.01; time × temperament effect: F(2,36) = 4.24, P < 0.05; time × temperament × treatment effect: F(4,36) = 6.40, P < 0.001). There were no independent effects of treatment or temperament on this variable (P>0.1).
There was an independent effect of time and an interaction between time and treatment on the mean plasma concentration of LH (time effect: F(2,36) = 14.1, P < 0.001; time × treatment effect: F(4,36) = 2.83, P < 0.05). There was an independent effect of time and an interaction between time, temperament and treatment on basal plasma concentrations of LH (time effect: F(2,36) = 6.99, P < 0.01; time × temperament × treatment effect: F(4,36) = 2.57, P < 0.05). There were no independent effects of temperament or treatment or any further interactions between treatment, temperament and/or time on either the mean or basal plasma concentrations of LH (P>0.1).
Representative profiles of LH secretion in sheep from each combination of temperament and treatment are shown in .
Figure 3. Representative profiles of the plasma concentration of LH secretion in calm (left panels; black diamond) and nervous (right panels; grey square) sheep maintained with companion sheep (top panels; control), subjected to isolation stress (middle panels; isolation only) or the layered stressor paradigm (bottom panels). Isolation (I) is indicated by the pale grey shaded area. The onset of each of stressors in the layered stressor paradigm is indicated by the first letter of the stressor (i.e. M for mannequin, B for blower and R for restraint). Pulses of LH are indicated by the placement of an arrow at the peak of the pulse.
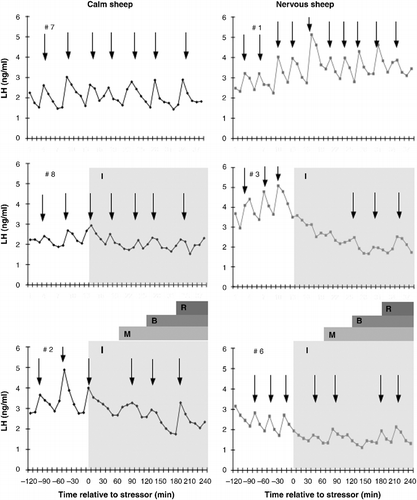
Control animals
None of the measures of LH secretion differed between calm or nervous sheep or changed over time throughout the experiment (P>0.1).
Isolation
Isolation decreased LH pulse frequency (P < 0.01) and LH pulse amplitude (P < 0.05) in nervous sheep compared to the control period. Pulse frequency at this time point was also lower in the isolated sheep than control sheep of the same temperament (P < 0.05) and calm sheep exposed to the same stressor (P < 0.05). In nervous sheep, the numerical decrease in the mean plasma concentration of LH associated with isolation (Period 1 vs. Period 2; P < 0.1) only differed significantly from the control period during Period 3 (Period 1 vs. Period 3; P < 0.05). In contrast, LH pulse frequency during Period 3 remained lower than control sheep of the same temperament (P < 0.05) but was only numerically lower than the control period (P < 0.1). Among calm sheep, no measures of LH secretion differed from the control period or control sheep of the same temperament at any time point during the experiment (P>0.1). The mean and basal plasma concentrations of LH did not differ between calm and nervous sheep at any point during the experiment (P>0.1).
Layered stressor
Exposure to the selection stressors decreased the mean and basal plasma concentrations of LH in both calm and nervous sheep compared to the control period (P < 0.05) but not compared to control sheep of the same temperament (P>0.1). Exposure to the selection stressors decreased LH pulse amplitude in both calm and nervous sheep compared to the control period (P < 0.05) and control sheep of the same temperament (P < 0.05) but had no effect on LH pulse frequency (P>0.1). During exposure of both calm and nervous sheep to the non-selection stressors, the mean and basal plasma concentrations of LH remained lower than the control period (P < 0.05) and control sheep of the same temperament (P < 0.05). LH pulse amplitude increased during Period 3 in both calm and nervous sheep (Period 2 vs. Period 3; P < 0.05) and did not differ from the control period (Period 1 vs. Period 3; P>0.1) or control sheep of either temperament (P>0.1). There were no differences in any measures of LH secretion between calm or nervous sheep throughout the experiment (P>0.1).
