Abstract
This study was undertaken to elucidate possible relationships between menstrual cycle stage, neuroticism and behavioral and physiological responses to a cognitive challenge. The study investigated the differences between high neuroticism and low neuroticism groups across the menstrual cycle (luteal, menstrual and ovulatory stages). The Stroop color-naming task was used as a stressor. During the task, the galvanic skin response (GSR), heart rate (HR) and HR variability (HRV) were simultaneously recorded by a polygraph. The results showed a significant difference in reaction times (RT) on the Stroop task between the high- and low-neuroticism groups during menstruation. However, there were no significant RT differences between groups during the luteal or ovulatory cycle stages. The GSR of the high-neuroticism group during menstruation was significantly lower than it was in the luteal and ovulatory stages. Moreover, during menstruation, the cardiovascular responses (high-frequency HRV (HF) and low-frequency HRV (LF)) and accuracy on the Stroop task were positively correlated, while the correlations between HF, LF and the RT were negative. The results demonstrate that during menstruation, there were consistent variations in female behavior and physiology when facing a cognitive stressor. Specifically, the high-neuroticism group was more sensitive to the stressor than the low neuroticism group, with decreased reaction time on the Stroop task, and increased GSR and HRV.
Introduction
Stress responses have been defined as an individual’s psychological and physiological responses in the face of a threatening and challenging environment for which a response is required (Healey & Picard, Citation2005). Signs of a stress response are increased activation of the sympathetic nervous system (SNS) and the hypothalamic–pituitary–adrenocortical (HPA) axis. Increase in heart rate (HR), blood pressure (BP) and other markers can be triggered by an elevation in catecholamine release, caused by stimulation of the SNS (Nater & Rohleder, Citation2009; Schwabe & Wolf, Citation2009). According to the cognition-evaluation theory of a stress response, the main impact of stress on an individual relates to the individual’s evaluation of the stressor, meaning that faced with the same stressor, individuals with different personalities react differently (Conway et al., Citation2012).
Previous studies have shown that under stress, personality influences not only an individual's behavioral response (Plessow et al., Citation2011) but also the characteristics and extent to which the individual’s endocrine stress response system is activated (Derijk & de Kloet, Citation2008). Hence, blunted cortisol responses are associated with higher neuroticism in women and with lower extraversion in men (Oswald et al., Citation2006). Moreover, the effect of personality on physiology under stress may be expressed through reactions and restoration of activity of the cardiovascular system (Hutchinson & Ruiz, Citation2011). Bibbey et al. (Citation2012) conducted an experiment on 352 participants to explore the effects of personality on physiological responses to acute psychological stress. They found that cortisol and HR reactivity to stress were negatively associated with neuroticism but positively associated with agreeableness and openness. The above studies indicate that when investigating the effect of stress on an individual’s physiological responses, the neuroticism factor should be taken into consideration. Thus, this study explored physiological differences when facing a stressor between individuals who differ in neuroticism. The goal was to establish specific correlations between the performance on the stress task and physiological activity.
Furthermore, studies emphasizing an individual’s physiological responses under stress have often been controversial due to the factor of gender. The complication lies in controlling the influence of hormonal changes during the menstrual cycle. Litschauer et al. (Citation1998) conducted an experiment on the effect of sex and menstrual cycle stage on individual stress responses and found that physiological differences between men and women when facing stressors were not significant. Similar results were found by Sato & Miyake (Citation2004), whose data revealed only menstrual cycle differences rather than gender differences. Specifically, sympathetic nervous activity in the luteal phase was significantly greater than in the follicular phase, whereas parasympathetic nervous activity was predominant in the follicular phase. However, Goldstein et al. (Citation2010) used functional magnetic resonance imaging (FMRI) to investigate the same issue and concluded that females are endowed with a natural hormonal capacity to regulate the stress response that differs from that of males (Goldstein et al., Citation2010). Another study, which also used FMRI, reported similar findings of a neural mechanism that may mediate increased stress-sensitivity during the late luteal phase (Ossewaarde et al., Citation2010).
