Abstract
Perturbations in the perinatal environment have been shown to significantly alter mesolimbic dopamine (DA) and hypothalamic–pituitary–adrenal (HPA) responses to stressors in adulthood. We have previously demonstrated that adult offspring exposed to high fat during the last week of gestation and throughout lactation display permanent alterations in mesolimbic DA function and behavior. The goal of the present study was to investigate nucleus accumbens (NAc) DA and HPA responses to acute and repeated stress in high fat exposed (HFD, 30% fat) and control (CD, 5% fat) offspring. Using microdialysis to monitor extracellular DA, we report that adult HFD offspring show an enhanced NAc DA response to acute tail-pinch compared to CD offspring. With repeated tail-pinch, the response of the HFD animals remains unchanged while CD offspring exhibit a sensitized DA response. The pattern of the DA response to both acute and repeated stress is also significantly altered by early diet exposure with an earlier peak and faster return to baseline levels in CD compared with HFD offspring. Similarly, neuroendocrine adaptations to repeated tail-pinch are observed in CD animals, but not in HFD animals. While controls display a habituated adrenocorticotropic hormone (ACTH) response to repeated tail-pinch, and an exacerbated ACTH response to a novel stressor, this effect was not observed in the HFD offspring. Together, our data demonstrate that exposure to high fat during early development impairs adaptations in NAc DA and HPA responses usually observed with repeated stress.
Introduction
Environmental influences during early life are important determinants of adult stress responsiveness. For example, repeated neonatal maternal separation (Brake et al., Citation2004; Francis et al., Citation2002), neonatal handling (Brake et al., Citation2004) and naturally occurring variations in maternal care (Liu et al., Citation1997; Zhang et al., Citation2005) are associated with significant alterations in hypothalamic–pituitary–adrenal (HPA) axis and mesocorticolimbic dopamine (DA) responses to stress. The ability of early environmental factors to “program” adult behavioral, neuroendocrine and autonomic responses to stressors depends, in part, on the postnatal functional integration of the HPA axis (Walker et al., Citation2001) as well as maturation of mesocorticolimbic DA projections (Antonopoulos et al., Citation2002; Benes et al., Citation2000). During this critical period of development, maternal dietary changes can signal available resources to the offspring and alter the nutritional and hormonal environment of developing young. For instance, maternal high-fat feeding increases the lipid content of the maternal milk and subsequently increases plasma concentrations of leptin and corticosterone as early as postnatal day (PND) 10 (D’Asti et al., Citation2010). Other authors and we have previously demonstrated that increasing the maternal fat content of the diet induces long-lasting alterations in mesolimbic DA function (Naef et al., Citation2008, Citation2011, Citation2012; Teegarden et al., Citation2009; Vucetic et al., Citation2010). Adult offspring exposed to 30% fat (versus 5%) during early life display blunted locomotor (Naef et al., Citation2008) and nucleus accumbens (NAc) DA responses to acute amphetamine (Naef et al., Citation2011), reduced behavioral sensitization to repeated amphetamine administration (Naef et al., Citation2008) and reduced anticipatory NAc DA responses to high-fat pellets (Naef et al., Citation2012). The neural substrates subserving the differential responses in high fat exposed offspring include increased activity of the DA transporter (DAT) and decreased expression of the DA D2 inhibitory pre-synaptic receptor in the ventral tegmental area (VTA) (Naef et al., Citation2011). In addition to its well-documented effects on DA function, perinatal high-fat feeding reduces stress responses in neonates (Trottier et al., Citation1998), although adolescent rats from high-fat feeding mothers display higher adrenocorticotropic hormone (ACTH) and corticosterone responses to stress. Exposure to high-fat diet in adulthood consistently increases HPA axis activity (Tannenbaum et al., Citation1997) and enhances vulnerability to diseases, yet the long term effect of perinatal high fat on adult stress responsiveness is unknown.
In this study, we tested the hypothesis that early exposure to high fat significantly alters the NAc DA and HPA responses to acute and repeated stress. We used a repeated stress paradigm as it allowed us to test the capacity of the systems to either adapt or sensitize to repeated challenges. We report that, compared to controls, adult offspring exposed to high-fat diet in early development showed an enhanced NAc DA response to acute stress, but failed to sensitize to repeated stress. Similarly, ACTH responses to tail-pinch stress failed to habituate in high-fat adult offspring and did not show facilitation to a novel stressor as observed in control animals. These data demonstrate that exposure to high fat during early development impairs adaptations in both NAc DA and HPA responses to repeated stress, highlighting a potential for increased vulnerability to stress-related disorders in these offspring.
