Abstract
Stress is known to lead to metabolic and behavioral changes. To study the possible relationships between stress and dietary intake, male Sprague-Dawley rats were fed one of three diets for 6 weeks: high carbohydrate (HC), high fat (HF), or “Cafeteria” (CAF) (Standard HC plus a choice of highly palatable cafeteria foods: chocolate, biscuits, and peanut butter). After the first 3 weeks, half of the animals from each group (experimental groups) were stressed daily using a chronic variable stress (CVS) paradigm, while the other half of the animals (control groups) were kept undisturbed. Rats were sacrificed at the end of the 6-week period. The effects of stress and dietary intake on animal adiposity, serum lipids, and corticosterone were analyzed. Results showed that both chronic stress and CAF diet resulted in elevated total cholesterol, increased low-density lipoprotein (LDL), and lower high-density lipoprotein (HDL). In addition, increases in body weight, food intake, and intra-abdominal fat were observed in the CAF group compared with the other dietary groups. In addition, there was a significant interaction between stress and diet on serum corticosterone levels, which manifest as an increase in corticosterone levels in stressed rats relative to non-stressed controls in the HC and HF groups but not in the CAF group. These results show that a highly palatable diet, offering a choice of food items, is associated with a reduction in the response to CVS and could validate a stressor-induced preference for comfort food that in turn could increase body weight.
Introduction
Stress is defined as a state of threatened homeostasis that is restored by a complex repertoire of physiological and behavioral adaptive responses (Chrousos Citation1998). Stresses can give rise to changes in eating behavior in humans as well as in animals. In humans, it was shown that stress can lead to hyperphagia (nibbling, bulimia) as well as hypophagia (Morley et al. Citation1983). In rodents, stress is known to inhibit food intake both acutely and chronically (Armario et al. Citation1984, Citation1990; Marti et al. Citation1994; Meerlo et al. Citation1996; Rybkin et al. Citation1997; Diane et al. Citation2008).
Chronic application of the same stressor induces habituation in rodents (Dal-Zotto et al. Citation2000; Marin et al. Citation2007; Stewart et al. Citation2008), leading to a diminution of behavioral and physiological responses to the stressor, including attenuated stress-induced hypophagia (Krahn et al. Citation1990). Chronic variable stress (CVS) is a model in which variable acute stress paradigms are applied unpredictably over several weeks. Traditionally, CVS has served as a model of depression in rodent studies (Katz Citation1982; Willner Citation2005). In addition, it is considered as a relevant stress model designed to be resistant to habituation, since it induces chronic hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, as indicated by persistent elevated corticosterone levels (Rai et al. Citation2003; Marin et al. Citation2007). Furthermore, CVS is known to systematically decrease body weight gain (Ulrich-Lai et al. Citation2006; Marin et al. Citation2007). In rats or mice, CVS leads to anhedonia, characterized by a decrease in consumption of a palatable sweet solution (measured by a sucrose preference test) (Willner Citation1997).
Dietary characteristics can also influence the adaptation to chronic stress. Intake of palatable food is known to dampen stress responses (Adam and Epel Citation2007), especially when a choice of food items is offered (la Fleur et al. Citation2005). There appears to be a link between palatable “comfort” food and positive emotional feelings (sense of security, well-being). Indeed, it has been demonstrated that comfort foods improve stress symptoms in rats exposed to different types of acute or chronic stresses (Ulrich-Lai et al. Citation2007; Foster et al. Citation2009; Maniam and Morris Citation2010). For example, palatable diets were shown to improve anxiety and depression-like symptoms following an adverse early environment in rats (Maniam and Morris Citation2010). Similarly, in humans, increased consumption of sweet and fatty foods during stress was found to reduce the stress response (Epel et al. Citation2001; Zellner et al. Citation2006).
The objective of this study was to evaluate the effect of the availability of a palatable diet, as compared with monotonous high-fat or standard balanced diets, on the response to CVS in rats. We used a CVS model in which rats were exposed sequentially, over a period of weeks, to a variety of mild stressors. We compared three types of diet: a standard high-carbohydrate diet, a high-fat diet, and a cafeteria diet (Shafat et al. Citation2009). The ‘Cafeteria’ diet refers to the offering of a number of ‘cafeteria’ type foods (high fat, energy, and sugar contents) (Rothwell and Stock Citation1988; Shafat et al. Citation2009). We chose the cafeteria diet because of its greater palatability and its lack of monotony when compared with the other diets.
