Abstract
The concept of stress is relevant to magnetic resonance imaging (MRI) examination in various ways. First, levels of stress to staff and patients have not been quantified in ultra-high magnetic fields. Second, research is increasingly interested in experimentally defining regional brain activity during stress. It is therefore important to know whether exposure to the ultra-high static magnetic fields per se might also lead to neurohormonal responses in the hypothalamus–pituitary–adrenal axis and the sympathoadrenal systems. In the present blinded case cross-over study with 41 healthy participants, we measured cortisol not only before and after but also during static magnetic field exposure in MRI scanners. Measures of catecholamines before and after exposure were also part of the study protocol. Using three different field strengths (1.5, 3 and 7 T) and a mock scanner (0 T), we examined whether not only the MRI procedure but also the static magnetic field per se has an influence on the neuroendocrine responses. We found no significant differences in the course of cortisol or catecholamine concentrations between the different static magnetic fields. Our study suggests that the results of MRI studies using stress-paradigms are not influenced by the static magnetic field itself.
Introduction
‘Stress’ in biological systems can be broadly defined as an actual or anticipated disruption of homoeostasis or an anticipated threat to well-being (Ulrich-Lai and Herman Citation2009). Physiological stress responses in humans involve a coordinated set of reactions that result in removal of the organism from, or adaptation to, the stressful situation. The major constituents of neuroendocrine stress signalling in humans involve the locus coeruleus (norepinephrine/sympathetic nervous system) and the hypothalamus–pituitary–adrenal (HPA) axis. These systems are in turn under the influence of multiple neural pathways coming from both rostral and caudal sources, e.g. the noradrenergic neurons of the nucleus tractus solitarii in the brainstem (Ulrich-Lai and Herman Citation2009; Kudielka and Wüst Citation2010). The effector hormone of the HPA axis is cortisol. It is now broadly accepted that stress throughout the lifespan may be linked to mental illnesses such as depression, psychosis, bipolar and anxiety disorders and cognitive decline, as well as somatic sequelae (e.g. osteoporosis, diabetes, visceral obesity and coronary heart disease) (Björntorp Citation2001; Brown et al. Citation2004; Lupien et al. Citation2009; Goh and Agius Citation2010; Cizza et al. Citation2012).
Magnetic resonance imaging (MRI) procedures are loud, uncomfortable and liable to induce a claustrophobic response and have been reported to cause moderate anxiety in up to 30% of the subjects (Melendez and McCrank Citation1993). Indeed, elevated cortisol levels have been found following MRI scans in young adolescents (Eatough et al. Citation2009) and in adults (Tessner et al. Citation2006). Enhanced stress-induced cortisol levels have been correlated with regional brain activity in the amygdala, hippocampus (Pruessner et al. Citation2005; van Stegeren et al. Citation2007; Lederbogen et al. Citation2011) and prefrontal cortex (Wang et al. Citation2005; Kern et al. Citation2008). Thus, it is conceivable that changes in field- or MR-procedure-dependent, but paradigm-independent neurohormonal responses may influence regional brain activity.
The field strengths of MRI scanners have increased continuously in the last years aiming at an improved signal-to-noise ratio. As a result, possible physiological activation induced by ultra-high static magnetic fields per se might compromise the results of imaging investigations in studies with stress paradigms. Apart from that, safety aspects and the hormonal stress responses related to ultra-high static magnetic fields are of high practical significance for scanner staff that is permanently exposed to such magnetic fields as well as patients.
There is no published evidence indicating that magnetic field strength is associated with a stress response. However, increasing field strengths are associated with an increased occurrence of side effects. There have been reports of occurrence of a metallic taste, nausea as well as vertigo (Schenck et al. Citation1992; Glover et al. Citation2007) and, to a lesser extent, head ringing and nystagmus (Chakeres et al. Citation2003a; de Vocht et al. Citation2006). In a meta-analysis by Heinrich et al., the effects of static magnetic fields on sensory perception were evaluated in all existing studies between 1992 and 2007 that had applied different field strengths ranging from 0.7 T (stray field of a 1.5 T magnet; de Vocht et al. Citation2003) to 8 T (Chakeres et al. Citation2003b); the main findings on sensory perceptions included an increase of dizziness and vertigo (Heinrich et al. Citation2011).