Experimental procedures
Animals
Pregnant female Sprague–Dawley rats (Charles River, St-Constant, Quebec) were received in our animal facility on gestation day 13 and immediately placed on either a control diet (CD; 5% fat, 60% carbohydrate, 15% protein, 3.45 kcal/g) or a high-fat diet (HFD; 30% fat, 24% carbohydrate, 15% protein, 4.54 kcal/g) until postpartum Day 22. Both diets were powdered semipurified diets from Harlan Teklad (IN). Litters were culled to 10 pups on PND 1. On PND 22, male offspring were weaned from their mother, caged by two and maintained on the CD until tested in adulthood (Postnatal day 90–120). Animals were housed under controlled conditions of light (12:12 h light/dark cycle, lights on at 08:00 h), temperature (24–26 °C) and humidity (70–80%). All procedures were approved by the Animal Care Committee at McGill University in accordance with the guidelines of the Canadian Council on Animal Care.
Mesolimbic (NAc) DA responses to acute and repeated tail-pinch stress
In-vivo microdialysis was used to measure NAc DA responses to tail-pinch stress. Adult (>PND 90) male rats (six animals per diet group) were implanted with 20 gauge cannula aimed at the NAc (AP = 6.5 mm, ML = 1.4 mm, DV = −6.5 mm from Bregma) according to the atlas of Paxinos & Watson (Citation1998). On the experimental day, rats were placed in opaque circular (30 cm diameter) chambers containing 2 cm of bedding and a microdialysis probe was inserted into the guide cannula. Microdialysis probes (active membrane = 2.5 mm) and probe assembly were constructed as previously described (Lupinsky et al., Citation2010; Naef et al., Citation2011). Flow rate of artificial cerebrospinal fluid was set at 1.5 μl/min and samples were collected every 15 min for 1 h prior to stress and for 2 h post-tail-pinch stress. During the 30-min tail-pinch stress, a plastic clothespin was secured to the animal’s tail. Importantly, attachment of the clothespin to the tail of the animals did not elicit a pain response, as inferred from a lack of audible vocalization, flinching or jumping. Most animals gnawed the clothespin and if they successfully removed it before the end of the stress session, it was immediately replaced. Rats were exposed to the same tail-pinch stress in the same environment for the next 4 days consecutively, but dialysate collection was only conducted on the first (Day 1) and last (Day 5) day of repeated stress. Following the experiment, correct probe placement was verified for each animal. Examples of probe placements using these coordinates and these probe assemblies can be found in Naef et al. (Citation2011).
Dialysate levels of DA were measured using high-performance liquid chromatography with electrochemical detection as previously described (Naef et al., Citation2011). Chromatographic peak analysis was conducted using ESA CoulArray software (Chelmsford, MA) which identified unknown peaks in samples and matched these peaks with the retention time of the known standards for DA. Baseline measurements of DA were calculated using the mean concentration of four time points prior to stress initiation. For each time point examined, data are represented as a percent of mean baseline concentration.
Neuroendocrine responses to acute and repeated stress
In this experiment, separate groups of animals were tested for either acute or repeated exposure to stress. In the acute stress condition (Day 1: CD n = 6; HFD n = 7), naive animals were implanted with a jugular cannula, allowed to recover for 2 d, then subjected to 30 min of tail-pinch stress (as described above) followed 1 h later by 30 min of restraint, which was performed by placing the rat in a plastic restraint bag. For both tail-pinch and restraint, blood samples were collected through the jugular catheter at 5, 15, 30, 60 and 90 min post-stress initiation. The 90 min post-tail-pinch sample served as baseline for the restraint stress. In the repeated stress groups (Day 5: CD n = 7; HFD n = 6), animals were repeatedly stressed with 30 min of tail-pinch stress for 4 d (jugular implantation on Day 3), then tested for tail-pinch and restraint, as described above on Day 5.
Male rats (600–700 g) were equipped with indwelling jugular catheters under isoflurane anesthesia. A silicone catheter (I.D.: 0.025 inches, O.D.: 0.047 inches) was inserted ∼3 cm into the jugular vein aimed at the top atrium of the heart, secured to the vein by silk thread and exteriorized between the scapulae. Each catheter was filled with heparinized (Hep) saline (50 U/ml) and flushed the day after surgery with Hep-saline. On the day of testing, animals were moved to a separate testing room and the jugular catheter was connected to ∼30 cm of PE50 tubing that exteriorized to the cage in order to allow for the sampling of plasma without disturbing the animals. Animals were left undisturbed for 90 min to acclimatize to the testing environment before stress onset. At each sampling time, the volume of blood withdrawn (∼200 µl) was replaced by 150–200 µl of heparinized saline.