Hence, some differences between individuals are dependent on the stressor used, while the HPA and SNS responses show marked and consistent differences according to gender across the menstrual cycle. Ordaz & Luna (Citation2012) have summarized that sex differences in physiological reactivity to acute psychological stress change directionality over development. Therefore, further studies assessing the effect of the menstrual cycle on individual stress responses are needed. Furthermore, Schallmayer & Hughes (Citation2010) showed in a study on the impact of oral contraception and neuroticism on cardiovascular stress reactivity across the menstrual cycle, the usefulness of controlling for personality when studying cardiovascular reactivity. Therefore, the menstrual cycle and personality were chosen as the pivotal factors in our study of differences in stress responses between individuals.
This study combined behavioral and physiological indices, reflecting adrenomedullary and SNS activation, to measure responses to a stressor in women. Such activation can be measured by recording HR, BP and other physiological indices (Hlavacova et al., Citation2008). Previous studies have mainly measured HPA axis (cortisol) responses and behavior performance to reflect individuals’ physiological and behavioral activities under stress, and less frequently behavior, HR and other SNS indices (Derijk & de Kloet, Citation2008; Kudielka & Wust, Citation2010; Oswald et al., Citation2006; Suridjan et al., Citation2012). For this study, we considered measurement of SNS and adrenomedullary responses to be more appropriate than HPA measurements because of the short duration of the stressor (Verhasska et al., Citation2004).
Weidner & Helmig (Citation1990) used cognitive tasks to induce stress responses with the aim to investigate cardiovascular stress reactivity and mood during the menstrual cycle. Their findings indicated that hormonal variations characteristic of the luteal and follicular phases did not influence commonly used measures of cardiovascular stress reactivity. McKinley et al. (Citation2009) found that compared to the ovulatory stage, women showed reductions in HR and a rise in HR variability (HRV) during the luteal period, while Sherwood et al. (Citation2010) found that SNS activity varied in the face of psychological or physiological stressors in participants at different stages of the menstrual cycle. However, another study showed no specific correlation between the menstrual cycle stage and SNS activity (Jonassaint et al., Citation2009). Hence, in this study, we focused on the variations in indicators of SNS and adrenomedullary responses to an acute stressor across the menstrual cycle. The physiological measures of SNS and adrenomedullary activation used in our experiment were the galvanic skin response (GSR) and the electrocardiogram (ECG). The GSR is a measure of the electrical resistance of the skin, which is proportional to sweat secretion and acts as an indicator of sympathetic activation during stress (Jo et al., Citation2012). HRV is the beat-to-beat variation in HR, which has been used as a biomarker of autonomic nervous system activity associated with mental stress (Zhong et al., Citation2005). Spectral analysis of HRV was computed for cardiac parasympathetic modulation, sympathetic modulation and sympathovagal balance (Simplicio et al., Citation2012). The sympathetic activity changes across seconds, whereas the parasympathetic activity changes more rapidly across milliseconds (Lane et al., Citation2009). The high-frequency (HF) bandwidth of HR occurs at 0.15 Hz ∼ 0.40 Hz and is accepted as mainly representing the flexibility of vagal (parasympathetic) tone. The low-frequency bandwidth (LF) of HR occurs at 0.04 Hz ∼ 0.15 Hz and is a product of both sympathetic and parasympathetic activity. The LF/HF ratio of R–R variability is used to represent the balance between sympathetic and parasympathetic activity (Appelhans & Luecken, Citation2006).
Our main objective was to explore variation in SNS activity of high- and low-neuroticism females in the face of a stressor at different menstrual cycle stages. The Eysenck personality questionnaire (EPQ) was used to divide women into high and low neuroticism groups. The Stroop color-naming task was used as a psychological stressor to trigger a stress response during the luteal, menstrual and ovulatory cycle stages. GSR, HR and HRV were simultaneously recorded by a polygraph. The behavioral responses and physiological indices were combined to represent the correlation of SNS activities and cognitive performance under stress. We hypothesized that the cognitive performance of women, when facing a stressor, would be influenced by their neuroticism levels. Moreover, we hypothesized that the physiological responses of women to the stressor would differ across their menstrual cycle.
Methods
Participants
Ninety-six female undergraduates between the ages of 23 and 27 years (mean ages, M ± SD, 24.9 ± 0.24 years) were recruited to this study. The participants were recruited via flyers in the University and campus network. Exclusion criteria were being currently pregnant or lactating, taking oral contraceptives or being under medical treatment. The final sample comprised 27 participants.
All participants provided written informed consent to participate in this experiment. Experimental procedures were approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neurosciences and Learning of Beijing Normal University. The study was performed in accordance with the Declaration of Helsinki.