Especially, during movement within high field strengths—such as during medical examinations—side effects have been described, including dizziness, metallic taste and phosphenes. It is conceivable that perception of such sensory effects may affect stress responses; these responses may in turn be mirrored by the changes in peripheral cortisol and catecholamine levels. In this study, dizziness was significantly higher in the 7 T scanner than all other field strengths (p < 0.001) (CitationHeinrich et al. in press). In addition, in order to avoid future legislatory decisions limiting the application of high field strength magnetic tomographs due to the lack of evidence, studies like the present one are important to provide the necessary proof on possible effects (or their absence) of high field strengths on human stress responses.
In this study, we conducted cortisol assessments before, during and after MRI scans. Measures of catecholamines before and after the scans were also part of the study protocol. In addition, we examined whether not only the MRI procedure but also the magnetic field strength per se (1.5, 3 and 7 T and a mock scanner) had an influence on the neuroendocrine responses of the participants.
Material and methods
Study population
We included 41 healthy participants between the ages 18 and 34 years (25.6 ± 0.58 years; 21 males and 20 females). Before any examinations took place, a semi-standardized interview was carried out in order to exclude current or past psychiatric diagnoses (DSM-IV). All participants underwent a thorough physical examination during a first screening visit. Their educational level varied from 12 to 18 years of education (13.2 ± 1.2 years). Participants were also given a thorough neuropsychological examination during the screening visit using the Cambridge neuropsychological test automated battery (http://www.camcog.com) and an intelligence test [the ‘Hamburg Wechsler Intelligenztest für Erwachsene—revised edition (HAWIE-R)’]. To minimise possible stressful reactions as well as learning effects, all tasks later presented during the testing procedure in the scanners were practiced during the screening visit. Following tasks were performed: line bisection, pursuit aiming (eye–hand coordination); Freiburg visual acuity and contrast test, visual discrimination, visual tracking (visual system); N-back (working memory), digit span (short-term memory), recognition memory (long-term memory); attention network task and simple reaction time (attention and reaction) (CitationHeinrich et al. in press). Exclusion criteria were the following: irremovable metal objects inside or on the body, mental or physical illness, amblyopia and red-green blindness, pregnancy, current cannabis use (validation by cannabis test in urine) and IQ < 80. None of the participants had previous exposure to MRI procedures. The study was approved by the ethics committee of the Medical Faculty Heidelberg and participants signed informed consent.
Procedures
We instructed all participants to rest well and refrain from consuming alcohol on the night before the examinations. All tests were conducted at the German Cancer Research Center in Heidelberg where three MR systems with different field strengths (1.5, 3 and 7 T) were available. During the examinations, the participants were exposed only to the static magnetic field. A mock scanner consisting of the bore and cabin of a disused MRI scanner with non-static magnetic field was set up as the control condition. Pumping noise in the mock scanner was generated by speakers. No switching gradients and no radiofrequency pulses were applied.
Participants were blindfolded before entering the building and scanner room to ensure that they were unaware of the scanner and of the strength of the static magnetic field in which they would be tested. Participants were placed inside the scanner still wearing the blindfold which was only removed when the final examination position had been reached. Additionally, all signs indicating the field strength were covered before each testing day. We removed the blindfold in the changing rooms (between tests). At the end of every testing session, we asked participants to rate the probability that they had been in a 0, 1.5, 3 or 7 T condition to test the efficacy of our blinding procedure.
All participants were tested in each of the four scanner conditions with a 1-week interval in between. The order of scanners was randomised to eliminate order and learning effects. Each testing session consisted of two runs of approximately 50 min each, in which the effects of the different field strengths on cognitive functions were assessed by means of neuropsychological tests (Heinrich et al., unpublished observations). The first run was conducted while participants were lying still inside the scanner, and the second run was carried out while the examination table was moved back and forth by a motor connected to the examination table. The movement condition always followed the static condition in order to avoid possible negative effects of movement on well-being that might lead to impairment in the subsequent tests. The details of this 4 (field strength) × 2 (movement state) case cross-over design are displayed in .