Hypothalamic tissue collection
Baseline and stress-induced corticotropin releasing hormone (CRH) messenger ribonucleic acid (mRNA) and heteronuclear ribonucleic acid (hnRNA) expressions were examined following 30 min of tail-pinch (CD n = 3, HFD n = 4) or 30 min of restraint (CD n = 7, HFD n = 7) in separate cohorts of animals. This time point was chosen to coincide with peak ACTH secretion observed in similar experimental groups. With both stressors, animals were sacrificed by rapid decapitation at 30 min post-stress and expression levels were compared with unstressed animals (tail pinch: CD n = 4, HFD n = 5; restraint: CD n = 6, HFD n = 8). Upon decapitation, brains were removed, snap frozen with isopentane and then kept at −80 °C until processing for CRH mRNA and hnRNA using in situ hybridization (ISH). ISH was conducted according to previously published methods (mRNA: Mansi et al., Citation1998; hnRNA: Chen et al., Citation2001). Twenty µm brain sections were collected onto Superfrost Plus slides (Fisher Scientific, Ottawa, Canada) and stored at −80 °C until processed.
ISH for hypothalamic PVN CRH mRNA and hnRNA
Synthesis and labeling of the CRH mRNA probes was performed as previously described (Mansi et al., Citation1998). A plasmid containing a 659-bp fragment of exonic CRH was kindly provided by Dr G Drolet (Laval University). Briefly, radioactive antisense cRNA was synthesized by incubating 8 µl (100 uCi) 35S-UTP (Perkin Elmer, Woodbridge, ON, Canada) with 1.5 µl specific cDNA (250 ng/µl), 1 µl 100 mM dithiothreitol (DTT), 2 µl GTP/ATP/CTP, 1 µl Protector RNase inhibitor (Roche Pharmaceuticals, Mississauga, ON, Canada) and 1 µl SP6 RNA Polymerase for 1 h at 37 °C. Following this initial step, 1 µl DNAse (DNA1 RNase-free, Roche) was added and incubation continued for an additional hour. The probe was then purified by column chromatography using a G-25 Sephadex spin column saturated with Tris NaCl EDTA buffer (STE, Roche).
For CRH mRNA ISH, tissue sections were fixed in 4% paraformaldehyde for 20 min, then digested by proteinase K (10 μg/ml in 100 mM Tris–HCl pH 8.0 and 50 mM EDTA) at 37 °C for 30 min. Next, the brain sections were rinsed in a solution of 0.1 M triethanolamine (TEA), acetylated in 0.25% acetic anhydride in 0.1 M TEA and dehydrated through graded concentrations of ethanol (50, 70, 95 and 100%). After vacuum drying for 2 h, 100 µl of hybridization mixture containing 35S-CRH cRNA (l07 cpm/ml) was spotted on each slide, sealed under a coverslip and incubated at 56 °C overnight. The following day, coverslips were removed and the slides were rinsed in 4 × standard saline citrate (SSC, Roche) at room temperature. Sections were digested by RNase A (20 μg/ml, 30 min at 37 °C), rinsed in descending concentrations of SSC (2×, 1×, 0.5 × + 1 mM DTT), dipped 10 times in 0.1 × SSC + 1 mM DTT and dehydrated through graded concentrations of ethanol.
For the synthesis and labeling of the CRH hRNA probes, a plasmid containing a 530-bp fragment of intronic CRH was kindly provided by Dr Tallie Baram (UCI, Chen et al., Citation2001). Radioactive antisense cRNA was synthesized by incubating T7 RNA polymerase (30 U, Promega, Madison, WI) with 250 ng of plasmid linearized with Hind III in 0.5 mM ATP/GTP/UTP, 35S-CTP, 10 mM DTT, 40 mM Tris-HCl, (pH 7.9), 6 mM MgCl2, 2 mM spermidine, 10 mM NaCl and 20 U RNAse inhibitor (RNasin, Promega, Madison, WI). After 2 h at 37 °C, 3 U of RNase-free DNase (RQ1-DNase, Promega Madison, WI) was added for 15 min at 37 °C. The probe was subjected to mild alkaline hydrolysis and purified by column chromatography using a STE Select-D G-25 spin column from Thomas Scientific (Swedesboro, NJ).
