Abstract
Providing care for people with autism spectrum disorder (ASD) is particularly stressful and frequently associated with disturbances in the hypothalamic–pituitary–adrenal (HPA) axis of the caregiver. This study examined whether the stress response is modulated by factors such as age of the care recipient and number of years spent by the caregiver in providing care for the ASD individual. Caregivers of children (n = 15), adolescents (n = 12), and adults (n = 11) with ASD were exposed to two episodes of acute psychosocial stressor in a 1 day session. Salivary cortisol samples were obtained before, during, and after the stressor episodes. Psychological characteristics (states of anxiety, anger, and mood) were measured before and after the stressor episodes. The characteristics of the ASD individuals (age, degree of autism, global activity, and level of autonomy) were also registered. A difference in stress response was found when caregivers of ASD children were compared with those of ASD adolescents and adults, ε = 0.25, F(2.24,53.65) = 5.82, p < 0.004; ε = 0.23 and F(2.11,48.43) = 4.88, p < 0.01, respectively. Thus, upon acute-stressor exposure, caregivers of ASD individuals presented a stress response that correlated with the age of the patient (the older the patient, the lower the cortisol response). Additional factors, such as number of years spent providing care and level of autonomy of the recipient, also significantly contributed to the stress response. Together, the results demonstrate that recipient characteristics contribute to the detection of high-risk individuals within a caregiver population.
Introduction
Providing care for patients with autism spectrum disorder (ASD) is a particularly stressful situation due to the specific demands of the care recipient. Parents of children with ASD are at greater risk of depression and other mental health problems, suffer greater stress, anxiety, and depression, and report more physical health complaints than parents of children with other disabilities (Abbeduto et al., Citation2004; Lovell et al., Citation2012; Montes & Halterman, Citation2007).
Prolonged exposure to stressful situations, such as caring for an offspring with developmental disabilities, is also associated with disturbances in the mechanisms for managing stress-related demands, such as alterations in the hypothalamic–pituitary–adrenal (HPA) axis (measured by cortisol levels), although the results of these measurements are far from being homogeneous. Indeed, there are reports of elevated daytime cortisol levels in caregivers of children with disabilities (Seltzer et al., Citation2009, Citation2010) and low cortisol levels in caregivers of children with cerebral palsy (Bella et al., Citation2011).
Given that caregivers have to cope repeatedly with acute stressors in their daily lives, studies that focus on the role of the HPA axis response to acute events are particularly relevant for understanding the health cost of caregiving. Nevertheless, studies that investigate the effect of acute stressors on the HPA axis response in caregivers of offspring with ASD are scarce. A previous study carried out in our laboratory compared the health complaints and the endocrine, immunological, and psychological responses to a stressor between a group of 41 parents of offspring with ASD and a group of 37 non-caregiver parents (De Andrés-García et al., Citation2012). The results of the study demonstrated a dysregulation in both immune [measured by immunoglobulin-A (IgA) levels] and hormonal stress-induced (measured by cortisol levels) responses in the caregiver group. In addition, caregivers reported more health complaints and more perceived stress and fatigue than the controls. Nevertheless, these responses varied greatly among caregivers, indicating that factors involved in care were modulating the psychological and endocrine responses in this population.
In previous studies of non-formal caregivers of persons suffering from different pathologies several factors related to the care context were suggested to explain, at least in part, the variability observed within the caregiver population. Among these factors, the diagnosis and symptoms of the patient and the years spent by the caregiver caring for the patient were considered to be of particular importance (Benson & Karlof, Citation2009; Tooth et al., Citation2005).
The degree of challenge posed to the carer by the patient has been shown to be positively related to the health status of the caregiver (Bromley et al., Citation2004; Lecavalier, Citation2006; Woodgate et al., Citation2008), and to an altered antibody response to vaccination in younger caregivers (Gallagher et al., Citation2009). Yet, in those studies, the diagnosis of the patient itself as a contributing factor to the etiology of acute stress in caregivers has not been considered. ASD covers a wide spectrum of symptoms and pathologic categories (Haglund & Källén, Citation2011). In some cases, the diagnostic features of ASD cannot be completely defined because of the complexity of the symptoms and the developmental status of the patient. This uncertainty may also affect caregivers and be a factor that overlaps with the possible effect of caregiving on health.
In the general population, the length of exposure to stress determines the consequence of stress on the organism (Tsigos & Chrousos, Citation2002). In particular, prolonged exposure to stressful situations is associated with a blunted stress-induced cortisol response (Burke et al., Citation2005; Matthews et al., Citation2001). By contrast, there were no differences between the adrenocorticotropic hormone (ACTH) levels of caregivers of patients with dementia and those of controls (Cacioppo et al., Citation2000; Epel et al., Citation2010). The amount of time spent caring for a patient is a factor that may be relevant in caregiver stress studies (Tooth et al., Citation2005). For instance, caregivers who had spent 14 years caring for patients with schizophrenia had a blunted cortisol awakening response (González-Bono et al., Citation2011). By contrast, caregivers who had spent up to 11 years caring for patients with ASD had a lower cortisol response to stress than that of the controls (De Andrés-García et al., Citation2012).