Figure 1. A synopsis of the study protocol including the baseline visit and the sequence of events during the experimental procedure [beginning with the (1) cortisol sample followed by exposition to and then movement in static magnetic fields and ending with the (7) cortisol sample]. Each study participant took part in the experimental procedure in four different conditions of the magnetic field (0, 1.5, 3, 7 T); the order of the conditions was random.
![Figure 1. A synopsis of the study protocol including the baseline visit and the sequence of events during the experimental procedure [beginning with the (1) cortisol sample followed by exposition to and then movement in static magnetic fields and ending with the (7) cortisol sample]. Each study participant took part in the experimental procedure in four different conditions of the magnetic field (0, 1.5, 3, 7 T); the order of the conditions was random.](/cms/asset/a36a724c-4402-4ff0-8b0e-0b2d0b47b753/ists_a_708949_f0001_b.gif)
At the beginning of each testing day, participants first had to provide a saliva sample for measurement of cortisol (saliva sample no. 1; time point: 0 min). They were then asked to complete questionnaires assessing mood and well-being and were medically examined, followed by a second saliva sample as well as blood collection for assessment of catecholamines (saliva sample no. 2, blood sample no. 1; time point: 10 min). Following this, participants were placed in the scanner (approximately 5 min) and after an additional 5-min interval without any intervention in the scanner, the third saliva sample was collected (saliva sample no. 3; time point: 20 min). Next, the neuropsychological tests were performed while the participants were lying still on the examination table (approximately 50 min). At the end of the tests, and before leaving the scanner, another saliva sample was collected (saliva sample no. 4; time point: 70 min). After a short break of approximately 15 min, participants were again placed inside the scanner (5 min). A saliva sample was collected after a 5-min interval in the scanner under movement conditions (saliva sample no. 5; time point: 100 min). The participants again participated in all neuropsychological tests during movement of the examination table (approximately 50 min). At the end of this second run, saliva was collected (saliva sample no. 6; time point: 150 min). After leaving the scanner, participants again answered questionnaires indicating which side effects or sensory perceptions occurring during the examination were medically examined and had to rate the probabilities for each field strength (20 min). Afterwards, the last saliva sample was collected, and blood was drawn for the assessment of catecholamines (saliva sample no. 7, blood sample no. 2; time point: 170 min). An electrocardiography (ECG) was recorded during the whole procedure.
Due to logistic reasons (41 participants × 4 scanner conditions × 1-week interval between scanners), we carried out part of the examinations in the early afternoon. Thus, while part of the procedures was started at 8 am (n = 24; 14 females and 10 males), the remaining trials were initiated at 1 pm (n = 17; 7 females, 10 males). All participants were examined either in the morning or in the afternoon with the exception of two subjects whose testing took part both in the morning and in the afternoon due to subject-related scheduling conflicts. These two participants had originally been randomised to be examined in the morning hours, and only their examinations taking place in the morning were later introduced in our statistical analysis.
Medical examination
We carried out a heart and lung auscultation and a neurological examination during the screening visit and repeated the neurological examination on the experimental days (before and at the end of the experimental procedure). The neurological examination included the cranial nerves (II, III, IV, V, VI, VII, XI, XII), including a test for eyesight and red-green sight, pupillomotor responses and assessment of nystagmus, as well as a cursory strength (bicep muscle, toe walk and heel walk) and sensory examination, the evaluation of reflexes (biceps, brachioradialis, knee and ankle), Babinski sign, cerebellar functions (rapid alternating movements, finger-to-nose testing and Romberg sign) and gait.
Field strength exposure
In the static condition, participants were positioned with their heads in the iso-centre of the respective MR system (1.5, 3 and 7 T), and during the movement condition the examination table was moved back and forth in a sinusoidal motion with a maximum deflection of 20 cm at the point of the highest spatial changing of the magnetic field (highest magnetic gradient of the specific MR system; 1.5 T: 823 mm, 3 T: 917 mm and 7 T: 1383 mm distance to iso-centre). The strength of the magnetic field was reduced to about 60% at this point and this resulted in 942, 1828 and 4414 mT, respectively. Maximum speed of movement in all scanners (0, 1.5, 3 and 7 T) was V max = 9.2 cm/s; in the 1.5, 3 and 7 T scanners, this resulted in a maximum temporal change of magnetic field of dB/dt = 0.8 T/s.