For CRH hnRNA ISH, the sections were fixed for 20 min in 4% paraformaldehyde, followed by dehydration and rehydration through graded concentrations of ethanol. Sections were then acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0) for 8 min, dehydrated through graded ethanol rinses, immersed in chloroform (5 min), and then rehydrated (100 and 95% ethanol). The slides were allowed to dry, then 60 µl of the prehybridization mixture was added and sections were allowed to incubate for 1 h. Prehybridization and hybridization was performed at 55 °C in a solution of 50% formamide, 5 × SET, 0.2% sodium dodecyl sulfate (SDS), 5 × Denhardt’s, 0.5 mg/ml salmon DNA, 0.25 mg/ml yeast tRNA, 100 mM DTT and 10% Dextran sulfate. Following prehybridization, sections were hybridized overnight with 2.5 × 107 cpm/ml 100 µl per slide of 35S-labeled ribonucleotide probe. The following day, the slides were rinsed with 2× SSC, RNase digested (30 min at 37 °C), rinsed with decreasing concentrations of SSC at 62 °C (2 × 5 min, 1 × 5 min, 0.25 × 30 min, 0.1 × 1 h, 0.03 × 1 h at 62 °C), then dehydrated with increasing concentrations of ethanol. Following hybridization for both mRNA and hnRNA, slides were vaccum-dried for 2 h and exposed to X-ray film (Eastman Kodak, Rochester, NY) for 7 (mRNA) and 17 (hnRNA) d. Hybridization signal was quantified in the paraventricular nucleus (PVN) using computerized densitometry of X-ray films by means of an MCID image analyzer system (Imaging Research Inc., St Catherine, ON, Canada). Three to four PVN sections per animal were used for analysis.
Hormonal determinations
Plasma concentrations of ACTH and corticosterone MP Biomedicals) were assayed with rat-specific radioimmunoassay kits (ACTH: DiaSorin, Stillwater, MN; corticosterone: MP Biomedicals, Santa Ana, CA), as previously described (Buwembo et al., Citation2012; Proulx et al., Citation2001). The limit of detection for ACTH and corticosterone were 15 pg/ml and 0.3125 µg/dl, respectively.
Statistical analysis
In repeated sampling experiments, two-way analysis of variance (ANOVA) was conducted with time during testing as the repeated measures variable. Bonferroni post hoc analysis was used when appropriate. Detailed use of statistical tests is described in the “Results” section. Values are reported as means ± SEM.
Results
NAc DA responses to acute and repeated tail-pinch stress
NAc DA responses to acute and repeated tail-pinch were measured using in vivo microdialysis. Application of tail-pinch stress elicited increases in extracellular NAc DA in both CD and HFD offspring (), although the pattern and amplitude of these responses were influenced by previous experience with the stressor (Day 1 versus Day 5) and early diet (CD versus HFD). (CD n = 6; HFD n = 6) depicts NAc DA concentrations as a percent of baseline during the 30-min tail-pinching and for 90 min post-stress on Days 1 and 5 of tail-pinch. While both diet groups showed a significant time effect when analyzed with a two-way repeated measures ANOVA (across time and days [CD: F (7, 35) = 7.45 p < 0.00001; HFD: F (7, 35) = 3.65 p < 0.01), a significant day effect was only observed in the CD offspring (CD F (1, 35) = 7.77, p < 0.05; HFD (F (1, 35) = 0.62).Thus, a sensitized NAc DA response was observed with repeated stress in the CD, but not in the HFD offspring. Peak DA concentrations were observed at 15 min in CD while DA peaked at 30 min in the HFD group and never fully recovered to baseline after 2 h.
Figure 1. (A) NAc DA responses (percent of baseline DA concentrations) of CD and HFD offspring on Day 1 (white) and Day 5 (black) of 30-min tail-pinch stress (gray shaded area). In the CD offspring, tail-pinch stress led to an increase in NAc DA (p < 0.01) with peak DA concentrations observed at 15 min post-stress and a sensitized DA response was observed on Day 5 versus Day 1 (p < 0.0001). In the HFD offspring, tail-pinch increased NAc DA concentrations (p < 0.01), but the peak was observed at 30 min post-stress and repeated stress did not sensitize NAc DA. (B) Mean increase (mean change from baseline, 15–120 min) in DA concentrations following tail-pinch stress was significantly higher in HFD offspring on Day 1 (p < 0.05), but not on Day 5 of tail-pinch stress. **p < 0.01, ***p < 0.0001 represent the simple main effects in two-way repeated measures ANOVA, #p < 0.05 CD versus HFD offspring Bonferroni post hoc. Values represent the mean ± SEM of six animals per diet group.