Materials and methods
Animals and diets
Forty-eight adult male Sprague-Dawley rats (Lebanese American University Stock) aged initially 8–10 weeks old were housed in groups of four in a temperature and humidity-controlled room under a 12:12 light/dark cycle (lights on at 08:00 h) during the whole length of the experiment. The animals were weight-matched and assigned to one of the three following dietary groups: standard high-carbohydrate (HC), high-fat (HF), or cafeteria diet (CAF; n = 16 per group). The cafeteria diet consists of the standard high-carbohydrate and a choice of highly palatable cafeteria-style foods with known caloric intake: chocolate, biscuits, and peanut butter (). The diets were given ad libitum for 6 weeks. All experimental protocols were approved by the Animal Ethical Committee of the Lebanese American University, which complies with the Guide for the Care and Use of Laboratory Animals (National Research Council of the United States Citation2011). Food intake and body weight gain were monitored three times a week throughout the study. Food intake, corrected for spillage, including individual CAF diet items, was recorded at 8 a.m. by measuring the difference in food cup weight before and after presentation to the rats.
Table I. Nutrient composition of the HC and HF diets and of cafeteria diet food items.
CVS procedure
After the first 3 weeks of diet, rats from each dietary group were again divided into two groups after being weight-matched (n = 8): control and stressed. Controls were kept undisturbed in their home cages during the following 3 weeks of treatment, and the others were stressed daily for the remaining period of the study using a CVS model. This stress model was modified from existing models (Duncko et al. Citation2001; Gamaro et al. Citation2003) and consisted of a 21-day variable stressor paradigm during which different individual stressors were applied each day for different lengths of time (one stressor per day). The following stressors were used: (a) cage tilt for 3 or 4 h (home cages were tilted to 30° from the horizontal); (b) space reduction for 4 or 5 h (eight rats were placed in a collective cage usually designed for four to five rats, dimensions 50 cm × 40 cm × 21 cm); (c) restraint for 1–3 h (rats were placed into a perforated plastic tube of 6 cm × 20 cm in which they could not turn over); (d) forced swimming for 10 min in cold water (five rats were placed together in a plastic tank, dimensions 100 cm height × 50 cm diameter containing 75 cm of water at 18°C) or in (e) warm water (same procedure at 26°C); (f) flashing light for 3 or 4 h (flashes at 40 W with frequency of 5 flashes/s); and (g) neighbor cages (move to an unclean unoccupied neighbor cage for 14 h). Animals remained on their diets throughout stress exposure. Stress application started at different times every day in order to minimize its predictability. reports the detailed stressor schedule.
Table II. Schedule of stressor agents used during the chronic stress treatment.
Sucrose preference test
A sucrose preference test was conducted every week to assess hedonic behavior and monitor the effectiveness of the CVS model (Willner Citation1997; Duncko et al. Citation2001). The test consists of a 12-h period of water deprivation, after which animals are exposed to two bottles: one containing water and the other a 1% sucrose solution. The amount of sucrose solution ingested during the subsequent 2 h, corrected for body weight, represents the hedonic behavior. The sucrose preference test was initiated for three sessions prior to the start of the stress procedure and was conducted in the animals' home cages.
Blood collection and abdominal fat dissection
The rats were fasted overnight at the end of the CVS procedure. The following morning they were deeply anesthetized with pentobarbital (100 mg/kg), blood was drawn from the inferior vena cava 5–6 min after the induction of anesthesia, and the rats were then euthanized by exsanguination. The intra-abdominal fat (epididymal, mesenteric, and retroperitoneal) was subsequently removed and weighed.
Serum lipid and serum corticosterone analysis
The blood samples were centrifuged at 2000g for 15 min. The collected serum was then stored at − 80°C for subsequent analysis. Blood lipid analysis (total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides) was performed using Spinreact kits (Spinreact, Girona, Spain). Serum corticosterone levels were determined using the Double Antibody 125I Radioimmunoassay Kit for rats and mice (MP Biomedicals, LLC, Solon, OH, USA). All samples were run in duplicate and analyzed within the same assay.