Laboratory assays
Salivary cortisol measurements were acquired as a reliable indicator of total free plasma cortisol (Kirschbaum and Hellhammer Citation1994). Saliva samples were obtained using Salivettes (Sarstedt, Leicester, UK) which contained an untreated cotton swab. All samples were stored at − 20°C. After thawing, the samples were centrifuged for 5 min at 3000 rev/min, resulting in a clear supernatant of low viscosity. Salivary cortisol was measured by means of a time-resolved immunoassay with fluorescence detection. The lower limit of detection was 0.43 nmol/l, with interassay and intraassay coefficients of variation of less than 10% across the expected range of cortisol levels.
Blood samples were immediately centrifuged (10 min at 4000 rev/min) for separation of serum and were stored in aliquots at − 20°C. After completion of the study, samples were shipped deep frozen on dry ice to the laboratory (Labor Limbach, Heidelberg, Germany) for quantification of epinephrine and norepinephrine concentrations using a commercially available radioimmunoassay (RIA). The assay procedure comprises extraction and acylation of samples followed by a competitive RIA (Labor Diagnostika Nord, Nordhorn, Germany) for final quantification.
Statistical analyses
For catecholamines, we used the repeated measures analysis of covariance (rm-ANCOVA) in order to assess changes in catecholamine concentrations within each field strength; in this case, the ‘pre- and post-scan catecholamine concentrations’ constituted the two-level repeated measures factor, ‘gender’ and ‘time point of the investigation’ (am vs. pm) were the fixed factors and ‘age’ was the covariate. The rm-ANCOVA with two repeated measures factors was applied for the evaluation of catecholamine value differences between the magnetic field strengths; in this case, the four ‘field strengths’ depicted the first four-level repeated measures factor and the ‘pre- and post-scan catecholamine concentrations’ and the second two-level repeated measures factor, while ‘gender’ and ‘time point of the investigation’ (am vs. pm) were the fixed factors and ‘age’ was the covariate.
In order to examine mean cortisol value differences within the respective magnetic field strength, we applied a generalised linear model with repeated measures instead of a repeated measures analysis of variance (rm-ANOVA), as the time intervals between cortisol samples varied in length throughout the experimental procedure (); in this case, the saliva ‘cortisol value’ was the dependent variable, the corresponding ‘time point of cortisol sampling’ (in minutes) was an independent variable, ‘gender’ and ‘time point of the investigation’ (am vs. pm) were factors and the ‘age’ of the participants was a covariate. The generalised linear model with repeated measures was also applied for evaluation of value differences between the different magnetic field strengths; in this case, the ‘cortisol value’ was the dependent variable, the corresponding ‘time point of cortisol sampling’ (in minutes) was an independent variable, the four ‘field strengths’ were the within-subject factor, ‘gender’ and the ‘time point of the investigation’ (am vs. pm) were factors and ‘age’ was the covariate.
Effect sizes for each one of the cortisol (no. 1 to no. 7) and catecholamine (no. 1 and no. 2 for norepinephrine and epinephrine, respectively) samples in each field strength were determined as the mean difference in cortisol (or catecholamine) concentrations between corresponding sample numbers in a specific (1.5 or 3 or 7 T) field strength and the reference field strength divided by the standard deviation (SD) found in the reference field strength for the same cortisol (or catecholamine) sample number ( = Cohen' s d; Cohen Citation1988). The cortisol and catecholamine concentrations during the procedures in 0 T served as the reference.
All analyses were carried out using SAS 9.2 and PASW 18.
Results
Medical examination
In our group of participants, no influence of the different magnetic field strengths on the results of the medical examination could be observed.
Cortisol
In each of the field strengths, we noticed a significant change in the cortisol concentrations from samples 1–7 (0 T: Z = − 6.70, p = 0.02; 1.5 T: Z = − 10.04, p < 0.0001; 3 T: Z = − 8.43, p < 0.0001; 7 T: Z = − 6.43, p < 0.0001); this change was shown to be a decrease, probably due to habituation to the experimental procedure. The ‘time point of investigation’ (am vs. pm) had a significant main effect (p < 0.0001) in all analyses. ‘Age’ had no impact, while there was a significant effect of ‘gender’ in the case of 1.5 T (Z = 2.88, p < 0.01) and 7 T (Z = 1.96, p = 0.05).