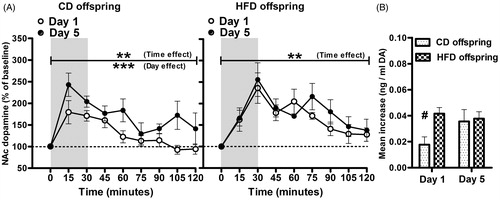
Diet group differences in NAc DA responsiveness to stress () were directly compared as mean increase (mean change from baseline between 15 and 120 min) with a two-way repeated-measures ANOVA. Although NAc mean DA increase did not show a significant diet [F (1, 10) = 2.506, p > 0.05] or day [F (1, 10) = 2.877] effect, a significant diet × day interaction was apparent [F (1, 10) = 6.950, p = 0.0249]. Bonferroni post hoc tests revealed that mean NAc DA increases were significantly elevated in HFD offspring compared to CD offspring on Day 1 of stress (p < 0.05). Baseline DA concentrations (data not shown) did not vary as function of diet [F (1, 10) = 0.2328, p > 0.05] or day of stress [F (1, 10) = 4.331, p > 0.05].
Neuroendocrine responses to acute tail-pinch and restraint stress and repeated tail-pinch stress
Plasma ACTH and corticosterone responses to tail-pinch followed by restraint stress are depicted in for control () and high-fat () adult offspring subjected to stress acutely (Day 1) or after 4 d of repeated tail pinch (Day 5). For each diet group, ACTH and corticosterone responses were analyzed using a two-way repeated measures ANOVA with time (0–180 min) as the repeated-measure variable. In the CD offspring, ACTH responses to tail-pinch decreased following repeated exposure to the same stressor, but increased in response to a novel stressor (restraint stress). In this diet group, the ANOVA showed a significant time effect [F (10, 121) = 36.66, p < 0.0001] and a time × day interaction [F (10, 121) = 7.171 p < 0.0001]. In the repeated stress group (Day 5), t-tests with a Bonferroni correction revealed a significant reduction in ACTH concentrations at 15 and 30 min after the onset of tail-pinch, suggesting habituation to this stressor. However, in response to restraint stress, a significant increase in the ACTH response was observed at 5, 15 and 30 min after restraint onset (95, 105 and 120 min in ) indicative of facilitation of the response to a novel, heterotypic stressor. In contrast, the HFD offspring did not display significant changes in the magnitude of their responses to either stressor when acute and repeated responses were compared [day effect: F (1, 100) = 0.013 p = 0.9128], although as expected, a significant time effect was detected [F (10, 110) = 30.63 p < 0.0001]. Corticosterone concentrations showed a significant time effect for both diet groups [CD: F (10, 110) = 11.51 p < 0.0001; HFD: F (10, 120) = 9.693 p < 0.0001], but no significant day effect.
Figure 2. Plasma ACTH and corticosterone concentrations of CD (A) and HFD (B) offspring in response to tail-pinch and restraint stress on Day 1 (white) versus Day 5 (black) of repeated tail-pinch stress. Gray shaded areas represent the timing of stressors’ exposure. In the CD offspring, with repeated tail-pinch, we observed significant reductions in ACTH levels at 15 and 30 min post tail-pinch, but increased concentrations at 5, 15 and 30 min post restraint stress. This effect was not observed in HFD offspring. Repeated tail-pinch stress did not significantly alter corticosterone responses for both CD and HFD offspring. (C) Area under the curve in CD and HFD offspring computed for each stressor (tail pinch: 0–90 min, restraint: 90–180 min) on Day 1 and Day 5 of tail-pinch stress. t-Tests (Day 1 versus Day 5) revealed habituation and facilitation in CD but not in HFD offspring. Values are means ± SEM of CD (Day 1: n = 6, Day 5: n = 7) and HFD (Day1: n = 6, Day 5: n = 6) adult offspring. *p < 0.05 and ***p < 0.001 represent significant time points differences (Day 1 versus Day 5) using Bonferroni post hoc analysis, #p < 0.01 Day 1 versus Day 5.
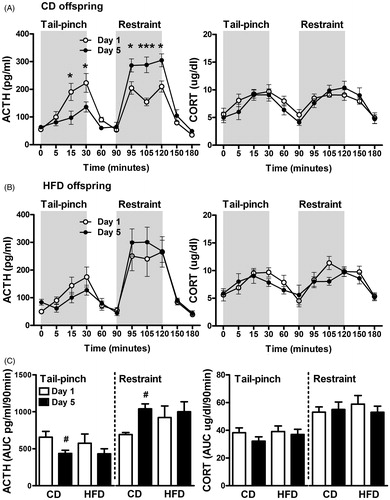
To directly compare diet groups, we computed the area under the curve for plasma ACTH and corticosterone responses to acute (Day 1) and repeated (Day 5) exposure for each stressor (tail-pinch 0–90 min, restraint 90–180 min) and a t-test was conducted for each stressor and diet group. We confirmed that the overall stress response in the CD group was lower with the homotypic stressor (tail-pinch) on Day 5 (p = 0.017, t-test) and increased with the novel stressor on Day 5 (p = 0.008, t-test). No significant differences between days were detected in the HFD offspring. There were no overall differences between diet groups in either ACTH or corticosterone responses as determined by repeated measures ANOVA. However, while CD offspring displayed habituation to tail-pinch and facilitation to restraint, these neuroendocrine adaptations were not observed in the HFD group.