Agitation score
There was an independent effect of time (F(1,31) = 19.9, P < 0.001) and interactions between temperament and treatment (F(1,12) = 4.56, P < 0.05) and time and temperament (F(1,12) = 12.4, P < 0.01), but no independent effects of temperament or treatment (P>0.1) on the cumulative agitation score.
Isolation
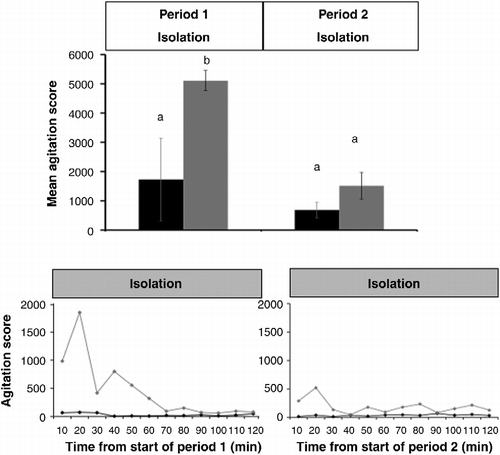
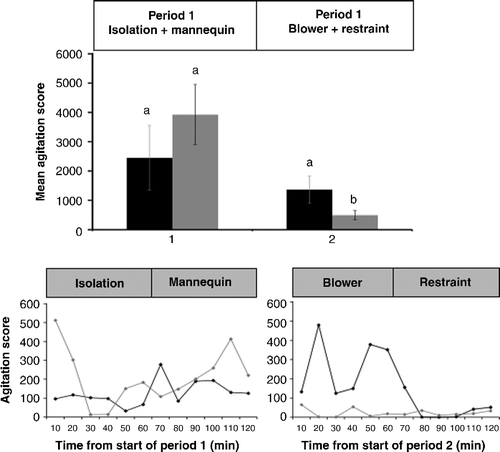
During the first 2 h of isolation, the cumulative agitation score was higher in nervous sheep than in calm sheep (nervous: 5108 ± 346 vs. calm: 1728 ± 1420; P < 0.05; ). However, during the second 2 h of isolation, the agitation scores declined in nervous sheep (P < 0.05) and no longer differed significantly between calm and nervous sheep (calm: 688 ± 266 vs. nervous: 1513 ± 455; P>0.1; ).
Figure 4. Mean ( ± SEM) cumulative agitation score during Periods 1 and 2 in calm sheep (black bars) and nervous sheep (grey bars) subjected to isolation stress. Superscripts indicate differences between temperaments or within temperament over time within treatment (P < 0.05). The changes in the mean agitation score over time (10 min intervals) are shown below for Period 1 (left panel) and Period 2 (right panel) for calm sheep (black diamond) and nervous sheep (grey diamond).
Layered stressor
During exposure to the selection stressors, the cumulative agitation score was elevated in both calm and nervous sheep and was not affected by temperament (calm: 2450 ± 1109 vs. nervous: 3923 ± 1031; P>0.1; ). During exposure to the non-selection stressors, the cumulative agitation score remained elevated in calm sheep (Period 1: 2450 ± 1109 vs. Period 2: 1362 ± 464; P>0.1; ) but declined in nervous sheep (Period 1: 3923 ± 1031 vs. Period 2: 492 ± 159; P < 0.05; ). Consequently, the mean agitation score during exposure to the non-selection stressors (Period 2) was higher in calm sheep than in nervous sheep (calm: 1362 ± 464 vs. nervous: 492 ± 159; P < 0.05; ).
Figure 5. Mean ( ± SEM) cumulative agitation score during Periods 1 and 2 in calm sheep (black bars) and nervous sheep (grey bars) subjected to the layered stressor paradigm. The sheep were subjected to isolation combined with a mannequin during Period 1 with the addition of a novel object (blower) and restraint during Period 2. Superscripts indicate differences between temperaments or within temperament over time within treatment (P < 0.05). The changes in the mean agitation score over time (10 min intervals) are shown below for Period 1 (left panel) and Period 2 (right panel) for calm sheep (black diamond) and nervous sheep (grey diamond).