Furthermore, during informal care of patients with developmental disabilities, the length of period taking care of the patient was not an isolated aspect. Indeed, the length of this period was associated with the age of the caregiver, most frequently parents, and with the age of the patient. Thus, all of these factors, either alone or in combination, could modulate the stress response in caregivers. With respect to the age of the caregivers, most previous studies, which focused on caregivers of patients with dementia, have been inconsistent in their results (Cacioppo et al., Citation1998, Citation2000; Epel et al., Citation2010). Furthermore, care should be exercised when comparing the results obtained from caregivers of dementia patients with those obtained from caregivers of offspring with developmental disabilities: studies of caregivers of patients with dementia failed to show significant differences in cortisol levels between caregivers of 60 years or older and those of controls in the same age group (Cacioppo et al., Citation2000). By contrast, studies of caregivers of offspring with ASD reported that the cortisol levels of 40-year-old caregivers were lower than those of controls in the same age group (De Andres-García et al., Citation2012). Several factors, such as the emotional attachment between the patient and the caregiver and the duration of the illness (among other factors), differ between caregivers of patients with dementia and those caring for individuals with developmental disabilities. This fact could be a basis for differences in the data obtained for both types of caregivers.
Several correlational studies have examined the possible effect of age of the patient on the health status of the caregiver; however, no significant associations were found (Benson and Karlof, Citation2009; Bromley et al., Citation2004; Lecavalier et al., Citation2006), although positive associations were found between the age of children with ASD and feelings of pessimism experienced by their mothers (Abbeduto et al., Citation2004).
To clarify this issue, the present study aimed to evaluate the impact of the age of the care recipient on the stress response of the caregiver. To reduce potential interference by factors such as the symptoms of the care recipient, only caregivers of patients who had a confirmed diagnosis of either classic autism or Asperger syndrome were included in the present study. Despite this, however, the symptoms of ASD varied considerably among patients. Therefore, the potential effects of behavioral and cognitive disruption were statistically controlled in the present study. To minimize the possible influence of the age of the caregiver on the HPA axis response, the groups were composed of middle-aged caregivers only; thus, any possible explained variance by this factor was covaried. Taking the above into consideration, and because caregivers of older patients have been exposed to stress for a longer period than caregivers of younger offspring, a dysregulation in the HPA axis was hypothesized to be more pronounced in caregivers of older patients than in caretakers of younger patients.
Methods
Participants
Contact with participants was established following an informative meeting of a local association of relatives of ASD patients (Valencian Association of Parents of Persons with Autism, APNAV). The criteria for participating in the study as a caregiver were to be the main caregiver of a patient medically diagnosed with ASD on the basis of the 10th revision of the International Statistical Classification of Diseases (ICD-10; World Health Organization, Citation1993) criteria and to be living in the same household as the patient. All participants were born in Spain, and the Spanish language was used throughout the duration of the protocol. Participants did not receive any medical treatment(s) that may have influenced the endocrine system 1 year prior to or during the experiment. The final sample of the present study followed an exhaustive medical screening of patients as previously reported (De Andrés-García et al., Citation2012).
The total sample, composed of parents of individuals with ASD (n = 38), was comprised of 15 caregivers (nine women, six men) of child patients (aged ≤11 years), 12 caregivers (eight women, four men) of adolescent patients (between 12 and 16 years), and 11 caregivers (nine women, two men) of adult patients (≥17 years). The characteristics and descriptive statistics of care recipients are presented in , and those of caregivers are presented in . All participants, or their legal guardians, signed an informed consent form, and the experimental protocol followed ethical norms based on the 1964 Declaration of Helsinki. The protocol described in the present study was approved by the Ethical Committee for Human Research of the University of Valencia prior to performing the research. Information about care recipients was provided by their caregivers (legal guardians of the patients).
Table 1. Mean ± SEM anthropometrical and socio-demographic data, and social autonomy of the care recipient in three groups (child, adolescent and adult patients).
Table 2. Mean ± SEM or count for characteristics and socio-demographic data in caregivers of child patients, adolescent patients and adult patients.
Procedure
Participants were instructed to abstain from eating, taking stimulants, drinking beverages (such as tea, coffee, or alcohol), brushing their teeth, or smoking, for 2 h prior to arrival at the laboratory. The experimental procedure was always carried out between 16:00 and 19:00 h to minimize hormonal variations due to circadian rhythms. Each session lasted ∼2.5 h.
After the participants arrived, anthropometrical variables were measured, and compliance with the instructions was checked. An initial, pre-stress saliva sample to measure cortisol level (Csal-1), was then collected, and participants were conducted to the stress room. After a 10-min habituation period, another sample (Csal-2) was collected while the participants filled in the psychological questionnaires for the evaluation of pre-stress states of anxiety, anger and mood. Participants received general information about the stress stimuli and, after a rest period of 3 min, another saliva sample (Csal-3) was collected.
All participants were exposed to a psychosocial stressor consisting of four mental and arithmetic tasks in the same order. In the middle of the stress period, a saliva sample (Csal-4) was collected. Immediately after the end of exposure to the stressor, a saliva sample (Csal-5) was collected while the participants completed questionnaires for the evaluation of post-stress anxiety, anger and mood. Task appraisal (perceived stress, dissatisfaction with the outcome, and internal and external attribution of performance) was evaluated. Participants were conducted into the first room where salivary samples were collected 10, 20, 30, 45 and 60 min after the stressor (Csal-6, Csal-7, Csal-8, Csal-9 and Csal-10, respectively).
Finally, caregivers completed some personality batteries and an extended interview about marital status, educational level, smoking, socio-economic status, level of psycho-educational information about ASD, their care status and information about care recipient status (descriptive data, behavioral aspects and functionality, cognitive aspects, and social autonomy).