Hypothalamic responses to acute tail-pinch and restraint stress
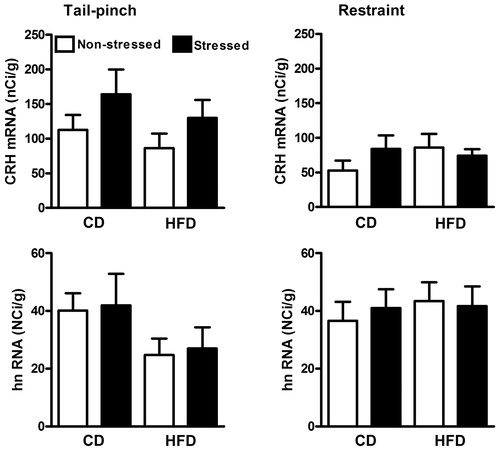
depicts CRH mRNA and hnRNA expressions measured at 30 min post tail-pinch and restraint stress in CD and HFD offspring. Two-way ANOVAs were used for analysis of all measures. The CRH mRNA response to stress showed a small, but nonsignificant response to tail-pinch [stress versus nonstressed: F (1, 12) = 3.466 p = 0.0873] and no response to restraint [F (1, 24) = 0.33, >0.05]. Furthermore, tail-pinch [F (1, 12) = 0.07, p > 0.05] and restraint [F (1, 22) = 0.04, p > 0.05] failed to increase CRH hnRNA at the 30 min time point. No diet group differences were observed in either mRNA or hnRNA CRH measures.
Figure 3. Hypothalamic PVN levels of CRH mRNA and hnRNA in response to either 30 min of tail-pinch (left) or 30 min of restraint (right) in CD and HFD offspring. Brain tissues were collected at the end of the stressor (30 min) in both conditions. Tail-pinch tended to increase CRH mRNA in both CD and HFD rats although there were no significant differences between diet groups in stress responses of either RNA transcripts. Values represent the mean ± SEM (baseline tail-pinch CD n = 4 HFD n = 5, tail-pinch CD n = 3 HFD n = 5; baseline restraint CD n = 6 HFD n = 7, restraint CD n = 7 HFD n = 7. About 3–4 sections containing the PVN were analyzed per animal.
Discussion
The goal of this study was to examine the long-term consequences of early exposure to high fat on NAc DA and HPA responses to acute and repeated tail-pinch stress. While CD offspring displayed a sensitized NAc DA response with repeated exposure to daily tail-pinch stress (Day 1 versus Day 5), this phenomenon was not observed in the HFD offspring, possibly as a result of a higher DA response that was observed on the first day of stress in this group. In the CD offspring, DA sensitization with repeated tail-pinch was associated with habituation of the ACTH response to tail-pinch but facilitation when challenged with a novel stressor, restraint. Together, these findings suggest that early exposure to high fat through the maternal milk impairs adaptations in HPA activity and DA neurotransmission after repeated stress and suggests that HFD offspring might be more vulnerable to repeated stress-induced pathologies.
NAc dopamine response to acute stress
The mesolimbic DA system has been most extensively studied in the context of drug and food reinforcement. However, there is convincing evidence that mesolimbic DA is also involved in the appraisal, integration and behavioral responses to psychogenic stressors (Cabib & Puglisi-Allegra, Citation2012). Tail-shock (Abercrombie et al., Citation1989; Gresch et al., Citation1994), tail-pinch (Budygin et al., Citation2012; Doherty & Gratton, Citation1992; Rouge-Pont et al., Citation1998) and restraint (Doherty & Gratton, Citation1992; Puglisi-Allegra et al., Citation1991) stress have been previously shown to significantly modulate the release of DA in the NAc. This is confirmed in this study in which we observe significant increases in NAc DA concentrations on Day 1 and Day 5 of repeated tail-pinch in both CD and HFD adult offspring. However, the NAc DA response of HFD offspring was higher in magnitude on Day 1 of testing and a different pattern of response was observed relative to controls. While the CD DA response peaked at 15 min after tail-pinch initiation and quickly returned to baseline, peak DA concentrations were observed at 30 min in HFD animals and DA levels never recovered. An increase in the magnitude of the NAc DA response and a deficit in recovery in the HFD offspring suggest impairments in the regulation of NAc DA in HFD offspring. Several mechanisms might explain this observation, including changes in regulatory inputs to the NAc from the medial prefrontal cortex (mPFC) and local synaptic modulation of extracellular DA concentrations.