Design
The study employed a mixed-factorial design. The EPQ short scale for Chinese (EPQ–RSC) was used to assess participants’ neuroticism (Qian et al., Citation2000). We divided the participants into different groups based on their scores on the neuroticism subscale of the EPQ–RSC. The norm of the neuroticism scale of EPQ–RSC was 4.81. Therefore, those participants with scores from 5 to 10 on neuroticism were placed in the high neuroticism group and those participants with scores from 0 to 4.81 (norm), together with scores on psychoticism (another subscale of EPQ–RSC) lower than 3 formed the low neuroticism group. Inclusion criteria also included having no physical or psychological problems. In the final sample of 27 participants, 11 participants were assigned to the high-neuroticism group (neuroticism scores, M ± SD, 7.00 ± 1.61), and 16 participants were assigned to the low-neuroticism group (neuroticism scores, M ± SD, 0.56 ± 0.81). Before the experiment, we used basic information about the menstrual cycle to test the menstrual regularity of the participants. Based on the physiological character of the menstrual cycle, the test dates occurred during menstruation (days 1–4), ovulation (days 11–13) and the luteal stage (days 21–22). All of the 27 participants accepted the physiological (GSR and ECG) and psychological tests (Stroop color-naming test) during each stage, hence all women were tested three times on 3 days during the menstrual cycle across a 2-month timeframe. The study used a repeated-measures protocol (thus ensuring that between-menstrual stage differences were not affected by sampling heterogeneity), with order of testing by cycle stage counterbalanced across participants (thus obviating order effects, such as habituation). For menstrual cycle stage verification, we obtained prospective self-reports about when their menstruation started.
Participants had no color blindness or family history of psychiatric disorders and had normal or corrected-to-normal vision. Participants also took the Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) (Wang et al., 1999) at three different stages. No participant showed any clinical symptoms of anxiety or depression (total anxiety scores <45 and total depression scores <10). Strenuous exercise was avoided before participants entered the laboratory.
Instruments
Eysenck personality questionnaire
The EPQ short scale for Chinese (EPQ–RSC) has four subscales: extraversion/introversion (E), neuroticism/stability (N), psychoticism/socialization (P) and lying (L). There are 12 items in each subscale and 48 items in total. This scale has good reliability and validity according to the evaluation results of the Committee of Psychometrics, Chinese Psychology Society (Qian et al., Citation2000). Internal consistency as measured by Cronbach’s alpha values were 0.75 (E), 0.77 (N), 0.54 (P) and 0.75 (L).
Stroop color-naming task
The E-prime software program (Psychology Software Tools, Pittsburg, PA) was used to run the Stroop color-naming task, and we simultaneously collected physiological data from the participants with a MP150 multipurpose polygraph. The classical Stroop color-naming task used the words “red” and “green” as word stimuli and red and green as font colors. Participants were required to name the color of the word correctly and as quickly as possible. Accuracy rate (ACC) and response time (RT) were recorded simultaneously. There were two conditions: in the conflicted condition, the font color of “red” was green; in the consistent condition, the font color of “green” was green. There were 240 trials in total with 120 trials in each condition. Each trial was presented for 1500 ms. The trial interval was 500 ms with a white fixation point “+” in the middle of the black screen. Participants were asked to respond with the right index and middle fingers. Fingers were randomly matched with colors before experiments. The formal experiment took place after practice. Reaction time outliers were filtered using a <150 and >1000 ms cut-off, and subsequent removal of all RTs exceeding 3.0 SD from the mean.
Procedure
The laboratory was kept clean and quiet. All experimental sessions were conducted between 14:00 h and 18:00 h in a temperature-controlled (comfortable value of approximately 25 °C) dimly lit room. Physiological activity data (GSR, HR and HRV) were collected using the BIOPAC MP150 system polygraph (Biopac Systems, Inc., Goleta, CA) (Hussain et al., Citation2012). The physiological recordings in the baseline, stress and recovery periods were included as part of the experiment, which lasted for 15 min. Participants completed informed consent forms, EPQ–RSC and self-report emotion scales (BAI and BDI) before the recordings of their physiology activity. Then, the participants were asked to relax and keep their eyes closed for 5 min, while physiological data were collected under the resting-state (baseline period). Next, the Stroop color-naming task was conducted for 5 min, during which physiological indices were recorded (stress period). Finally, participants were asked to relax and keep their eyes closed for another 5 min. This was the recovery period during which physiological activity was expected to return to the baseline levels.