Psychosocial stressor
All participants were exposed to a psychosocial stressor, consisting of a set of mental tasks to be completed within 20 min in front of a committee composed of two men and three women. During the stress period, a video camera was switched on to increase the evaluative threat and to simulate a recording. Raters remained motionless and did not provide feedback to participants until the stressor was completed. Participants were requested by the committee to concentrate and make an effort to obtain the best results. During the 20-min duration of the stressor, participants had to perform attention, declarative, and timed arithmetic tasks. These tasks were adapted from the Trier Social Stress Task (TSST), a standardized protocol for the induction of psychosocial stress (Kirschbaum et al., Citation1993) in laboratory settings. The adaptation consisted in the replacement of the speech part by cognitive and visuo-spatial tasks, while the mental arithmetic task was maintained. At the end of the stress period, the video camera was switched off and participants were told that no recording had been made and that the aim of the video recording simulation had been to enhance the evaluative context of the stressor. The variation of the psychosocial stress protocol was demonstrated to elicit psychophysiological responses in previous studies (De Andrés-García et al., Citation2012).
Perceived characteristics of the care recipient
Descriptive data about the care recipient were collected by means of caregiver interviews. Data included age, gender, educational level, confirmed diagnosis (Asperger or classical autism), use of medication, years elapsed since last medical diagnosis was performed, and percentage of disability. Caregivers also filled out questionnaires aimed to assess the degree of autism, global activity, and dependence level of the care recipients.
The degree of autism of the care recipient was assessed with the Spanish version of the Autism Spectrum Quotient–Adolescent Version questionnaire (Baron-Cohen et al., Citation2006). This questionnaire was answered by caregivers and is composed of 50 items ranged on a 4-point Likert scale from A (definitely agree) to D (definitely disagree), with a reliability coefficient higher than 0.76. The maximum possible score on this scale is 50, and higher scores indicate higher degrees of autism.
The global activity of the care recipient was evaluated using the Spanish version of the Global Assessment of Functioning scale (Bobes et al., Citation2002; Endicott et al., Citation1976) with a reliability coefficient ranging from 0.69 to 0.91. The evaluation is composed of 10 sentences describing symptoms ranged on a 10-point Likert scale from 1 (severe symptoms and risk of suicide attempt) to 100 (no symptoms) and offers a single item for global functionality.
Patient dependence was evaluated using the Spanish version of the Barthel Index (Mahoney & Barthel, Citation1965). This scale measures the functional independence and mobility of individuals with chronic pathologies and evaluates whether the individuals can perform certain tasks independently of others. The scale is composed of 10 items (feeding, bathing, grooming, dressing, bladder and bowel control, movement, chair/bed transference, mobility, and climbing stairs) and has a Cronbach’s α higher than 0.87. Total scores range from 0 to 100 with higher scores indicating greater levels of independence by the care recipient.
Finally, the social autonomy of patients was evaluated as part of the interview with the caregiver, and was based on three questions concerning institutionalized patients (yes or no), hospital admission (yes or no), and the number of leisure activities of the patient.
Psychological and biochemical measurements on caregivers
Caregiver status
The care status of the caregiver was assessed by means of a structured interview. Information about the caregiving process was obtained and included (1) the weekly time spent caring for the patient (how many hours per week do you spend caring for your son/daughter?), (2) worries about the patient’s future [on a scale from 0 (not at all) to 10 (very worried), how worried are you about the future of your son/daughter?] and (3) degree of suffering [on a scale from 0 (not at all) to 10 (very much), how much have you suffered owing to the disease of the care recipient?].
Caregiver burden was evaluated by the Spanish version of the Caregiver Burden Inventory created by Zarit et al. (Citation1980). This instrument is composed of 22 items ranked on a 5-point Likert scale from 0 (never) to 4 (nearly always). The reliability coefficient is 0.92. Items are related to health, social, and personal lifestyle, and to the interpersonal relationships of patients with functional and behavioral disabilities. According to these domains, caregivers express their feelings of burden and higher scores represent a greater burden.
The caregiver’s perceived availability of social support was assessed using the Spanish version (De la Revilla et al., Citation2005) of the Medical Outcome Social Support Survey (MOS-SSS). This questionnaire is a 20-item measurement of the perceived availability of social support that was developed by Sherbourne et al. (Citation1991) as a part of a medical outcome study. The questionnaire is composed of four subscales (1) emotional/informational, (2) tangible, (3) positive social interactions and (4) affectionate. All items but one are ranked on a 5-point Likert scale from 1 (none of the time) to 5 (all of the time). The reliability coefficient is higher than 0.91.
Psychological traits
Anxiety was assessed using the Spanish adaptation of the State-Trait Anxiety Inventory (Spielberger et al., Citation1970). This instrument is composed of 20 items ranked on a 4-point Likert scale from 1 (almost never) to 4 (almost always). Higher scores represent greater anxiety with 60 representing a maximum score and the highest level of anxiety. The reliability coefficients range from 0.82 to 0.92.
Anger was evaluated using the Spanish version of the State-Trait Anger Expression Inventory-2 (STAXI-2; Spielberger et al., Citation1983). This questionnaire provides two scores: the anger trait, composed of 10 items and distributed into angry temperament and angry reaction (with 20 as a maximum for both); and the expression of anger, composed of 24 items organized into four subscales of internal and external control of anger, and internal and external expression of anger (all with 24 as the maximum score). All items were ranked by a 4-point Likert scale from 0 (almost never) to 3 (almost always) with reliability coefficients ranging from 0.65 to 0.86.
Resilience was evaluated using the Brief Resilient Coping Scale (Sinclair & Wallston, Citation2004). This instrument is designed to evaluate the ability of the individual to cope with stress in an adaptive manner. It is composed of four items: (1) I look for creative ways to alter difficult situations, (2) regardless of what happens to me, I believe I can control my reactions, (3) I believe I can grow in positive ways by dealing with difficult situations, and (4) I actively look for ways to replace the losses I encounter in life. These items are ranked on a 5-point Likert scale with a reliability coefficient higher than 0.69.