In the simultaneous analysis of the four magnetic field strengths using the generalised linear model, we found again a significant decrease in cortisol concentrations from samples 1 to 7 (Z = − 8.67, p < 0.0001) and a significant effect of the ‘time point of investigation’ (Z = 7.86, p < 0.0001). ‘Age’ and ‘gender’ had no significant impact on this simultaneous analysis. Moreover, there was no significant curve progression deviation between the fields 1.5, 3 and 7 T compared to the 0 T condition (1.5 vs. 0 T: Z = 1.39, p = 0.17; 3 vs. 0 T: Z = 1.73, p = 0.08; 7 vs. 0 T: Z = 1.16, p = 0.25).
As shown in , the changes in cortisol concentrations from samples no. 1 to no. 7 in the whole study cohort and in all field strengths mirror on average a decrease in cortisol concentrations. Despite this decrease in mean values, we have found a varying fraction of subjects showing an increase in their cortisol responses. The percentage of subjects displaying such an increase in cortisol concentrations ranges from 0% (0 T, am) to 31.3% (3 T, pm).
Figure 2. The am mean values of saliva cortisol concentrations (samples 1–7) during exposure to the different static magnetic field strengths (0, 1.5, 3 and 7 T). A decrease in the cortisol response from samples 1 to 7 in all fields can be seen, probably due to habituation of the participants to the experimental procedure. Error bars indicate SDs.
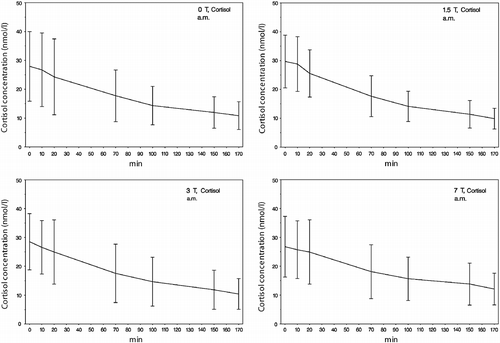
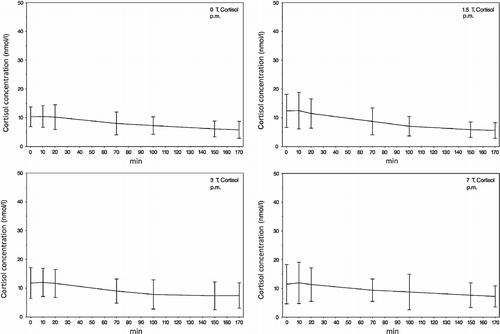
Figure 3. The pm mean values of saliva cortisol concentrations (samples 1–7) during exposure to the different static magnetic field strengths (0, 1.5, 3 and 7 T). A decrease of the cortisol response from samples 1 to 7 in all fields can be seen, probably due to habituation of the participants to the experimental procedure. Error bars indicate SDs.
Catecholamines
We found no significant main effect of ‘Δcatecholamine’ (Δ: pre- vs. post-scan differences) for norepinephrine and epinephrine within each field strength with the exception of norepinephrine concentrations during the 7 T experiments showing a pre-/post-level of significance p = 0.02, which was shown to represent a decrease. The ‘time point of investigation’ (am vs. pm) had no main effects. In detail, the results were as follows: 0 T—ΔNorepinephrine: F 1,35 = 0.36, p = 0.55; main effect ‘age’: F 1,35 = 6.60, p = 0.02; ΔEpinephrine: F 1,35 = 1.58, p = 0.22; 1.5 T—ΔNorepinephrine: F 1,36 = 1.71, p = 0.20; ΔEpinephrine: F 1,36 = 0.94, p = 0.34; main effect ‘gender’: F 1,36 = 12.95, p = 0.001; 3 T—ΔNorepinephrine: F 1,35 = 0.52, p = 0.48; ΔEpinephrine: F 1,35 = 0.81, p = 0.38; 7 T—ΔNorepinephrine: F 1,36 = 6.18, p = 0.02; ΔEpinephrine: F 1,36 = 0.00, p = 0.99; main effect ‘age’: F 1,36 = 6.51, p = 0.02; main effect ‘gender’: F 1,36 = 4.30, p = 0.05. Results are displayed in .