Discussion
Genetic differences in temperament directly affected both the magnitude and dynamics of the responses of the adrenocortical and reproductive endocrine axes of sheep to isolation. Specifically, in nervous sheep, isolation induced a greater increase in cortisol secretion and decrease in LH secretion than that observed in calm sheep. However, our hypothesis that nervous sheep would be more reactive to other stressors that were specific to the selection process was rejected. Instead, we found that the two lines were equally reactive, in terms of their behaviour and cortisol secretion, to isolation combined with stressors that were both specific and non-specific to the selection process.
The cortisol response of nervous sheep to isolation was greater in magnitude and duration than in calm sheep. Indeed, the plasma concentration of cortisol in nervous sheep was elevated above the control period for up to 4 h after initial imposition of the isolation stressor. This observation differed markedly from that reported in sheep not selected for temperament, where the cortisol response to isolation combined with restraint reached a peak within 30–40 min and returned to basal concentrations within 1–2 h of initial imposition of the stressor (Tilbrook et al. Citation1999; Rivalland et al. Citation2005). The sustained elevation in cortisol secretion in nervous sheep exposed to the isolation stressor indicates significant divergence between calm and nervous sheep in the positive feedforward or negative feedback systems within the HPA axis, which may reflect genetic differences in the strategies that they use to cope with stress. The high levels of locomotor's activity and vocalisations of nervous sheep, combined with their large cortisol response to the stress of isolation, indicate that they have developed a pro-active strategy to cope with stress. In contrast, calm sheep are more docile and less reactive to the isolation stressor, indicating a more passive coping strategy (Koolhaas et al. Citation2010).
Little is currently known about the feedforward or feedback mechanisms within the HPA axis of calm and nervous sheep. However, the variation in cortisol pulse amplitude that we observed in the control sheep may allow us to understand the processes involved: pulse amplitude increased briefly over the morning (11 am–1 pm) in calm sheep but not in nervous sheep, then decreased over the afternoon (1–3 pm) in nervous sheep but not in calm sheep. The afternoon coincides with the trough of the postulated circadian rhythm in sheep (Fulkerson and Tang Citation1979) suggesting that, contrary to observations in rodents (Windle et al. Citation1998; Windle et al. Citation2001), hyper-reactivity to isolation in nervous sheep appears to be associated with lower cortisol pulse amplitude during the trough of the 24-h cycle. The basal pattern of cortisol secretion is closely related to the neuroendocrine expression of the stress response (review; Lightman and Conway-Campbell Citation2010), so we propose that the reactivity of the two lines to isolation may be due to innate differences in the pattern of ACTH/cortisol secretion underlying the circadian rhythm.
In nervous sheep, the large and sustained cortisol response to isolation was associated with a rapid and sustained reduction in the frequency and amplitude of LH pulses. The effect of the psychosocial stressor on LH pulse amplitude is supported by previous studies (Breen et al. Citation2007; Wagenmaker et al. Citation2009) and is probably mediated through the same mechanisms reported for sheep that have not been selected for temperament (direct inhibition of GnRH, Breen et al. Citation2004; reduced pituitary sensitivity to GnRH, Breen et al. Citation2007; Wagenmaker et al. Citation2009). However, the relationship between psychosocial stress and changes in cortisol secretion and LH pulse frequency are less clear (Tilbrook et al. Citation1999; Stackpole et al. Citation2006; Pierce et al. Citation2008; Oakley et al. Citation2009). In this study, LH pulse frequency was only reduced by the combination of nervous temperament and the isolation stressor, in which the greatest increase in cortisol secretion was also observed. This relationship between the magnitude of the cortisol response and the suppression of LH pulse frequency supports the observations of Stackpole et al. (Citation2006) even though the plasma concentrations of cortisol in this study (60.1 ± 9.47 ng/ml) were markedly lower than those induced by the ‘low dose’ of exogenous cortisol (118 ± 20 ng/mL; Stackpole et al. Citation2006). The mechanism through which a psychosocial stressor such as isolation affects LH pulse frequency is thus not completely dependent on the adrenocortical axis and requires further investigation. To this end, the magnitude and duration of the cortisol response of nervous sheep to isolation provides a valuable experimental model.