Psychological response to psychosocial stress
Task appraisal was ad hoc assessed with a list of four items based on previous studies (Carrillo et al., Citation2001; De Andrés-García et al., Citation2011), ranked on a 10-point Likert scale from 0 (nothing) to 10 (a high level). The items questioned participants about internal and external attribution, degree of perceived stress, and dissatisfaction feelings with the performance of the task.
Mood was evaluated using the Spanish adaptation of the Profile of Mood States (McNair et al., Citation1992). This instrument is composed of 29 items distributed into five subscales (depression, vigor, tension, fatigue and anger) and ranked on a 5-point Likert scale from 0 (nothing) to 4 (a high level) with a Cronbach’s α >0.80.
Anxiety was assessed using the Spanish adaptation of the State-Trait Anxiety Inventory (Spielberger et al., Citation1970). This instrument is described above, in the “Psychological trait” section. Reliability coefficients ranged from 0.16 to 0.62.
Anger was evaluated by means of the Spanish version of STAXI-2, an instrument designed by Spielberger et al. (Citation1983) and composed of 15 items distributed on three subscales (physical expression, verbal expression and feeling of anger) ranked on a 4-point Likert scale from “not at all” to “a high level”. Reliability coefficients ranged from 0.65 to 0.86.
Cortisol analysis
Given the high number of biological samples obtained, and to avoid a stress-induced increase in cortisol levels caused by exposure to the venipuncture event (Aardal & Holm, Citation1995), the biomarkers were all measured using saliva samples. Salivary cortisol concentration correlates well with free plasma cortisol concentration (Kirschbaum et al., Citation1999), and sample collection is non-invasive. Saliva samples were collected with a Salivette cotton dental roll (Sastedt, Rommersdolf, Germany), immediately frozen at −20 °C and stored at this temperature until thawed for use in radioimmunoassay analysis. All samples from each given individual were run in duplicate in the same assay and values were averaged. The criterion for measurement replication was fixed as an inter-duplicate variation coefficient of 8%. Radioimmunoassays were performed with a Coat-A-Count Cortisol instrument (Diagnostic Products Corporation-Siemens Healthcare Medical Solutions Diagnostics, Bad Nauheim, Germany), which has a sensitivity of detection of cortisol levels as low as 1.4 nmol/l and uses a rabbit polyclonal antibody immobilized on the wall of a polypropylene tube. It is highly specific for cortisol (cross-reactivity with other peptide and steroid hormones is <1%). The intra- and inter-assay variation coefficients were 4.3 and 5.2%, respectively.
Data analysis
Chi-square tests were used to evaluate differences in the nominal socio-demographic variables of caregivers, care status (worries about the future), and nominal variables of the care recipient.
After checking the normality of the data using the Kolmogorov–Smirnov test, repeated ANCOVAs were carried out with time as a within-subjects factor, group as a between-subjects factor, and age of caregivers as the covariate for cortisol concentration and mood. Greenhouse–Geisser adjustments for the degree of freedom were considered, and univariate analysis of covariance (ANCOVAs) with age of the caregivers as the covariate were performed as a post hoc analysis. Estimated marginal means were used as descriptive data when ANCOVAs with covariate variables were performed.
For analysis of cortisol concentrations, the magnitude of responses was estimated by the area under the curve (AUC) with respect to increases calculated using the trapezoid formula (Pruessner et al., Citation2003). The AUC was calculated with reference to the first value. For analysis of mood states, the stress-induced response was calculated using post-stress minus pre-stress scores. Pearson and Spearman correlations were used to determine the relationships between variables where appropriate. Differences between groups in stress-induced mood responses were compared using univariate ANCOVAs with age of the caregivers as a covariate. One-factor analysis of variance (ANOVAs) were used to analyze the differences between groups in variables with a single measurement. Statistical analyses were performed with the SPSS 17.0 for Windows package software (SPSS Inc., Chicago, IL). The alpha level was fixed at 0.05. Data are expressed as mean (M) ± standard error of mean (SEM).
Results
Differences in the status of care recipients ()
There were significant differences between the care recipient groups in terms of the age of the patients [F(2,37) = 55.5, p = 0.0001] with the differences being significant between the three groups (for all, p < 0.0001). The means of the patient ages in each group were 8.7 years for children, 14.2 years for adolescents and 20.5 years for adults. Accordingly, there were significant differences in the number of years spent in caregiving following the definitive patient diagnosis [F(2, 37) = 16.25, p = 0.0001], particularly when the group of caregivers of child patients was compared to other groups (for both, p < 0.007). The mean care period from the definitive diagnosis was 6.8 years in caregivers for child patients, 12.5 years for caregivers of adolescent patients and 16.6 years for caregivers of adult patients. No differences were observed between care recipient groups in terms of gender, medication intake or educational level of the patients.
Regarding the autonomy of patients, there were no significant differences between groups in terms of global activity, disability percentage, autism quotient, institutionalized status, or the number of leisure activities of the patient (for all, p > 0.09). However, there were significant differences in the level of dependence [F(2,35) = 7.26, p = 0.002] and hospital admissions [χ2(2,n = 38) = 7.14, p = 0.03] with the adult patients being more independent and having more hospital admissions than the adolescent and child patients ().
Caregivers provided information about the definitive diagnosis as reported by specialized psychological and psychiatric staff. The distribution of the diagnosis among groups was statistically significant [χ2(2,n = 38) = 11.44, p = 0.003]. The entire group of child patients had a diagnosis of classic autism (n = 15). By contrast, in the group of adolescents, there was a larger proportion of patients diagnosed with Asperger syndrome (n = 7) than those diagnosed with classic autism (n = 5). In the group of adults, there was a larger proportion of patients diagnosed with classic autism (n = 7) than those diagnosed with Asperger syndrome (n = 4).