Data recording
Physiological activities were collected via a MP150 system amplifier module, which included specific modules for the acquisition, conversion, amplification and storage of signals. We used digital filtering to extract the wave bands of interest. The bipolar electrodes that were used to collect the GSR data were attached to the index and middle fingers of the left hand (VIN+ and VIN–), respectively. The amplifier gain of the GSR was 5 µmho/V, the high-pass filter was DC and the low-pass filter was 1 Hz. The sample rate was 250 Hz and the units were µmho. The HR of each participant was determined on the basis of the R–R interval that was measured from the ECG signals. Ground was connected to the right lower limb, the VIN+ lead was connected to the left lower limb and the VIN– lead was connected to the left upper limb. The amplifier gain was 500, the high-pass filter was 0.5 Hz, the low-pass filter was 35 Hz and the sample rate was 500 Hz. The ECG units were beats per minute. While the participants were in a comfortable, relaxed state, the resting-state GSR and ECG data were recorded serially for 5 min after all of the components of the apparatus had been attached.
Data analysis
A repeated-measures analysis of variance (ANOVA) was conducted using SPSS 16.0 software (SPSS Inc., Chicago, IL) followed by simple effect analysis to test significant interactions and paired sample t tests to analyze significant main effects. All statistical analyses described employed a two-tail alpha of 0.05 (p < 0.05). For within-subject analysis, the Greenhouse–Geisser correction was used where appropriate. Effect sizes are presented as partial eta squared for ANOVA effects and Pearson’s r for physiological and behavioral response correlations (Cohen, 1988). Data are expressed as mean ± SEM. The AcqKnowledge 4.1 software program (Biopac Systems Inc., Goleta, CA) was used to extract and analyze the GSR, HR and HRV data. ECG data were collected at 5-min intervals (Kuppens et al., Citation2010). From these data, the HRV spectral information, which included the HF component (0.15–0.40 Hz), the LF component (0.04–0.15 Hz) and the ratio of the frequencies (LF/HF), were obtained via Fast Fourier Transforms of the ECG signals. All spectral data were expressed in normalized units. The spectral components were evaluated in terms of frequency (Hz) and amplitude, which was assessed by the area (or power spectral density) of each component. Thus, squared units were used for the absolute values expressed in ms squared (ms2). Natural logarithms (ln) of the power values were used because of the skewness of the distributions (Buchanan et al., Citation2010). LF and HF powers were expressed in absolute values (ms2) or in normalized values (nu). The normalization of LF and HF was performed by subtracting the VLF component from the total power. It tended to reduce, on the one hand, the effects of noise due to artifacts and, on the other hand, to minimize the effects of the changes in total power on the LF and HF components. Normalized units were obtained as follows (Sztajze, Citation2004):
Results
Effects of neuroticism and menstrual cycle stage on the behavioral stress response
A two-way repeated-measures ANOVA was conducted on the Stroop task performance (accuracy, ACC and reaction time, RT) with neuroticism (high and low) as the between-subjects factor and menstrual cycle stage (luteal, menstrual and ovulatory) as the within-subject factor. The values of the ACC and RT of high- and low-neuroticism participants on the Stroop task across the menstrual cycle are listed in .
Table 1. The percentage of accurate responses (%ACC) and reaction time (RT) on the Stroop task of the high-neuroticism (N = 11) and low-neuroticism (N = 16) groups in the luteal, menstrual and ovulatory stages.
The ANOVA results showed that the main effects of the menstrual cycle on ACC (F(2,50) = 1.090, p = 0.34 and = 0.042) and RT (F(2,50) = 0.630, p = 0.54 and
= 0.025) were not significant. The main effects of neuroticism on ACC (F(1,25) = 0.079, p = 0.78 and
= 0.003) and RT (F(1,25) = 1.902, p = 0.18 and
= 0.071) were not significant either. The only significant interaction was the neuroticism × menstrual cycle interaction on RT (F(2,50) = 3.182, p = 0.050 and
= 0.113). Further simple effect analysis found that in menstruation, the RT on the Stroop task of the high-neuroticism group was significantly shorter than the RT on the Stroop task of the low-neuroticism group (F(1,25) = 5.62 and p = 0.026; ).