We expected nervous sheep to secrete more cortisol than calm sheep during the first 2 h of the layered stressor paradigm because isolation and human presence are used to select the two lines at weaning. However, there are several reasons why these stressors, as imposed in this study, may not have been truly analogous to those used in the selection process. First, human presence is combined with isolation in an arena during the test at weaning rather than the isolation box, and the sheep are able to move towards or away from the human to gain access to a pen of companion sheep. Second, in this study, a mannequin was used to simulate human presence and, in spite of its attire and appearance, the sheep may not have responded in the same way as they would have to a human. Finally, the door of the isolation box remains closed during the selection test, whereas we opened the door to expose the mannequin and may thus have altered their perception of the stressors. The nature of the stressor clearly affects the reactivity of rodent species to stress (Veenema and Neumann Citation2007), so we propose that calm and nervous sheep did not differ in their response to the ‘selection stressors’ because the presentation of stressors was different from that used in the selection process.
Layering of stressors at hourly intervals has previously been shown to induce a robust and sustained increase in the plasma concentrations of cortisol in sheep (Breen et al. Citation2007; Pierce et al. Citation2008; Wagenmaker et al. Citation2009). However, in this study, both calm and nervous sheep showed signs of acclimatisation to the non-selection stressors within the layered stressor paradigm. This observation was unexpected and may be due to the nature of the stressors selected for this portion of the layered stressor paradigm. Both the noisy white flapping plastic or ‘blower’ (Romeyer and Bouissou Citation1992; CitationBickell 2005) and restraint (Tilbrook et al. Citation1999) are proven stressors of sheep and would be expected to activate the HPA axis. However, the impact of restraint on the HPA axis is relatively transient when it is not combined with additional stressors. For example, the concentration of cortisol typically returned to basal levels within 1–2 h of imposition of restraint, even when sheep were only able to move their head (Tilbrook et al. Citation1999; Rivalland et al. Citation2005). The initial impact of restraint in this study is also likely to have been less than previous studies (Tilbrook et al. Citation1999; Rivalland et al. Citation2005) because the sheep was still able to move its body and legs to some degree. Little is known about the capacity of sheep to acclimatise to the ‘blower’, but the suddenness, novelty and intrinsic unpleasantness of a stressor affect its impact on the physiology of sheep (Desire et al. Citation2004). The ‘blower’ posed no physical threat to the sheep, so we propose that the stressor initially startled the sheep but that both lines of sheep quickly acclimatised to its presence.
With respect to locomotor activity, isolation was associated with a greater agitation score in nervous sheep than in calm sheep, as reported in previous studies (Murphy et al. Citation1994; Bickell et al. Citation2009a,Citationb). However, the agitation score declined over time and did not differ significantly between the two lines during the second half of the isolation stressor. This observation contrasts markedly with the plasma concentrations of cortisol that remained higher in nervous sheep than in calm sheep, indicating a disconnection between the behavioural and neuroendocrine expression of the stress response. This hypothesis is re-enforced by the higher agitation score in calm sheep than in nervous sheep during the second half of the layered stressor paradigm, when cortisol concentrations were similar between the two lines. CitationSarabdjitsingh et al. (2010) reported that pretreatment of rats with corticosterone resulted in a normal neuroendocrine response to a stressor but disturbed the behavioural response compared to control rats. The relationship between behaviour and activation of the HPA axis warrants further investigation.
In conclusion, sheep selected for calm or nervous temperament clearly respond differently to the stress of isolation. Isolation induced greater increases in locomotor activity and cortisol secretion, and a greater decrease in LH secretion, in nervous sheep than in calm sheep. However, when isolation was combined with stressors that were not directly analogous to those used during the genetic selection for temperament, calm and nervous sheep showed similar adrenocortical and reproductive responses to the stressors. The reduction in LH secretion in nervous sheep exposed to the isolation stressor was mediated by reduced LH pulse amplitude and LH pulse frequency, a response that is likely to directly affect reproductive function.