Impact of the age of the patient on psychobiological responses of caregivers
The experimental session (16:00 h versus 18:00 h) had no impact on psychobiological responses to stressors. Furthermore, there were no differences between groups in terms of gender [χ2(2, n = 38) = 1.42, p = 0.49], anthropometrical and socio-demographic data, care status, burden, social support, resilience, and psychological traits (). Significant differences were found between groups in terms of the age of caregivers [F(2,37) = 18.91, p = 0.0001], with the caregivers for adults being older than the caregivers of adolescents and child patients (for all, p < 0.001). To isolate the possible confounder effect of the caregiver’s age on the HPA axis response, this variable was controlled using the age of the caregivers as a covariate.
In response to the stressor, as shows, no significant effects of group or its interactions were found for appraisal, mood, anxiety or anger (for all, p > 0.07). Repeated ANCOVAs only showed a significant effect of time on the physical expression of anger [F(1,34) = 7.58, p = 0.009] and the effect was higher after the stress period than before in the three groups of caregivers.
Table 3. Mean ± SEM psychological response to psychosocial stress in caregivers of child patients, adolescent patients and adult patients.
Salivary cortisol concentrations
shows a significant main effect of group [F(1,34) = 5.14, p = 0.0001] for cortisol concentration and for the time–group interaction [ε = 0.24, F(4.3,73.3) = 5.64, p = 0.0001]. Between-group comparisons revealed that these differences were statistically significant at 0, 10, 20, 30, 45 and 60 min after the stress period [F(2,37) = 6.42, p = 0.004; F(2,37) = 8.59, p = 0.001; F(2,37) = 7.43, p = 0.002; F(2,37) = 7.45, p = 0.002; F(2,37) = 3.96, p = 0.03 and F(2,37) = 4.96, p = 0.01, respectively]. When group-to-group comparisons were carried out, a significant time–group interaction was found when caregivers of child patients were compared with caregivers of adolescents and adults [ε = 0.25, F(2.24,53.65) = 5.82, p = 0.004; ε = 0.23, F(2.11,48.43) = 4.88, p = 0.01, respectively]. In summary, caregivers of child patients showed a significant cortisol response to an acute stressor. By contrast, caregivers of adolescent and adult offspring showed a buffered response. Accordingly, the AUC (arbitrary units, a.u.) for cortisol also differed between groups [F(2,37) = 6.71, p = 0.004]: the AUC in caregivers of child patients was 37.8 ± 109.9 a.u., the AUC in caregivers of adolescent patients was −47.8 ± 35.3 a.u., and the AUC in caregivers of adult patients was −61.9 ± 109.1 a.u. Post hoc analyses revealed that increases in caregivers of child patients were significantly different from the decreases in the other two groups (for all, p < 0.01).
Figure 1. Estimated marginal means of cortisol concentration in caregivers of child, adolescent and adult patients. Psychosocial stressor: a set of mental tasks lasting 20 min in front of a committee of two men and three women. Participants performed attention, declarative, and timed arithmetic tasks (adapted from TSST). For all times measured numbers of caregivers were: for child patients, n = 15; for adolescent patients, n = 12; for adult patients, n = 11; ’= minutes; Csal = salivary cortisol sample. Bars indicate standard error of mean. * = significant differences (p < 0.05) versus pre-stressor and other groups at same time, ANOVA.
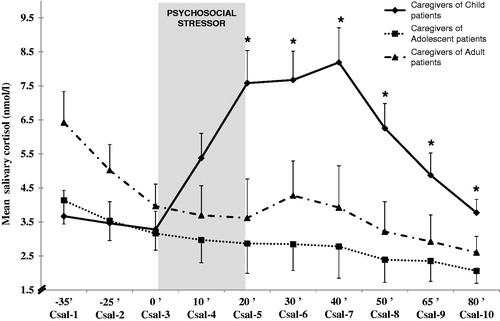
No relationships between the AUC for cortisol and the characteristics of the patient were found in any of the groups of caregivers. The number of years spent caring for the patient was positively associated with cortisol concentrations before and after the stress episode in caregivers of adolescents (for all, p < 0.02), and for cortisol concentrations during and after the stress in caregivers of adults (for all, p < 0.05), although no associations were found in caregivers of children.
Therefore, if the age of the patient is considered as a significant factor in the stress response, then other aspects of the care context, such as the number of years spent providing care to the patient and the autonomy of the patient must also be taken into account. When these variables were included as covariates in the analysis, the same results were obtained.
Discussion
The results herein show that care parameters, such as the age of patients, may modulate the cortisol response of caregivers to stress, even when the number of years spent providing care is statistically controlled. The caregiving process is a human model of chronic stress (Lupien et al., Citation2009). Consequently, the caregiver population may be affected by a major allostatic load as the price for coping with stressful situations and prolonged stress responses (McEwen & Stellar, Citation1993). This systematic and prolonged exposure to demands over-activates primary mediators, such as stress hormones, and this action may cause imbalances, diseases or mortality (McEwen, Citation1998). Previous studies carried out in our laboratory, in which caregivers of ASD patients with a high variability in symptoms were compared to controls in terms of the stress-induced response of the HPA axis, are in agreement with the findings of McEwen (Citation1998), as caregivers showed a buffered stress-induced response of the HPA axis (De Andrés-García et al., Citation2012). Nevertheless, in the study of De Andrés-García et al. (Citation2012), a high variability was observed in the cortisol profiles of caregivers, indicating that specific characteristics of the caregivers, care recipients, or of the context of the caregivers, may have a modulatory role on the cortisol response to stress. In this respect, the variability that was previously observed in the cortisol profiles of caregivers of ASD patients may also reflect the need to investigate additional factors, such as the characteristics of the patients. Indeed, from a biopsychosocial perspective, the consequences of the allostatic overload can be understood in transdisciplinary terms considering other variables apart from HPA activity (Picard et al., Citation2011). Thus, in the present study, characteristics of patients, such as a diagnosis intentionally limited to classic autism and Asperger syndrome, were investigated to assess the involvement of other potential factors in the stress response. Further identification of these additional factors may have clinical implications in detecting high-risk caregivers for therapeutic interventions.