The NAc DA response to stress is regulated in a large part by glutamatergic inputs from the mPFC, which are themselves the product of a delicate balance between mPFC norepinephrine (NE) and DA activity (Pascucci et al., Citation2007). While mPFC DA exerts an inhibitory influence on efferent inputs to the NAc, thus reducing the NAc DA response to stress (Doherty & Gratton, Citation1996), mPFC NE, through activation of alpha-1 adrenergic receptors, exerts the opposite effect leading to an enhancement of the NAc DA response to stress (Nicniocaill & Gratton, Citation2007). As HFD adult offspring displayed increased and prolonged DA stress responses, it is tempting to propose that a high mPFC NE tone combined with lower PFC DA might exist in these animals. Although we have not measured mPFC responses to stress in this study, we previously reported a lack of effect of perinatal diet on baseline and amphetamine-stimulated mPFC DA (Naef et al., Citation2011) concentrations. This suggests that increased mPFC NE activity might tilt the balance toward a greater input to NAc after stress exposure. Further experiments need to clearly establish this possibility.
Increased NAc DA responses to stress might also be due to local regulation of extracellular DA by the DA transporter (DAT) and presynaptic inhibitory DA D2 receptors. Stimulation of DA with acute (Copeland et al., Citation2005) and repeated stress (Copeland et al., Citation2005; El-Khodor & Boksa, Citation2002) enhances the expression and activity of DAT, which indicates that DAT might help buffer excessive DA release after stress. The higher integrated responses and deficits in the recovery of DA levels with the application of tail-pinch in HFD offspring indicate a potential reduced uptake capacity via DAT. However, under baseline conditions, we observed higher, but not lower DAT activity in HFD offspring (Naef et al., Citation2011), suggesting that baseline variations in DAT activity do not contribute to the regulation of DA during and following stress in these animals. Alternatively, the higher DA response and delayed recovery we observe in HFD animals might represent reduced inhibitory feedback via NAc presynaptic D2 receptors. Evidence to support this hypothesis was found in our previous experiment which showed that HFD rats had reduced expression of presynaptic D2 receptors mRNA in the VTA (Naef et al., Citation2011). This implies that a reduction in the concentration of D2 receptors in target areas such as the NAc and a weaker presynaptic inhibition of DA would allow for increased NAc DA release following stressful stimulation.
DA sensitization to repeated stress
Repeated stress has been reported to both sensitize (Brake et al., Citation1997; Doherty & Gratton, Citation1992) and reduce (Imperato et al., Citation1992) the NAc DA response to stress. Our data indicate that while the CD offspring sensitized to repeated tail-pinch, the DA response to the first and fifth episodes of tail-pinch was strikingly similar in HFD animals. It is possible that maximal DA levels were reached on Day 1 of tail-pinch in the HFD offspring and thus, precluded further increases on Day 5. However, we have observed considerably higher NAc DA concentrations following the administration of amphetamine in these animals (Naef et al., Citation2011), indicating that HFD animals have the capacity to mount higher responses with pharmacological stimulation than those observed with the application of tail-pinch on Day 1. Failure to show DA sensitization with repeated tail-pinch is consistent with our previous study (Naef et al., Citation2008) showing that, unlike the offspring of CD dams, HFD offspring do not sensitize to amphetamine’s locomotor stimulant effect with repeated drug administration. Thus, it would appear that early exposure to high fat interferes with the cascade of neuroadaptive changes that underlie the development of drug- and/or stress-induced sensitization of meso-Nac DA transmission. We can only speculate as to the nature of these mechanisms, but changes in neurotrophic factors within the mesocorticolimbic DA circuitry (Pierce & Barri, Citation2001) might be worth investigating, given that maternal obesity impairs hippocampal brain-derived neurotrophic factor (Tozuka et al., Citation2010).