The effects of neuroticism and menstrual cycle on physiological stress responses
The effects of neuroticism and menstrual cycle on GSR
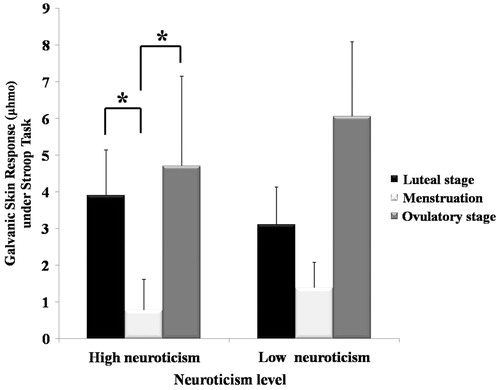
We conducted two-way repeated-measures ANOVA on the GSR with neuroticism (high and low) as the between-subjects factor and task type (resting state and Stroop task) and menstrual cycle stage (luteal, menstrual and ovulatory) as the within-subjects factors. The results indicated that the interaction of neuroticism × task type on GSR was significant (F(1,25) = 4.496, p = 0.044 and = 0.152). The simple effect analysis showed that under the stress state, the GSR of the high-neuroticism group differed significantly across the menstrual cycle (F(2,50) = 3.48 and p = 0.038); specifically, under the stress state, the GSR of the high-neuroticism group during menstruation was significantly lower than in the luteal and ovulatory stages ().
Figure 1. Galvanic skin response (μhmo) on the Stroop task in the high-neuroticism (N = 11) and low-neuroticism (N = 16) groups during the luteal, menstruation and ovulatory stages of the menstrual cycle. *p < 0.05, RM-ANOVA with menstrual cycle as within-subject factor (luteal, menstruation and ovulatory periods) and neuroticism as between-subjects factor (high-neuroticism and low-neuroticism). Values are mean ± SEM.
The effects of neuroticism and menstrual cycle on HR and HRV
The values of the HF, LF and LF/HF in the high- and low-neuroticism groups under the resting state and the stress state across the menstrual cycle are listed in . A two-way repeated-measures ANOVA was used to analyze the HR and HRV (LF, HF and LF/HF) with neuroticism (high and low) as the between-subjects factor and task type (resting state and Stroop task) and menstrual cycle stage (luteal, menstrual and ovulatory) as the within-subjects factors.
Table 2. High frequency of HRV (HF), low frequency of HRV (LF) and balance of HRV (LF/HF ratio) of women from high-neuroticism (N = 11) and low-neuroticism (N = 16) groups on the Stroop task and the resting-state of the luteal, menstruation and ovulatory stages across the menstrual cycle.
The ANOVA results indicate that the main effect of the task type on HF (F(1,25) = 30.137, p < 0.001 and = 0.547), LF (F(1,25) = 79.080, p < 0.001 and
= 0.760) and LF/HF ratio (F(1,25) = 8.987, p = 0.006 and
= 0.264) were significant. The significant interactions were the neuroticism × menstrual cycle interactions on HF (F(2,50) = 3.367, p = 0.042 and
= 0.119) and LF/HF ratio (F(2,50) = 3.653, p = 0.033 and
= 0.127). Further simple effect analysis showed a significant effect of the Stroop task on LF/HF ratio (F(1,25) = 4.97 and p = 0.035); specifically, in the ovulatory stage, the LF/HF ratio of the high neuroticism group was significantly greater than the LF/HF ratio of the low neuroticism group ().
Correlation between behavioral and physiological stress responses
Pearson correlation tests were conducted between the Stroop task performance (ACC and RT) and the physiological data (GSR, HR, LF, HF and LF/HF ratio) recorded under the stress state (Stroop task) in different stages of the menstrual cycle stage (luteal, menstrual and ovulatory). The Pearson correlation results are listed in .
Table 3. Correlations between accuracy (ACC), reaction time (RT) and physiological indices on the Stroop task for women of the high-neuroticism (N = 11) and low-neuroticism (N = 16) groups during the luteal, menstruation and ovulatory stages across the menstrual cycle.