The findings herein support the hypothesis that the age of the care recipient is relevant to the stress response of the caregivers. Thus, even when the symptoms of the care recipient and the age of the caregiver are controlled, caregivers of older offspring had lower cortisol levels than caregivers of younger care recipients, as hypothesized. Furthermore, the results herein show that caregivers of adolescent and adult patients have an alteration of HPA axis responsiveness since they did not show a significant change in cortisol concentration when exposed to a psychosocial stressor. Blunted cortisol responses to acute laboratory stress have been associated with a risk of suicide (McGirr et al., Citation2010) and a high risk of depression (Bouma et al., Citation2011) in a healthy population. The lack of response in healthy caregivers, observed herein, could be indicative of long-term mood disturbance and/or a mechanism to cope with daily stress. Furthermore, the results herein indicate that caregivers of adolescent or adult patients do not react psychologically or physiologically to challenges that are present in their daily lives. In this respect, longitudinal studies examining the impact of caregiving on the health of the caregiver while taking into consideration the different developmental time points of the care recipient are warranted. The results of the present study could be a starting point for future research on this issue.
It is unlikely that cortisol differences between groups were explained by mood alterations, because there were no differences between the caregiver groups in terms of mood. This dissociation between endocrine and psychological responses indicates that other factors are involved in the disruption of the stress response. Previous studies indicated that institutional support for caregivers may be a modulating factor for the normal activity of the HPA axis (González-Bono et al., Citation2011). However, in the present study, all participants came from a local association of relatives of ASD patients and therefore, the effect of institutional support over the hormonal stress response was a controlled factor.
ASD is a group of developmental disabilities with an early onset. The normal development of the child overlaps with the course of the pathology, and both processes progress concomitantly. It is possible that the role of the parents may have a greater weight than the role of caregiver for caregivers of ASD children. However, parents of adolescents and adults with ASD may perceive their additional responsibility in a more realistic manner and this may have implications for parent expectations about the disease progression of their offspring. Furthermore, access to a diagnosis is often a tortuous and lengthy process that can enhance the burden of feelings of caregivers, especially for caregivers of ASD offspring (Cadman et al., Citation2012). However, in the present study no differences in the burden of feelings were found between caregivers of child patients and caregivers of non-child patients, despite the difference in the time required to obtain a diagnosis.
To our knowledge, there are no other studies that have focused on the effects of the age of the patient on the caregiver cortisol response to stress, and this makes the interpretation of the results herein more difficult. In older caregivers of dementia patients, lower cortisol concentrations and higher ACTH concentrations have been reported after laboratory stress (Cacioppo et al., Citation1998; Epel et al., Citation2010), although in both cases, HPA axis activity was compared without the use of caregiver controls and no comparisons with younger caregivers were made. It is therefore difficult to compare the present findings with those of earlier studies.
Previous research on caregivers is scarce and mostly examined the circadian profiles of caregivers of children with cerebral palsy, attention deficit hyperactivity disorder (ADHD) or autism. For instance, lower concentrations of cortisol were found in caregivers of children with cerebral palsy (Bella et al., Citation2011) than in other carers. However, no differences in cortisol levels were found between caregivers of children with ADHD or autism and other carers (Lovell et al., Citation2012). In this context, the afternoon cortisol concentration of the caregivers in the present study was in the same range as that of caregivers of children with cerebral palsy, ADHD or autism (Lovell et al., Citation2012; Seltzer et al., Citation2010).
Although the impact of the age of the patient is a powerful factor that may alter the responses of caregivers of ASD offspring to stressful demands, it is not an isolated factor. In ASD caregivers, the age of the patient is inexorably linked with other possible mediators in the caregiving process, such as the time spent in providing care for the patient. Holland et al. (Citation2011) considered the time spent caring for the patient when evaluating HPA axis activity during two whole days and obtained an association between years spent caring and lower peaks of diurnal cortisol secretion in caregivers of dementia patients. In support of these findings, meta-analyses have demonstrated an inverse relationship between the time elapsed since stressor onset and the HPA axis activity (Miller et al., Citation2007). In the present study, the lack of capability to respond to stress in caregivers of adolescents and adult patients with ASD was replicated when the impact of the time spent caring for patients had been controlled for. In the study of Holland et al. (Citation2011), the average age of caregivers was >10 years older than that of carers in our study. The age of the care recipients in their study was also older than the care recipients in our study, and the care context was also different. We can therefore assume that in caregivers of ASD offspring the age of the patient is a more powerful HPA axis disruptor than the number of years spent providing care for the offspring.