Statistical analysis
Body weight gain and dietary intake data were analyzed separately using a three-way ANOVA, with treatment (no stress vs. stress) and diet (HC vs. HF vs. CAF) as the between-subject factors and time as the within-subjects factor.
Sucrose preference data, abdominal fat, serum corticosterone, and blood lipids were analyzed using a two-way ANOVA with treatment (no stress vs. stress) and diet (HC vs. HF vs. CAF) as factors. Descriptive data are presented as means ± standard error of the mean (SEM). Significant main effect differences were tested using Tukey–Kramer's post-hoc test for multiple comparisons. All data were analyzed using the SPSS 18 statistical package, with statistical significance defined as p < 0.05.
Results
Energy intake
There was a significant effect of diet on energy intake (F(2,47) = 4.25, p = 0.02). Animals fed the CAF diet had significantly higher energy intake normalized to their body weight (224.2 ± 9.9 kJ/100 g rat/day) compared with animals fed the HC (176.5 ± 6.8 kJ/100 g rat/day) or HF (152.5 ± 8.8 kJ/100 g rat/day) diets. Among stressed animals, energy intake was significantly affected by time (F(1,46) = 4.72, p = 0.035): stressed animals ate significantly less during the stress period (167.2 ± 8.5 kJ/100 g rat/day) compared with the baseline feeding period before stress (193.4 ± 7.1 kJ/100 g rat/day; ).
Figure 1. Energy intake (kJ/rat/day) of rats fed a high-carbohydrate, high-fat, or cafeteria diet and either submitted to a CVS paradigm or not during weeks 3–6. Data are expressed as the mean ± SEM. *Statistical difference with regard to the first 3 weeks. aStatistical difference with regard to the high-carbohydrate dietary group in the same stress condition. p < 0.05 is considered significant. Note: there was no significant difference between the energy intake of stressed and non-stressed animals.
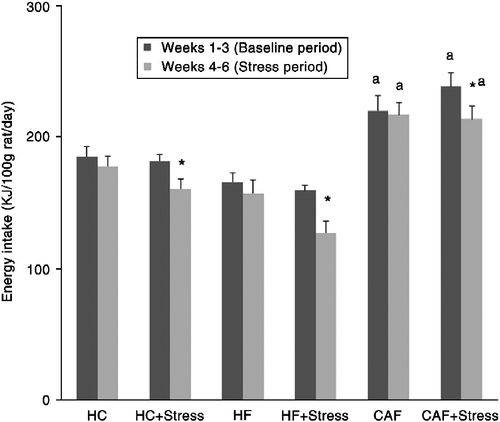
When comparing the average energy intake normalized to bodyweight from the cafeteria items, it appears that rats were consuming the biscuits the most, followed by HC food, chocolate, and peanut butter. There was no significant effect of stress on the consumption of any CAF item. However, within stressed animals, there was a significant effect of time on the consumption of peanut butter (F(1,5) = 34.7, p = 0.002), chocolate (F(1,5) = 7.6, p = 0.05), and standard HC diet (F(1,5) = 16.2, p = 0.013). Stressed animals consumed significantly less of these three items during the 3-week stress period compared with the 3-week baseline period ().
Table III. Energy intake (kJ/rat/day) from different cafeteria items of rats fed a cafeteria diet and either submitted to a CVS paradigm or not during weeks 4–6.