The results showed that in menstruation, there were significant correlations between HRV responses (HF: p = 0.007; LF: p = 0.018) and ACC on the Stroop task, while the significant correlations of HRV responses (HF: p = 0.022; LF: p = 0.009) and RT on the Stroop task were negative. These results indicate that the reductions in HRV (HF and LF) caused by stress were related to the decreases in ACC and increases in RT. Furthermore, there was significant negative correlation between LF/HF in the ovulatory stage and Stroop ACC in the luteal stage (p = 0.023), and a significant negative correlation between LF/HF ratio in the luteal stage and Stroop ACC during menstruation (p = 0.009). This indicates that when facing the stressor, the physiological responses of women in the Stroop task may predict their behavioral performance in the next stage of the menstrual cycle.
Discussion
The results show the impacts of neuroticism and menstrual cycle stage on cognitive performance and physical response caused by a cognitive stressor. When completing the Stroop task during menstruation, compared with low-neuroticism women, high-neuroticism women had shorter RT and lower GSR. Hence, the high-neuroticism women were more sensitive to the stressor than the low-neuroticism women. At different stages across the menstrual cycle, compared to the resting state, the women had lower HRV (HF, LF and LF/HF ratio) under the stress state, which indicates the validity of the stressor (Stroop color-naming test). Consistent with our hypothesis, performance in the Stroop task was influenced by neuroticism, while variations in participants’ physiology depended on the stage of the menstrual cycle.
The data also indicate a significant relationship between neuroticism and stress reactivity, contingent on stage of the menstrual cycle: specifically, neuroticism was associated with cognitive performance as well as the GSR and HRV during menstruation. As neuroticism is considered to lead to pessimism-related task disengagement and poor performance (Dobson, Citation2000), high-neuroticism participants might reasonably be expected to show less task-related physiological arousal. However, this correlation did not occur in previous studies in terms of the menstrual cycle (Schallmayer & Hughes, Citation2010), and our data do not support it either. One interpretation of this data is that neuroticism exacerbates menstrual effects on the SNS, such that the tendency for neurotic individuals to disengage from tasks is more pronounced during this cycle stage (Ussher & Wilding, Citation1992). As with other studies using the Stroop task as stressor, in our study performing the Stroop task, demanding cognitive performance, caused SNS activation (Jones et al., Citation2007; Matthews et al., Citation1995; Roelofs et al., Citation2007; Verhasska et al., Citation2004). Specifically, the main effect of the task on HRV (HF, LF and LF/HF ratio) clearly demonstrated that the Stroop task had induced stress responses. However, significant correlations between cognitive performance and physiological responses in the menstruation stage indicated that the combination of SNS and task performance may be more suitable to assess subjective and objective variation under stress.
Compared to the luteal and ovulatory stages, during menstruation, the RT of the high-neuroticism group was significantly shorter than the RT of the low-neuroticism group under stress. This indicated that the high-neuroticism females had higher arousal levels than the low-neuroticism females. Hlavacova et al. (Citation2008) reported a different effect. They used the Stroop test to explore the impact of the menstrual cycle (luteal and ovulatory stages) and trait anxiety on neuroendocrine system activation. They found no significant effect of menstrual cycle stage on performance in the Stroop test. These inconsistent results could be due to differences in dividing the stages of the menstrual cycle. In our study, the menstrual cycle was divided into three stages, and because of this, we found differences in stress responses between the low- and high-neuroticism groups during menstruation. This approach enhances the existing body of research on menstrual cycle stage on stress mechanisms.
The GSR of women in the high-neuroticism group differed across the three cycle stages, with GSR during menstruation significantly lower than in the luteal and follicular stages. Adams et al. (2003) reported results that are inconsistent with our findings. Their results showed that under the Stroop task (stress state), GSR increased from the resting state. Our findings about GSR in women during the Stroop task (stress state) also differed from those of Adams et al. (2003), which showed the effect of menstrual cycle and neuroticism level. Specifically, during menstruation, the cardiovascular response of high-neuroticism women was more sensitive than that of low-neuroticism women when facing a stressor. Furthermore, some researchers have reported gender differences without ruling out the impact of the menstrual cycle (O’Leary et al., Citation2007). Our results clearly indicate that the menstrual cycle should be taken into consideration when exploring mechanisms of stress from the perspective of effective monitoring.