Figure 4. Pre- and post-scan (T1 and T2) concentrations of the catecholamines norepinephrine and epinephrine in the four scanner conditions (0, 1.5, 3 and 7 T), separately for am and pm. The analysis of variance showed no significant main effect of ‘Δcatecholamine’ (Δ: pre- vs. post-scan differences) for norepinephrine and epinephrine within each field strength with the exception of norepinephrine concentrations during the 7 T experiments showing a pre-/post-level of significance p = 0.02. Error bars indicate SDs.
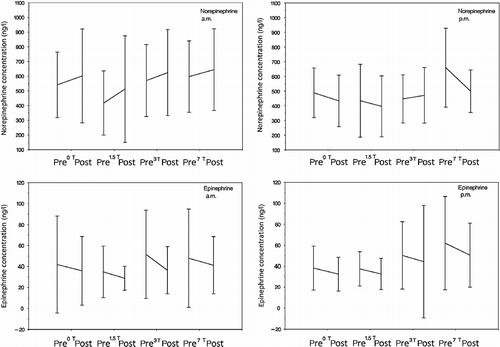
Looking at the individual curves, we found that subjects' catecholamines responses were variable. While the majority of subjects showed a decrease in catecholamines during the procedures, we found a minimum of 12.5% (0 T, norepinephrine, pm) and a maximum of 56.0% (7 T, norepinephrine, am) of subjects showing an increase.
Using the rm-ANOVA with two repeated measures factors (see ‘statistics’) in order to compare catecholamine pre-/post-scan level changes between the different field strengths, we found neither a significant effect of ‘Δcatecholamine’ nor any effect of ‘field strength’. In detail, the results are as follows: ΔNorepinephrine: F 1,34 = 1.99, p = 0.17; main effect of ‘field strength’: F 3,34 = 0.71, p = 0.55; main effect of ‘age’: F 1,34 = 4.28, p = 0.05; ΔEpinephrine: F 1,34 = 0.07, p = 0.80; main effect of ‘field strength’: F 1,34 = 0.62, p = 0.44; main effect of ‘gender’: F 1,34 = 4.96, p = 0.03).
Discussion
In summary, we found that cortisol saliva concentrations decreased during the course of the static field exposure in all scanners involved. We attribute the observed cortisol changes, which were similar in all field strengths and the mock scanner, in part to cortisol's circadian rhythm, as well as to the participants' habituation to the experimental procedure. This decrease was significant in all scanners.
However, by comparing all field strengths, we found that the scanner effect per se (0 vs. 1.5 or 3 or 7 T) had no significant influence on cortisol concentrations. With regard to the catecholamines, we found a significant pre-/post-decrease only in norepinephrine concentrations at the highest field strength (7 T). Again, neither the presence/absence nor the strength of the field had any significant effect on secretion of catecholamines. As all procedures (cortisol and blood sampling, neuropsychological tasks, etc) were strictly standardised in all scanners including the 0 T condition, any detected significant effects would have been attributed to the only altering condition, the field strength per se. Thus, the conclusion can be drawn that in our experimental setting a static magnetic field up to 7 T itself does not significantly increase cortisol or catecholamine concentrations in healthy participants.
The different time point of cortisol sampling (am vs. pm) was introduced in all statistical analyses as a confounding variable. The ‘time point of the investigation’ had significant main effects (p < 0.0001) on the analyses of cortisol both within and between field strengths' exposure, which is not surprising given that cortisol secretion has a pronounced circadian rhythm. However, the ‘time point of investigation’ had no effect, whatsoever on the analyses of catecholamines. Taking the cortisol and catecholamine concentrations during the procedures in 0 T as the reference, we have calculated effect sizes for each one of the cortisol (no. 1 to no. 7) and catecholamine (no. 1 and no. 2 for norepinephrine and epinephrine, respectively) samples in each field strength. In the case of cortisol, we found low effect sizes that ranged from a minimum of 0.01 to a maximum of 0.21. In the case of catecholamines, effect sizes were somewhat higher and could be considered low to moderate, ranging from 0.11 to 0.39 in the case of epinephrine and from 0.01 to 0.5 in the case of norepinephrine. Taken together, our null findings concerning the effect of field strength on cortisol and catecholamine levels are supported by the findings of low (to moderate) effect sizes for each of the samples in each field strength.