Sucrose preference
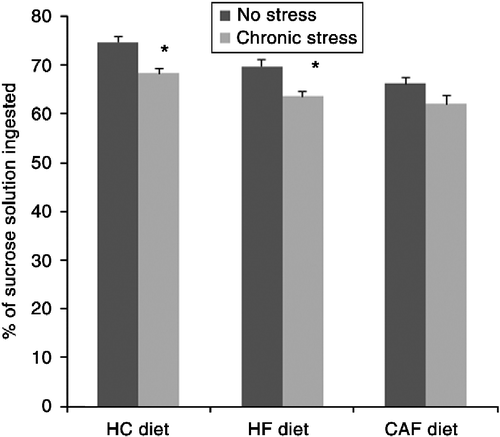
Stressed animals showed a decreased consumption of sucrose solution corrected for body weight (9.42 ± 1.3 ml/100 g body wt.) compared with non-stressed rats (14.7 ± 1.4 ml/100 g body wt.) F(1,35) = 4.1, p = 0.05. Similarly, sucrose preference, that is, the percentage of sucrose solution from the total liquid ingested, was significantly decreased in stressed animals compared with sucrose preference in controls (64.6% ± 1.3 vs. 70.1% ± 1.2). However, when looking at individual dietary groups, the percentage of sucrose solution ingested was only significantly lower in stressed animals of the HC (t(10) = 2.2, p = 0.04) and HF groups (t(10) = 2.4, p = 0.05) compared with their corresponding non-stressed groups (). In contrast, sucrose preference was not significantly affected by diet nor was there a significant interaction effect between treatment and diet.
Figure 2. Percentage of sucrose solution from the total liquid ingested during 2 h in rats fed a high-carbohydrate, high-fat, or cafeteria diet and either submitted to a CVS paradigm or not (n = 8). Data represent the average of three sucrose preference tests conducted during weeks 4–6 of the experiment. Results are expressed as mean ± SEM. *Statistical difference with regard to the non-stressed group having the same diet. p < 0.05 is considered significant.
Body weight and body composition
The final body weight of the rats was significantly affected by diet (F(2,47) = 3.9, p = 0.029), with CAF-fed animals having significantly higher body weights than both the HC and HF groups (401.5 ± 7.3 g for the CAF group vs. 376.2 ± 7.3 g for the HC, and 377 ± 7.3 g for the HF; ). There was no effect of stress on the final body weight of rats. However, daily body weight gain of the stressed animals (all dietary groups together) was significantly affected by time (F(1,47) = 6.4, p = 0.015). Indeed, stressed rats had a significantly lower daily weight gain during the 3-week stress period (2.3 ± 0.2 g/rat/day) compared with the 3-week baseline period without stress (3.9 ± 0.2 g/rat/day).
Table IV. Body weights, body weight gain, and abdominal fat for rats fed a standard chow, high-fat, or cafeteria diet and either submitted to a CVS paradigm or not (n = 8).
Intra-abdominal fat weight was significantly affected by diet (F(2,47) = 12.1, p < 0.001). Animals fed the cafeteria diet had significantly higher intra-abdominal fat percent compared with high-carbohydrate-fed (t(30) = 4.5, p < 0.001) and high-fat-fed animals (t(30) = 3.4, p = 0.002). There was no significant effect of stress on intra-abdominal fat (F(1,47) = 0.03, p = 0.87).
Serum lipids
Total serum cholesterol levels were significantly affected by stress (F(1,43) = 7.6, p = 0.009) as well as diet (F(2,43) = 3.1, p = 0.05; ). Stressed animals had significantly higher serum cholesterol levels than non-stressed animals (112.6 ± 6.6 vs. 87.7 ± 6.2 mg/dl). Also, cholesterol levels of animals in the CAF group and in the HF group (107.6 ± 7.4 and 109.4 ± 7.4 mg/dl) were significantly higher than the level of HC-fed animals (83.5 ± 8.6 mg/dl), t(26) = 2, p = 0.05 and t(26) = 1.9, p = 0.05, respectively. There was, however, no significant interaction between diet and stress.
Table V. Serum lipid parameters for rats fed a standard chow, high-fat, or cafeteria diet and either submitted to a CVS paradigm or not (n = 8).