Acknowledgements
This research was supported by operating funds from the EHB Lefroy Fellowship and the School of Animal Biology, University of Western Australia. We thank S. Gray for his assistance in the care and management of the animals, M. Blackberry for her assistance with the cortisol and LH assays and S. Plug for her assistance in data collection.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Barnett JL, Hemsworth PH. 1990. The validity of physiological and behavioural measures of animal welfare. Appl Anim Behav Sci.. 25 1–2: 177–187.
- Beausoleil NJ, Blache D, Stafford KJ, Mellor DJ, Noble ADL. 2008. Exploring the basis of divergent selection for ‘temperament’ in domestic sheep. Appl Anim Behav Sci.. 109 2–4: 261–274.
- Bickell SL. 2005. Calm sheep will be less stressed when faced with a novel, unknown object, Honours thesis, University of Western Australia, Perth, Australia.
- Bickell SL, Nowak R, Poindron P, Sebe F, Chadwick A, Ferguson D, Blache D. Temperament does not affect the overall establishment of mutual preference between the mother and her young in sheep measured in a choice test. Dev Psychobiol.. 2009a; 51 5: 429–438.
- Bickell SL, Poindron P, Nowak R, Chadwick A, Ferguson D, Blache D. Genotype rather than non-genetic behavioural transmission determines the temperament of Merino lambs. Anim Welfare.. 2009b; 18:459–466.
- Bickell SL, Nowak R, Poindron P, Chadwick A, Ferguson D, Blache D. 2011. Challenge by a novel object does not impair the capacity of ewes and lambs selected for a nervous temperament to display early preference for each other. Anim Prod Sci.. 51 6: 575–581.
- Blache D, Ferguson D. 2005. Increasing sheep meat production efficiency and animal welfare by selection for temperament. Sydney: Meat and Livestock Australia.
- Boissy A. 1995. Fear and fearfulness in animals. Q Rev Biol.. 70:165–191.
- Breen KM, Karsch FJ. 2006. New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrin.. 27 2: 233–245.
- Breen KM, Stackpole CA, Clarke IJ, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Young EA, Karsch FJ. 2004. Does the type II glucocorticoid receptor mediate cortisol-induced suppression in pituitary responsiveness to gonadotropin-releasing hormone?. Endocrinology.. 145 6: 2739–2746.
- Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ. 2007. Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress?. Endocrinology.. 148 4: 1882–1890.
- Desire L, Veissier I, Despres G, Boissy A. 2004. On the way to assess emotions in animals: Do lambs (Ovis aries) evaluate an event through its suddenness, novelty, or unpredictability?. J Comp Psychol.. 118 4: 363–374.
- Dobson H, Smith RF. 2000. What is stress, and how does it affect reproduction?. Anim Reprod Sci.. 60-61:743–752.
- Frank E, Salchner P, Aldag JM, Salome N, Singewald N, Landgraf R, Wigger A. 2006. Genetic predisposition to anxiety-related behavior determines coping style, neuroendocrine responses, and neuronal activation during social defeat. Behav Neurosci.. 120 1: 60–71.
- Fulkerson WJ, Jamieson PA. 1982. Pattern of cortisol release in sheep following administration of synthetic ACTH or imposition of various stressor agents. Aust J Biol Sci.. 35 2: 215–222.
- Fulkerson WJ, Tang BY. 1979. Ultradian and circadian rhythms in the plasma concentration of cortisol in sheep. J Endocrinol.. 81:135–141.
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. 2010. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front Neuroendocrin.. 31 3: 307–321.
- Landgraf R, Wigger A, Holsboer F, Neumann ID. 1999. Hyper-reactive hypothalamo-pituitary-adrenocortical axis in rats bred for high anxiety-related behaviour. J Neuroendocrinol.. 11 6: 405–407.
- Lightman S, Conway-Campbell B. 2010. The crucial role of pulsatile activity of the HPA axis for continuous equilibration. Nat Rev Neurosci.. 11:710–718.
- Martin GB, Oldham CM, Lindsay DR. 1980. Increased plasma LH levels in seasonally anovular Merino ewes following the introduction of rams. Anim Reprod Sci.. 3:125–132.