Another factor not directly related with the age of the care recipient is the diagnosis and the behavioral symptoms. Previous studies indicate that differences in behavior between Asperger and non-Asperger patients are a factor that profoundly modifies the health status of caregivers (Haglund & Källén, Citation2011). In the present study, both adolescent and adult patient groups had Asperger or classic autism diagnoses and yet there was no significant effect of diagnosis on either psychological or endocrine responses in the carers. Furthermore, the clinical staff confirmed the patient diagnoses to the parents and the diagnoses were based on ICD-10 medical coding. Caregivers reported the diagnoses in the present study, and this information was not directly checked with clinical specialists at the time of the experiment with acute stressors. This can be considered as a limitation of the present study. In a similar vein, the behavioral problems displayed by the patients may modulate carer HPA axis activity, as previously suggested (Seltzer et al., Citation2010). The results herein show that there were no differences between the caregiver groups in terms of the behavioral symptoms of the care recipient, making it difficult to attribute the endocrine differences to this aspect. However, the small sizes of the groups limit the extent to which the results can be interpreted. Future research will be required to examine the impact of the diagnosis of patients with ASD and non-ASD on the health status of their caregivers.
From a biopsychosocial perspective, other factors were identified as possible modulators of psychological consequences in the caregiving process. These factors include a lower internal locus of control related to higher levels of depression and isolation in caregivers (Dunn et al., Citation2001), as well as social support (Benson & Karlof, Citation2009; Bromley et al., Citation2004; Higgins et al., Citation2005; Lovell et al., Citation2012) and resilience (Bayat, Citation2007), both of which show a positive association with psychological well-being in ASD caregivers. In non-caregivers, trait anxiety and negative affect are related to a buffered cortisol response to psychosocial stress (de Rooij et al., Citation2010). However, in the present study, no differences were observed between the groups in terms of anger, anxiety, resilience, or social support. It is possible that other personality traits could be contributing to these differences. Further research is warranted to obtain an integrative model for this population.
Conclusions
Upon exposure to an acute psychosocial stressor, caregivers of ASD patients showed a disrupted HPA axis response that correlated with the age of the patient (the older the patient – the lower the cortisol response to stress). The present study emphasizes the importance of this factor among care recipient characteristics and shows that it is relevant to the health of the caregiver from an integrative view that includes biomarkers, individual characteristics, and environmental parameters. Further investigation is required to extend our knowledge of the factors involved in mediating the allostatic overload in caregivers to help identify the at-risk population, reduce health risks for caregivers, and design improved psychosocial programs of intervention adapted to the specific requirements of caregivers.
Declaration of interest
This work was supported by the Ministry of Science and Education of the Spanish Government (PSI2008-04408/PSIC), the General Direction of Science Policy of the Ministry of Education of the Valencian Regional Government (ACOMP/2010/250 and PROMETEO/2011/048), and the University of Valencia Research Service (UV-INV-AE11-41173). The authors declare that they have no conflict of interest.
Acknowledgements
The authors wish to express their gratitude to the patients and participants of this study.
References
- Aardal E, Holm AC. (1995). Cortisol in saliva-reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem 33:927–32
- Abbeduto L, Seltzer MM, Shattuck P, Krauss MW, Orsmond G, Murphy MM. (2004). Psychological well-being and coping in mothers of youths with autism, down syndrome, or Fragile X syndrome. Am J Ment Retard 109:237–54
- Baron-Cohen S, Hoekstra RA, Knickmeyer R, Wheelwright S. (2006). The autism-spectrum quotient (AQ)—Adolescent version. J Autism Dev Disord 36:343–50
- Bayat M. (2007). Evidence of resilience in families of children with autism. J Intellect Disabil Res 51:702–14
- Bella GP, Garcia MC, Spadari-Bratfisch RC. (2011). Salivary cortisol, stress, and health in primary caregivers (mothers) of children with cerebral palsy. Psychoneuroendocrinology 36:834–42
- Benson PR, Karlof KL. (2009). Anger, stress proliferation, and depressed mood among parents of children with ASD: a longitudinal replication. J Autism Dev Disord 39:350–62
- Bobes J, Portilla MP, Bascaran MT, Saiz PA, Bousoño M. (2002). Global Activity Evaluation Scale. Basic tools bank for practicing clinical psychiatry. Madrid: Psychiatry Editors. p 142
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. (2011). Self-assessed parental depressive problems are associated with blunted cortisol responses to a social stress test in daughters. The TRAILS study. Psychoneuroendocrinology 36:854–63
- Bromley JO, Hare DJ, Davison K, Emerson E. (2004). Mothers supporting children with autistic spectrum disorders: social support, mental health status and satisfaction with services. Autism 8:409–23
- Burke HM, Davis MC, Otte C, Mohr DC. (2005). Depression and cortisol responses to psychologicalstress: a meta-analysis. Psychoneuroendocrinology 30:846–56
- Cacioppo JT, Poehlmann KM, Kiecolt-Glaser JK, Malarkey WB, Burleson MH, Berntson GG, Glaser R. (1998). Cellular immune responses to acute stress in female caregivers of dementia patients and matched controls. Health Psychol 17:182–9
- Cacioppo JT, Burleson MH, Poehlmann KM, Malarkey WB, Kiecolt-Glaser JK, Berntson GG, Uchino BN, Glaser R. (2000). Autonomic and neuroendocrine responses to mild psychological stressors: effects of chronic stress on older women. Ann Behav Med 22:140–8
- Cadman T, Eklund H, Howley D, Hayward H, Clarke H, Findon J, Xenitidis K, et al. (2012). Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 51:879–88