LDL cholesterol levels were significantly affected by both diet (F(2,43) = 4.1, p = 0.023) and stress (F(1,43) = 8.9, p = 0.005). LDL was higher in CAF- and HF-fed animals (68 ± 7.5 and 69.1 ± 7.5 mg/dl) compared with the HC group (38.8 ± 8.8 mg/dl), t(26) = 2.23, p = 0.034 and t(26) = 2.34, p = 0.027, respectively. In addition, stressed animals had significantly higher LDL levels than non-stressed animals (72.4 ± 6.7 compared with 44.9 ± 6.3 mg/dl, p = 0.005). There was no significant interaction of diet and stress with regard to LDL levels. There was no significant effect of stress on HDL cholesterol levels. However, HDL was affected by diet F(2,40) = 3.7, p = 0.034, with CAF-fed animals having significantly lower HDL levels compared with HC-fed rats (39.6 ± 2.1 vs. 48.2 ± 2.6 mg/dl), t(25) = 2.2, p = 0.037.Serum triglyceride levels were not affected by dietary treatment (F(2,42) = 0.6, p = 0.80) or stress (F(1,42) = 0.2, p = 0.11).
Corticosterone levels
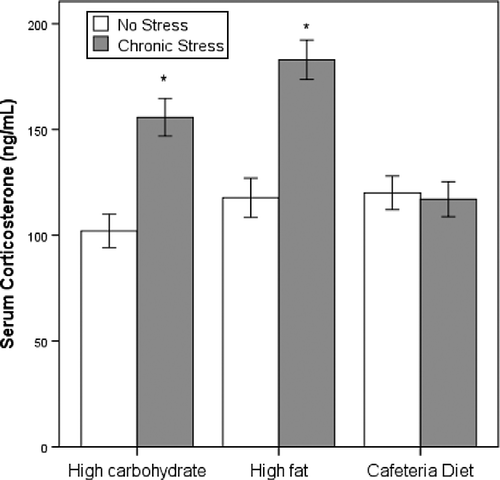
There was a significant interaction effect between stress and diet on rat serum corticosterone levels (F(2,47) = 8.9, p = 0.001; ). Stressed animals (all diets included) showed increased serum corticosterone (152 ± 4.9 ng/ml) levels compared with non-stressed animals (113.3 ± 4.9 ng/ml) (F(1,47) = 30.1, p < 0.001). However, the corticosterone levels were normalized in cafeteria-fed animals: there was no significant difference between the corticosterone levels of stressed compared with non-stressed animals fed the cafeteria diet.
Figure 3. Serum corticosterone concentration (ng/ml) of rats fed a standard chow, high-fat, or cafeteria diet and either submitted to a CVS paradigm or not (n = 8). Data are expressed as the mean ± SEM. *Statistical difference with regard to the non-stressed group having the same diet. p < 0.05 is considered significant.
Discussion
The objective of this study was to investigate the influence of different diets on the response to stress. CVS and the CAF diet were associated with worsened lipid profiles. Moreover, a significant interaction was found between diet intake and stress, as demonstrated by decreased serum corticosterone levels in CVS animals on the CAF diet. These findings suggest a reduction in the response to CVS following palatable food consumption.
Animals fed the cafeteria diet had higher body weight and intra-abdominal fat percentages compared with the other dietary groups. Body weight and fat depot, particularly intra-abdominal fat, were already shown to increase after access to a palatable diet containing lard and sucrose (Foster et al. Citation2009). In our study, the CAF diet offers a choice of foods, thereby increasing its palatability and justifying the increase in body weight and fat. CAF- and HF-fed animals had higher total cholesterol and LDL cholesterol levels compared with the other animals. Similar effects of HF (Manting et al. Citation2011) and cafeteria diets (Sugatani et al. Citation2008) on blood lipids have previously been described. CAF-fed animals showed lower HDL cholesterol levels as well. The cafeteria diet therefore worsened the blood lipid profile of animals to a greater extent than the high-fat feeding. This difference is probably due to the higher overall consumption of the CAF diet, owing to its increased palatability as well as its higher saturated and trans-fat content.