- Martin GB, Scaramuzzi RJ, Lindsay DR. 1983. Effect of the introduction of rams during the anoestrous season on the pulsatile secretion of LH in ovariectomized ewes. J Reprod Fertil.. 67 1: 47–55.
- Merriam GR, Wachter KW. 1982. Algorithms for the study of episodic hormone secretion. Am J Physiol.. 243 4: E310–E318.
- Murphy PM, Purvis IW, Lindsay DR, Le Neindre P, Orgeur P, Poindron P. 1994. Measures of temperament are highly repeatable in Merino sheep and some are related to maternal behaviour. Proc Aust Soc Anim Prod.. 20:247–250.
- Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. 2009. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: Influence of ovarian steroids. Endocrinology.. 150 1: 341–349.
- Pierce BN, Hemsworth PH, Rivalland ET, Wagenmaker ER, Morrissey AD, Papargiris MM, Clarke IJ, Karsch FJ, Turner AI, Tilbrook AJ. 2008. Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Horm Behav.. 54 3: 424–434.
- Rivalland ET, Iqbal J, Clarke IJ, Turner AI, Tilbrook AJ. 2005. Co-localization and distribution of corticotrophin-releasing hormone, arginine vasopressin and enkephalin in the paraventricular nucleus of sheep: A sex comparison. Neuroscience.. 132 3: 755–766.
- Romeyer A, Bouissou MF. 1992. Assessment of fear reactions in domestic sheep, and influence of breed and rearing conditions. Appl Anim Behav Sci.. 43:93–119.
- Sarabdjitsingh RA, Spiga F, Oitzl MS, Kershaw Y, Meijer OC, Lightman SL, de Kloet ER. 2010. Recovery from disrupted ultradian glucocorticoid rhythmicity reveals a dissociation between hormonal and behavioural stress responsiveness. J Neuroendocrinol.. 22 8: 862–871.
- Stackpole CA, Clarke IJ, Breen KM, Turner AI, Karsch FJ, Tilbrook AJ. 2006. Sex difference in the suppressive effect of cortisol on pulsatile secretion of luteinizing hormone in sheep. Endocrinology.. 147 12: 5921–5931.
- Tilbrook AJ, Clarke IJ. 2006. Neuroendocrine mechanisms of innate states of attenuated responsiveness of the hypothalamo-pituitary adrenal axis to stress. Front Neuroendocrin.. 27 3: 285–307.
- Tilbrook AJ, Canny BJ, Serapiglia MD, Ambrose TJ, Clarke IJ. 1999. Suppression of the secretion of luteinizing hormone due to isolation/restraint stress in gonadectomised rams and ewes is influenced by sex steroids. J Endocrinol.. 160 3: 469–481.
- Tilbrook AJ, Turner AI, Clarke IJ. 2000. Effects of stress on reproduction in non-rodent mammals: The role of glucocorticoids and sex differences. Rev Reprod.. 5 2: 105–113.
- Tilbrook AJ, Turner AI, Clarke IJ. 2002. Stress and reproduction: Central mechanisms and sex differences in non-rodent species. Stress.. 5 2: 83–100.
- Tsigos C, Chrousos GP. 2002. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res.. 53 4: 865–871.
- Veenema AH, Neumann ID. 2007. Neurobiological mechanisms of aggression and stress coping: A comparative study in mouse and rat selection lines. Brain Behav Evol.. 70 4: 274–285.
- Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. 2009. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology.. 150 2: 762–769.
- Watson RH, Radford HM. 1960. The influence of rams on the onset of oestrus in Merino ewes in Spring. J Agric Res.. 11:65–71.
- Windle RJ, Wood SA, Lightman SL, Ingram CD. 1998. The pulsatile characteristics of hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology. 139 10: 4044–4052.
- Windle RJ, Wood SA, Kershaw YM, Lightman SL, Ingram CD, Harbuz MS. 2001. Increased corticosterone pulse frequency during adjuvant-induced arthritis and its relationship to alterations in stress responsiveness. J Neuroendocrinol.. 13 10: 905–911.