Indeed, technical MRI specifications may elicit anxiety and increased neuroendocrine responses. In our set of experiments, minor sensory effects occurred also in the mock scanner (0 T), a fact that argues for an effect of a crowded and noisy scanner environment per se on the participants' subjective perception of side effects (Heinrich et al. Citation2011, unpublished observations), which in turn may lead to stress responses. In order to assess the effect of field strength per se on neuroendocrine responses, we applied strictly standardised procedures in the scanning environment. We did not use cramped head coils in our experiments. Subjects were wearing the same type of earphones and had their head lying directly on the scanner examination table. The neuropsychological tasks were presented on a monitor seen via a mirror, which in turn was secured to the examination table by flexible retaining clips. Thus, different experimental procedures (e.g. using cramped head coils) may cause additional stress responses, but this was not part of our investigational protocol. Moreover, participants were not able to guess the field strength they were tested in (Heinrich et al., unpublished observations).
In contrast, to previous studies on the subject, we present cortisol data collected before, after and during exposure to a static magnetic field. Furthermore, advantages of this study are the large study sample, balanced gender ratio and the acquisition of catecholamine concentrations in addition to cortisol. To date, there are no studies comparing different magnetic fields—including a real control condition in a mock scanner—with regard to possible influences on the neuroendocrinological stress responses of the examined individuals. Besides, in our experimental paradigm, there was no noticeable influence of the field strength on body functions as shown in neurological examinations before and after the experimental procedures.
In accordance with our results, a recent study by Peters et al. (Citation2011) examined acute effects of MRI scans by acquiring cortisol measurements immediately preceding and following the scan in comparison with basal cortisol levels. Repeated MRI scans were used to test novelty effects. The authors reported that the MRI experience is stressful, particularly for the initial scan, but the stress response was reduced in subsequent scans. Elevated cortisol levels have also been found following an MRI scan in young adolescents (Eatough et al. Citation2009) and in adults (Tessner et al. Citation2006).
In research on mental disorders, stress paradigms are increasingly employed in conjunction with brain imaging methods such as MRI or functional MRI (Cannistraro and Rauch Citation2003; Lederbogen et al. Citation2011). The non-invasive neuroimaging of a subjects' brain in response to stress-inducing psychological stimuli can be utilised in clinical studies to predict individual stress reactivity. Given that ultra-high-field MRI systems are becoming more widely used—providing shorter testing times and improved resolution—it is important to specifically analyse the effects of these scanners on the neuroendocrinological stress response.
Our single blinded study design with three different scanners (1.5, 3 and 7 T) and a mock scanner should be an efficient and thorough method to analyse possible effects of static magnetic fields on the stress axis of humans. Our data suggest that the results of MRI studies using stress-paradigms are not influenced by the static magnetic field itself. However, as the study was carried out on healthy young subjects, results may differ in older or psychiatric cohorts that are considerably less stress-resilient and where neuroendocrinological stress responses may be altered due to the psychiatric condition per se (Ising et al. Citation2005; Bradley and Dinan Citation2010). Future studies should attempt to clarify how gradients and high frequency (HF)-fields could influence neurohormonal responses of participants during MRI examinations. Furthermore, our results are of high practical relevance for the further development of interventional MRI and for scanner staff (doctors, nurses etc) who are exposed to high magnetic field strengths in corresponding facilities.
Declaration of interest The work was supported, in part, by the federal ministry for the Environment, Nature Conservation and Nuclear Safety (StSch 30,009). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Björntorp P. 2001. Do stress reactions cause abdominal obesity and comorbidities?. Obes Rev. 2 2: 73–86.
- Bradley AJ, Dinan TG. 2010. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: Implications for mortality. Psychopharmacology. 24 4: 91–118.
- Brown ES, Varghese FP, McEwen BS. 2004. Association of depression with medical illness: Does cortisol play a role?. Biol Psychiatry. 55:1–9.