Chronic exposure to mild unpredictable stress decreases the consumption of palatable sweet solutions (Willner et al. Citation1987; Willner Citation1997; Duncko et al. Citation2001; Wang et al. Citation2010). As expected, in our experiment, CVS induced anhedonia, manifested by decreased sucrose preference relative to non-stressed rats. However, when considering the CAF groups alone, the difference between the stressed and non-stressed groups was not significant. Similar results regarding sucrose preference were found in a study investigating the effects of palatable foods on stress in rats Maniam and Morris (Citation2010). CVS models are known to induce chronic hyperactivity of the HPA axis, a conclusion supported by persistent elevated corticosterone levels (Rai et al. Citation2003; Marin et al. Citation2007). Increased corticosterone was also observed in our study, verifying the effectiveness of the stress regimen. In addition, among stressed animals, both food intake and body weight gain were lower during the stress period compared with the control period. Our results are in accordance with other studies that show a reduction in body weight gain and food intake in response to CVS (Gamaro et al. Citation2003; Solomon et al. Citation2010). Rats from the CAF groups were consuming biscuits the most, followed by standard HC food, chocolate, and finally peanut butter. Similar consumption patterns were found for these items in other studies (Shafat et al. Citation2009). Food preference was not based on sugar content, since the biscuits and HC diet are lower in sugar relative to chocolate. The preference for biscuits over chocolate could be due to their texture. In addition, stressed animals consumed significantly less of the standard HC diet, chocolate, and peanut butter during the 3-week stress period compared with the 3-week baseline period but maintained the same biscuit consumption, indicating a CVS-related change in preference.
Stressed animals had higher total cholesterol and LDL levels than non-stressed animals, with similar HDL and TG levels. These results agree with a recent study showing that chronic mild unpredictable stress across a 3-week period leads to an increase in rat LDL cholesterol and TG levels but no modification in HDL levels (Neves et al. Citation2009). In addition, a recent study investigating the effects of HF diets on blood lipid profiles (Manting et al. Citation2011) found that HF diets and chronic stress (once a day) act together and induce abnormalities in blood and tissue lipids referred to as the lipid metabolism disorder. Exposure to both a HF diet and chronic stress was found to induce even higher levels of cholesterol, TG, and liver TG than exposure to a HF diet only. Conversely, in a recent study by Paternain et al. (Citation2011), a milder form of the chronic stress regimen used in our study (stressors administered three times a week) did not cause any changes in the plasma lipid profile of rats fed a standard or cafeteria diet. This could suggest the existence of a stress intensity threshold for CVS-related dyslipidemia.
There was a significant interaction between stress and diet on serum corticosterone levels. There was an increase in corticosterone levels of stressed rats compared with non-stressed rats in all the groups except the cafeteria-fed group. Therefore, CVS causes an increase in corticosterone, but this increase is attenuated by the CAF diet. These findings are also in agreement with the recent study by Maniam and Morris (Citation2010) in which a cafeteria diet was shown to reduce anxiety and depression-like symptoms in rats following exposure to an adverse environment (maternal separation). Similarly, another study demonstrated that the consumption of a highly palatable and highly caloric diet (chocolate) prevented an increase in adrenal gland weight after exposure to chronic restraint stress in rats (Fachin et al. Citation2008). Finally, a study on mice revealed that the consumption of a high-fat and high-sugar diet was associated with decreased corticosterone levels in mice exposed to restraint stress (Kuo et al. Citation2008). The results of all these studies are in agreement with our own, suggesting that palatable food consumption reduces the overall impact of stress. The fact that the CAF diet was not only palatable but also offered a choice of items compared with the high-fat diet, which might be of great importance in our results. Similar results were found with acute restraint stress in rats. Indeed, it was shown that rats provided with a choice of lard or sucrose demonstrated an inhibition of ACTH and corticosterone responses, whereas rats on a monotonous high-fat diet did not experience such changes after a 1-week dietary exposure (la Fleur et al. Citation2005). It must be noted that the relatively high baseline corticosterone values obtained in our experiment may be due to both the pentobarbital injected [previously shown to increase serum corticosterone levels in rats (Vahl et al. Citation2005)] and the actual act of injecting it.