- Carrillo E, Moya-Albiol L, González-Bono E, Salvador A, Ricarte J, Gómez-Amor J. (2001). Gender differences in cardiovascular and electrodermal responses to public speaking task: the role of anxiety and mood states. Int J Psychophysiol 42:253–64
- De Andrés-García S, González-Bono E, Sariñana-González P, Sanchis-Calatayud MV, Romero-Martínez A, Moya-Albiol L. (2011). La valoración del resultado modula la respuesta del cortisol a una tarea cooperativa de laboratorio en mujeres. [Internal attribution of outcome moderates the cortisol response to a cooperative task in women]. Psicothema 23:196–202
- De Andrés-García S, Moya-Albiol L, González-Bono E. (2012). Salivary cortisol and immunoglobulin A responses to stress as predictors of health complaints reported by caregivers of offspring with autistic spectrum disorder. Horm Behav 62:464–74
- De la Revilla L, Luna J, Bailón E, Medina I. (2005). Validation of the MOS social support questionnaire in primary care. Family Medicine 6:10–18
- de Rooij SR, Schene AH, Phillips DI, Roseboom TJ. (2010). Depression and anxiety: associations with biological and perceived stress reactivity to a psychological stress protocol in a middle-aged population. Psychoneuroendocrinology 35:866–77
- Dunn ME, Burbine T, Bowers CA, Tantleff-Dunn S. (2001). Moderators of stress in parents of children with autism. Community Ment Health J 37:39–52
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. (1976). The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33:766–71
- Epel ES, Lin J, Dhabhar FS, Wolkowitz OM, Puterman E, Karan L, Blackburn EH. (2010). Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun 24:531–9
- Gallagher S, Phillips AC, Drayson MT, Carroll D. (2009). Parental caregivers of children with developmental disabilities mount a poor antibody response to pneumococcal vaccination. Brain Behav Immun 23:338–46
- González-Bono E, De Andrés-García S, Moya-Albiol L. (2011). The cortisol awakening response in caregivers of schizophrenic offspring shows sensitivity to patient status. Anxiety Stress Coping 24:107–20
- Haglund NGS, Källén KBM. (2011). Risk factors for autism and Asperger syndrome: perinatal factors and migration. Autism 15:163–83
- Higgins DJ, Bailey SR, Pearce JC. (2005). Factors associated with functioning style and coping strategies of families with a child with an autism spectrum disorder. Autism 9:125–37
- Holland JM, Thompson LW, Cucciare MA, Tsuda A, Okamura H, Spiegel D, Rasgon NL, Gallagher-Thompson D. (2011). Cortisol outcomes among caucasian and latina/hispanic women caring for a family member with dementia: a preliminary examination of psychosocial predictors and effects for a psychoeducational intervention. Stress and Health 27:334–46
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61:154–62
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Lecavalier L. (2006). Behavior and emotional problems in young people with pervasive developmental disorders: relative prevalence, effect of subjects characteristics and empirical classification. J Autism Dev Disord 36:1101–14
- Lecavalier L, Leone S, Wiltz J. (2006). The impact of behaviour problems on caregivers stress in young people with autism spectrum disorders. J Intellect Disabil Res 50:172–83
- Lovell B, Moss M, Wetherell M. (2012). The psychosocial, endocrine and immune consequences of caring for a child with autism or ADHD. Psychoneuroendocrinology 37:534–42
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–45
- Mahoney FI, Barthel DW. (1965). Functional evaluation: the Barthel index. Md State Med J 14:61–5
- Matthews KA, Gump BB, Owens JF. (2001). Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychol 20:403–10
- McEwen BS. (1998). Protective and damaging effects of stress mediators. N Engl J Med 338:171–9
- McEwen BS, Stellar E. (1993). Stress and the individual. Mechanisms leading to disease. Arch Intern Med 153:2093–101
- McGirr A, Diaconu G, Berlim MT, Pruessner JC, Sablé R, Cabot S, Turecki G. (2010). Dysregulation of the sympathetic nervous system, hypothalamic-pituitary-adrenal axis and executive function in individuals at risk for suicide. J Psychiatry Neurosci 35:399–408
- McNair DM, Lorr M, Droppleman LF. (1992). POMS manual: profile of mood states. San Diego, CA: Educational and Industrial Testing Service. p 27
- Miller GE, Chen E, Zhou ES. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol Bull 133:25–45
- Montes G, Halterman JS. (2007). Psychological functioning and coping among children with autism: a population-based study. Pediatrics 119:1040–6
- Picard M, Sabiston CM, McNamara JK. (2011). The need for a transdisciplinary, global health framework. J Altern Complement Med 17:179–84
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Stawski RS, Hong J, Taylor JL. (2009). Psychosocial and biological markers of daily lives of midlife parents of children with disabilities. J Health Soc Behav 50:1–15
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Col C, Stawski RS. (2010). Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disor 40:457–69
- Sherbourne CD, Stewart AL. (1991). The MOS social support survey. Soc Sci Med 32:705–12
- Sinclair VG, Wallston KA. (2004). The development and psychometric evaluation of the Brief Resilient Coping Scale. Assessment 11(1):94--101
- Spielberger CD, Gorsuch RL, Lushene RE. (1970). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press
- Spielberger CD, Jacobs G, Russell S, Crane RS. (1983). Advancements in personality assessment. In: Spielberger D, Butcher JN, editors. Assessment of anger: the State-Trait Anger Scale. Hillsdale, NJ: L. Erlbaum Associates. p 159–87
- Tooth L, McKenna K, Barnett A, Prescott C, Muerphy S. (2005). Caregiver burden, time spent caring and health status in the first 12 months following stroke. Brain Injury 19:963–74
- Tsigos C, Chrousos GP. (2002). Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res 53:865–71
- Woodgate RL, Ateah C, Secco L. (2008). Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 18:1075–83
- World Health Organization. (1993). The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. Geneva: World Health Organization. p 147–54
- Zarit SH, Reever KE, Bach-Peterson J. (1980). Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 20:649–55