The combination of behavioral performance and physiological data in our experiment confirmed that sensitivity to stress is related to menstrual cycle stage and neuroticism type. Our design differed from that of Schallmayer & Hughes (2010), although we manipulated similar variables and obtained similar results. The objective of Schallmayer and Hughes was to circumvent the contaminating influence of the menstrual cycle and oral contraception, as the two factors were relevant for their study. Therefore, the target groups of their experiments were women who used oral contraception, with clinical relevance. However, we grouped participants into high- and low-neuroticism groups based on their EPQ–RSC scores, without taking other factors like trait anxiety or depression into consideration (Sherwood et al., Citation2010). The purpose of our design was to contribute to a growing body of evidence indicating that sensitivity of stress reactivity may be adaptive in controlling neuroticism (Bibbey et al., Citation2012). During menstruation, Stroop task performance correlated with physiological responses. Specifically, the increase in accuracy and the decrease in RT were related to the increase in HRV. This indicates that during menstruation, the increased HRV of women reflected central mechanisms that also enhanced cognitive performance. Furthermore, during menstruation, adjustments of physiological activity may aid coping with stressors successfully (Plessow et al., Citation2011). Thus, the correlation of task performance and physical response could be interpreted from the perspective of adaptability.
Overall, this study revealed an effect of neuroticism and menstrual cycle stage on SNS responses to a cognitive stressor. During menstruation, the high-neuroticism women had more sensitive cognitive performance and cardiovascular responses to the stressor than the low-neuroticism women.
Declaration of interest
The authors have declared that no competing interests exist.
The work was funded by the National Basic Research Program of China No. 2011CB505101 and the key lab open project of Beijing University of Chinese Medicine (2011-SYSKFKT03), as well as the Shangshan funding.
Acknowledgements
The authors would like to express their gratitude for the support of these projects. Dr. Oei is an Emeritus Professor of the University of Queensland and a visiting professor of Beijing Normal University and James Cook University in Singapore.
References
- Adams JM, Miller TW, Kraus RF. (2003). Exercise dependence: diagnostic and therapeutic issues for patients in psychotherapy. J Contemp Psychother 33(2):93–107
- Appelhans BM, Luecken LJ. (2006). Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol 10(3):229–40
- Bibbey A, Carroll D, Roseboom TJ, Phillips AC, de Rooij SR. (2012). Personality and physiological reactions to acute psychological stress. Int J Psychophysiol. http://dx.doi.org/10.1016/j.ijpsycho.2012.10.018
- Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, Thayer JF, Krischbaum C, Tranel D. (2010). Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology 35(1):56–66
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed). Hillsdale, NJ: Erlbaum
- Conway CC, Hammen C, Espejo EP, Wray NR, Najman JM, Brennan PA. (2012). Appraisals of stressful life events as a genetically-linked mechanism in the stress–depression relationship. Cogn Ther Res 36(4):338–47
- Derijk RH, de Kloet ER. (2008). Corticosteroid receptor polymorphisms: determinants of vulnerability and resilience. Eur J Pharmacol 583(2–3):303–11
- Dobson P. (2000). An investigation into the relationship between neuroticism, extraversion and cognitive test performance in selection. Int J Sel Assess 8(3):99–109
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. (2010). Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 30(2):431–8
- Healey JA, Picard RW. (2005). Detecting stress during real-world driving tasks using physiological sensors. IEEE T Intell Transp 6(2):156–66
- Hlavacova N, Wawruch M, Tisonova J, Jezovaa D. (2008). Neuroendocrine activation during combined mental and physical stress in women depends on trait anxiety and the period of the menstrual cycle. Ann New York Acad Sci 1148:520–5
- Hussain MS, Monkaresi H, Calvo RA. (2012). Categorical vs. dimensional representations in multimodal affect detection during learning. Lect Notes Comput Sci 7315:78–83
- Hutchinson JG, Ruiz JM. (2011). Neuroticism and cardiovascular response in women: evidence of effects on blood pressure recovery. J Pers 79(2):277–301
- Jo NY, Lee KC, Lee DS. (2012). Task performance under stressed and non-stressed conditions: emphasis on physiological approaches. Lect Notes Comput Sci 7198:19–26