The mechanism by which there is an interaction between stress response and diet palatability could be through an attenuation of the HPA axis stress response. The HPA axis is a conductor for stress responses, but is also tightly intertwined with the endocrine regulation of appetite. Stress and palatable food can stimulate endogenous opioid release. It was proposed that in turn, opioid release could be part of a powerful defense mechanism protecting from the detrimental effects of stress by decreasing the activity of the HPA axis and thus attenuating the stress response (Adam and Epel Citation2007). In this context, it was shown in rats that a daily limited consumption of sugar or a non-caloric sweetener leads to a decrease in corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Furthermore, sucrose intake was found to attenuate restraint-induced c-fos mRNA expression in the basolateral amygdala, infralimbic cortex, and claustrum of rats (Ulrich-Lai et al. Citation2007). Further experiments measuring neurotransmitter levels need to be conducted in order to better understand the underlying mechanism that leads to decreased corticosterone levels and whether or not the CAF diet interferes with the sympathetic nervous system.
Evidence suggests that the time of food availability could also play a role in affecting food intake and corticosterone response. Indeed, a recent study in rat shows that intermittent palatable food access induces a different corticosterone response to acute restraint stress than that seen following regular access (Kinzig et al. Citation2008). Future work could therefore study the effects of administering the cafeteria diet items discontinuously during the CVS paradigm. Also, a high-sucrose dietary group could be included to account for the higher sucrose content of the CAF diet compared with the HC and HF diets.
This study showed a decreased response to CVS in rats fed a cafeteria diet compared with both a standard high-carbohydrate diet and a monotonous high-fat diet. A cafeteria diet seems to interact with chronic stress to prevent an increase in corticosterone levels. While chronic stress alone tends to lower adiposity and increase corticosterone levels, and while a palatable cafeteria-type diet alone does not lead to changes in corticosterone levels, the combination of both leads to an attenuation of the stress-induced corticosterone upsurge. This interaction could validate a stressor-induced preference for comfort food. Furthermore, these mechanisms, determined in rats, may explain some of the obesity epidemic occurring in our society, especially given the relationship between socioeconomic conditions and obesity, which may be related to social stress (Wardle et al. Citation2010). A review article by Dallman et al. (Citation2003) has already suggested that people opt for comfort foods that are rich in fat and sugar in an attempt to reduce the activity of the chronic stress-response network. However, habitual use of these foods results in abdominal obesity, type II diabetes, cardiovascular disease, and stroke.
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adam TC, Epel ES. 2007. Stress, eating and the reward system. Physiol Behav. 91 4: 449–458.
- Armario A, Marti J, Gil M. 1990. The serum glucose response to acute stress is sensitive to the intensity of the stressor and to habituation. Psychoneuroendocrinology. 15:341–347.
- Armario A, Ortiz R, Balasch J. 1984. Effect of crowding on some physiological and behavioral variables in adult male rats. Physiol Behav. 32:35–37.
- Chrousos GP. 1998. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann NY Acad Sci. 851:311–335.
- Daher CF, Baroody KG, Baroody GM. 2006. Effect of Urticadioica extract intake upon blood lipid profile in the rats. Fitoterapia. 77 3: 183–188.
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. 2003. Chronic stress and obesity: A new view of “comfort food”. Proc Natl Acad Sci USA. 100 20: 696–11–701.
- Dal-Zotto S, Marti O, Armario A. 2000. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behav Brain Res. 114:175–181.
- Diane A, Victoriano M, Fromentin G, Tome D, Larue-Achagiotis C. 2008. Acute stress modifies food choice in Wistar male and female rats. Appetite. 50:397–407.
- Duncko R, Brtko J, Kvetnanský R, Jezová D. 2001. Altered function of peripheral organ systems in rats exposed to chronic mild stress model of depression. Cell Mol Neurobiol. 21 4: 403–411.
- Epel E, Lapidus R, McEwen B, Brownell K. 2001. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 26 1: 37–49.
- Fachin A, Silva RK, Noschang CG, Pettenuzzo L, Bertinetti L, Billodre MN, Peres W, Busnello F, Dalmaz C. 2008. Stress effects on rats chronically receiving a highly palatable diet are sex-specific. Appetite. 51 3: 592–598.
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. 2009. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 150 5: 2325–2333.
- Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C. 2003. Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int. 42 2: 107–114.
- Katz RJ. 1982. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol Biochem Behav. 16:965–968.
- Kinzig KP, Hargrave SL, Honors MA. 2008. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 95 1-2: 108–113.
- Krahn DD, Gosnell BA, Majchrzak MJ. 1990. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 27:1094–1102.
- Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnanský R, Zukowska Z. 2008. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann NY Acad Sci. 1148:232–237.
- la Fleur SE, Houshyar H, Roy M, Dallman MF. 2005. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 146 5: 2193–2199.
- Maniam J, Morris MJ. 2010. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 35 5: 717–728.
- Manting L, Haihong Z, Jing L, Shaodong C, Yihua L. 2011. The model of rat lipid metabolism disorder induced by chronic stress accompanying high-fat-diet. Lipids Health Dis. 28 10: 153.
- Marin MT, Cruz FC, Planeta CS. 2007. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 90:29–35.
- Marti O, Marti J, Armario A. 1994. Effects of chronic stress on food intake in rats: Influence of stressor intensity and duration of daily exposure. Physiol Behav. 55:747–753.
- Meerlo P, De Boer SF, Koolhaas JM, Daan S, Van den Hoofdakker RH. 1996. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiol Behav. 59:735–739.
- Morley JE, Levine AS, Rowland NE. 1983. Minireview. Stress-induced eating. Life Sci. 32:2169–2182.
- National Research Council. 2011. Guide for the Use and Care of Laboratory Animals. Washington, D.C., WA: NIH Publication.
- Neves VJ, Moura MJ, Tamascia ML, Ferreira R, Silva NS, Costa R, Montemor PL, Narvaes EA, Bernardes CF, Novaes PD, Marcondes FK. 2009. Proatherosclerotic effects of chronic stress in male rats: Altered phenylephrine sensitivity and nitric oxide synthase activity of aorta and circulating lipids. Stress. 12 4: 320–327.
- Paternain L, García-Diaz DF, Milagro FI, González-Muniesa P, Martinez JA, Campión J. 2011. Regulation by chronic-mild stress of glucocorticoids, monocyte chemoattractant protein-1 and adiposity in rats fed on a high-fat diet. Physiol Behav. 103 2: 173–180.
- Rai D, Bhatia G, Sen T, Palit G. 2003. Comparative study of perturbations of peripheral markers in different stressors in rats. Can J Physiol Pharmacol. 81:1139–1146.
- Rothwell NJ, Stock MJ. 1988. The cafeteria diet as a tool for studies of thermogenesis. J Nutr. 118 8: 925–928.
- Rybkin ZhouYII, Volaufova J, Smagin GN, Ryan DH, Harris RB. 1997. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol. 273 5 Pt 2: R1612–R1622.
- Shafat A, Murray B, Rumsey D. 2009. Energy density in cafeteria diet induced hyperphagia in the rat. Appetite. 52 1: 34–38.
- Solomon MB, Jankord R, Flak JN, Herman JP. 2010. Chronic stress, energy balance and adiposity in female rats. Physiol Behav. 102 1: 84–90.
- Stewart LQ, Roper JA, Young WSIII, O'Carroll AM, Lolait SJ. 2008. Pituitary-adrenal response to acute and repeated mild restraint, forced swim and change in environment stress in arginine vasopressin receptor 1b knockout mice. J Neuroendocrinol. 20:597–605.
- Sugatani J, Osabe M, Wada T, Yamakawa K, Yamazaki Y, Takahashi T, Ikari A, Miwa M. 2008. Comparison of enzymatically synthesized inulin, resistant maltodextrin and clofibrate effects on biomarkers of metabolic disease in rats fed a high-fat and high-sucrose (cafeteria) diet. Eur J Nutr. 47 4: 192–200.
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. 2007. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 148 4: 1823–1834.
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. 2006. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 291:E965–E973.
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. 2005. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 289 5: E823–E828.
- Wang SS, Yan XB, Hofman MA, Swaab DF, Zhou JN. 2010. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neurosci Bull. 26 4: 297–303.
- Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. 2010. Stress and Adiposity: A Meta-Analysis of Longitudinal StudiesObesity (Silver Spring) (e-publication ahead of print).
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. 1987. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 93 3: 358–364.
- Willner P. 2005. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 52:90–110.
- Willner P. 1997. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology. 134 4: 319–329.
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. 2006. Food selection changes under stress. Physiol Behav. 87 4: 789–793.