- Cannistraro PA, Rauch SL. 2003. Neural circuitry of anxiety: Evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 37 4: 8–25.
- Chakeres DW, Bornstein R, Kangarlu A. Randomized comparison of cognitive function in humans at 0 and 8 Tesla. J Magn Reson Imaging. 2003a; 18 3: 342–345.
- Chakeres DW, Kangarlu A, Boudoulas H, Young DC. Effect of static magnetic field exposure of up to 8 Tesla on sequential human vital sign measurements. J Magn Reson Imaging. 2003b; 18 3: 346–352.
- Cizza G, Ronsaville DS, Kleitz H, Eskandari F, Mistry S, Torvik S, Sonbolian N, Reynolds JC, Blackman MR, Gold PW, Martinez PE. 2012. Clinical subtypes of depression are associated with specific metabolic parameters and circadian endocrine profiles in women: The POWER study. PloS One. 781 e28: 912.
- Cohen J. 1988. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.07642.
- de Vocht F, van-Wendel-de-Joode B, Engels H, Kromhout H. 2003. Neurobehavioral effects among subjects exposed to high static and gradient magnetic fields from a 1.5 Tesla magnetic resonance imaging system-a case-crossover pilot study. Magn Reson Med. 50 4: 670–674.
- de Vocht F, van Drooge H, Engels H, Kromhout H. 2006. Exposure, health complaints and cognitive performance among employees of an MRI scanners manufacturing department. J Magn Reson Imaging. 23 2: 197–204.
- Eatough EM, Shirtcliff EA, Hanson JL, Pollak SD. 2009. Hormonal reactivity to MRI scanning in adolescents. Psychoneuroendocrinology. 34:1242–1246.
- Glover PM, Cavin I, Qian W, Bowtell R. 2007. Gowland. Magnetic-field-induced vertigo: A theoretical and experimental investigation. Bioelectromagnetics. 28 5: 349–361.
- Goh C, Agius M. 2010. The stress-vulnerability model how does stress impact on mental illness at the level of the brain and what are the consequences?. Psychiatr Danub. 22 2: 198–202.
- Heinrich A, Szostek A, Nees F, Meyer P, Semmler W, Flor H. 2011. Effects of static magnetic fields on cognition, vital signs, and sensory perception: A meta-analysis. J Magn Reson Imaging. 34:758–763.
- Heinrich A, Szostek A, Meyer P, Nees F, Rauschenberg J, Gröbner J, Gilles M, Paslakis G, Deuschle M, Semmler W, Flor H, Cognition and sensation in ultra-high static magnetic fields—a randomized case-crossover study with several scanners up to 7 Tesla (in press in Radiology).
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. 2005. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 29:1085–1093.
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. 2008. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 33:517–529.
- Kirschbaum C, Hellhammer DH. 1994. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 19:313–333.
- Kudielka BM, Wüst S. 2010. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus–pituitary–adrenal axis activity and reactivity. Stress. 13 1: 1–14.
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wust S, Pruessner JC, Rietschel M, Deuschle M, Meyer-Lindenberg A. 2011. City living and urban upbringing affect neural social stress processing in humans. Nature. 474:498–501.
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 10:434–445.
- Melendez JC, McCrank E. 1993. Anxiety-related reactions associated with magnetic resonance imaging examinations. JAMA. 270:745–747.
- Peters S, Cleare AJ, Papadopoulos A, Fu CH. 2011. Cortisol responses to serial MRI scans in healthy adults and in depression. Psychoneuroendocrinology. 36:737–741.
- Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lord C, Meaney M, Lupien S. 2005. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 28:815–826.
- Schenck JF, Dumoulin CL, Redington RW, Kressel HY, Elliott RT, McDougall IL. 1992. Human exposure to 4.0-Tesla magnetic fields in a whole-body scanner. Med Phys. 19:1089–1098.
- Tessner KD, Walker EF, Hochman K, Hamann S. 2006. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp. 27:889–895.
- Ulrich-Lai YM, Herman JP. 2009. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 10:397–409.
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. 2007. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem. 87:57–66.
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. 2005. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA. 102:17804–17809.