- Jonassaint CR, Why YP, Bishopm GD, Tong EM, Diong SM, Enkelmann HC, Khader M, Ang J. (2009). The effects of neuroticism and extraversion on cardiovascular reactivity during a mental and an emotional stress task. Int J Psychophysiol 74(3):274–9
- Jones A, Beda A, Ward AMV, Osmond C, Phillips DIW, Moore VM, Simpson DM. (2007). Size at birth and autonomic function during psychological stress. Hypertension 49(3):548–55
- Kudielka BM, Wust S. (2010). Human models in acute and chronic stress: assessing determinants of individual hypothalamus–pituitary–adrenal axis activity and reactivity. Stress 13(1):1–14
- Kuppens P, Allen NB, Sheeber LB. (2010). Emotional inertia and psychological maladjustment. Psychol Sci 21(7):984–91
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. (2009). Neural correlates of heart rate variability during emotion. NeuroImage 44(1):213–22
- Litschauer B, Zauchner S, Huemer K-H, Kafka-Lutzow A. (1998). Cardiovascular, endocrine, and receptor measures as related to sex and the menstrual cycle period. Psychosom Med 60(2):219–26
- Matthews KA, Caggiula AR, Mcallister CG, Berga SL, Owens JF, Flory JD, Miller AL. (1995). Sympathetic reactivity to acute stress and immune response in women. Psychosom Med 57(6):564–71
- McKinley PS, King AR, Shapiro PA, Slavov I, Fang Y, Chen IS, Jamner LD, Sloan RP. (2009). The impact of menstrual cycle period on cardiac autonomic regulation. Psychophysiology 46(4):904–11
- Nater UM, Rohleder N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 34(4):486–96
- O’Leary MM, Loney BR, Eckel LA. (2007). Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology 32(2):183–91
- Ordaz S, Luna B. (2012). Sex differences in physiological reactivity to acute psychosocial stress in adolescence. Psychoneuroendocrinology 37(8):1135–57
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson I-M, Backstrom T, Fernandez G. (2010). Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology 35(1):47–55
- Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. (2006). Relationship between cortisol responses to stress and personality. Neuropsychopharmacology 31(7):1583–91
- Plessow F, Fischer R, Krischbaum C, Goschke T. (2011). Inflexibly focused under stress: acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. J Cognitive Neurosci 23(11):3218–27
- Qian MY, Wu GC, Zhu RQ, Zhang P. (2000). Development of the revised Eyesenck personality questionnaire short scale for Chinese (EPQ-RSC). Acta Psychol Sin 32(3):317–23
- Roelofs K, Bakvis P, Hermans EJ, Pelt JV, Honk JV. (2007). The effects of social stress and cortisol responses on the preconscious selective attention to social threat. Biol Psychol 75(1):1–7
- Sato N, Miyake S. (2004). Cardiovascular reactivity to mental stress: relationship with menstrual cycle and gender. J Physiol Anthropol Appl Hum Sci 23(6):215–23
- Schallmayer S, Hughes BM. (2010). Impact of oral contraception and neuroticism on cardiovascular stress reactivity across the menstrual cycle. Psychol Health Med 15(1):105–15
- Schwabe L, Wolf OT. (2009). Stress prompts habit behavior in humans. J Neurosci 29(22):7191–8
- Sherwood A, Park SB, Hughes JW, Blumenthal JA, Hinderliter A, Trivedi R, McFetridge-Durdle J. (2010). Cardiovascular hemodynamics during stress in premenopausal versus postmenopausal women. Menopause 17(2):403–9
- Simplicio MD, Costoloni G, Western D, Hanson B, Taggart P, Harmer CJ. (2012). Decreased heart rate variability during emotion regulation in subjects at risk for psychopathology. Psychol Med 42(8):1775–83
- Suridjan I, Boileau I, Bagby M, Rusjan PM, Wilson AA, Houle S, Mizrahi R. (2012). Dopamine response to psychosocial stress in humans and its relationship to individual differences in personality traits. J Psychiatr Res 46(7):890–7
- Sztajze J. (2004). Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly 134(34–35):514–22
- Verhasska CM, Smeenkb JMJ, Minnenc AV, Kraaimaat FW. (2004). Neuroticism, preattentive and attentional biases towards threat, and anxiety before and after a severe stressor: a prospective study. Pers Indiv Differ 36(4):767–78
- Ussher JM, Wilding JM. (1992). Interactions between stress and performance during the menstrual cycle in relation to the premenstrual syndrome. J Reprod Infant Psyc 10(2):83–101
- Wang XD, Wang XL, Ma H. (1999). Rating scales for mental health. Chinese Journal of Mental Health. Beijing, China: Mental Health in Chinese Press, 191--4
- Weidner G, Helmig L. (1990). Cardiovascular stress reactivity and mood during the menstrual cycle. Women Health 16(3–4):5–21
- Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, DeMeersman RE, Basner RC. (2005). Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol 98(6):2024–32