Acute and habituated neuroendocrine and hypothalamic responses to stress
In response to acute stress (Day 1), both CD and HFD offspring showed significant and similar ACTH and corticosterone responses to tail-pinch and restraint. However, considerable diet group differences emerged with repeated stress in that HFD offspring failed to display either habituation to the homotypic stressor (tail-pinch) or facilitation to the heterotypic stressor (restraint stress). Habituation of neuroendocrine responses to repeated homotypic stress has been well documented for a number of stressors (restraint, cold, novel environment, etc.) and is thought to represent an adaptive protective process to avoid exposure to large amounts of circulating glucocorticoids as well as limit central activation of stress pathways (Nesse et al., Citation2007). Failure to adapt in response to repeated stressors is linked to psychiatric disorders such as post-traumatic stress disorder and major depression (Grissom & Bhatnagar, Citation2009). Several brain regions have been implicated in the process of habituation to repeated stress. In particular, lesions of the posterior (Bhatnagar et al., Citation2002), but not the anterior (Fernandes et al., Citation2002) paraventicular thalamus were found to prevent habituation to repeated restraint. This region projects heavily to several portions of the amygdala (including basolateral and central) which could relay inputs to the paraventricular pPVN neurons and modulate HPA activity (Jankord & Herman, Citation2008). It is currently unclear whether early HFD exposure modifies stress-induced activity in these key regions regulating the HPA axis. The participation of the mPFC and other limbic structures for stressor appraisal and learning the familiarity of the stressor appears to be critical in regulating HPA responses (Grissom & Bhatnagar, Citation2009). Indeed, transient inactivation of the mPFC (Weinberg et al., Citation2010) or right mPFC lesions (Sullivan & Gratton, Citation2002) eliminated corticosterone habituation to repeated stress, but not acute stress, suggesting adaptations in mPFC circuitry with repeated stress. The observation that HFD rats do not display habituation to repeated stress might thus indicate deficits in the function of the mPFC to regulate neuroendocrine adaptation. Previous studies suggest that the diffential effects of repeated stress on HPA activity and NAc DA release (at least in CD rats) may be mediated by region-specific changes in endocannabinoid signaling, specifically via 2-AG production (Patel & Hillard, Citation2008)
In order to test whether hypothalamic indices of HPA axis activation after acute stress were modified by early diet exposure, we measured CRH hnRNA and mRNA levels in brains from a separate cohort of naive animals subjected to either acute tail-pinch or restraint. CRH mRNA levels tended to increase 30 min after tail pinch in both diet groups and a modest, nonsignificant increase in CRH mRNA was observed after restraint only in the CD group, although this time point might not represent maximal CRH mRNA activation (Kovacs & Sawchenko, Citation1996). There were no stress or diet group effects on CRH hnRNA levels, possibly because of the very transient nature of hnRNA production which might not have been captured by our 30-min sampling time. Notably in this study, corticosterone secretion did not reflect changes in circulating ACTH levels reported in the repeated stress conditions. In both diet groups and conditions, a significant stress response was observed, but we did not find indices of either habituation or facilitation. Previous studies have often reported dissociation between plasma ACTH and corticosterone secretion (Doell et al., Citation1981) as adrenal corticosterone secretion is nonlinearly related to ACTH release and can saturate within the range of ACTH secretion observed in our study. We believe that measurement of plasma ACTH levels represents a more accurate reflection of central stress-induced activation compared to corticosterone, and should be used preferentially when experimental procedures allow for a rapid sampling of blood during stimulation.
Facilitation of the ACTH response to a novel stressor
While reduced (habituated) neuroendocrine responses observed with repeated stress might signal adaptation and coping, under these conditions, the neuroendocrine system displays exacerbated responses to novel stressors, highlighting a state of neuroendocrine sensitization or facilitation. With repeated tail-pinch, we showed a facilitated response to restraint, a heterotypic stressor in the CD, but not in the HFD animals. Facilitation within the HPA axis is also known to involve the recruitment and activation of the basolateral amygdala and the posterior paraventricular thalamus where there is increased orexigenic neurotransmission originating from the lateral hypothalamic neurons. (Heydendael et al., Citation2011). Although we have not investigated activation in these structures as a function of perinatal diet, the lack of either habituation or facilitation in HFD offspring suggests that mechanisms underlying adaptability and flexibility in the HPA axis are impaired by early exposure to high fat.
Early nutritional environment and stress: implications for obesity
The nutritional and hormonal milieu of developing young can have long-lasting consequences on energy homeostasis (Levin, Citation2006). In rodents, exposure of the mother to a high-fat diet promotes the development of obesity in the offspring (Walker et al., Citation2008; reviewed in Levin, Citation2006). This increased vulnerability to metabolic disturbances is thought to represent long-lasting adaptations within hypothalamic and dopaminergic brain circuits involved in the homeostatic and hedonic control of feeding behavior. In this article, we demonstrate that exposure to high fat during the perinatal period leads to reduced functional plasticity in mesolimbic DA circuits and impaired neuroendocrine adaptations to repeated tail-pinch stress. It is possible that such dysregulation in the face of repeated stress represents an inability to successfully adapt to stress and might thus increase vulnerability to develop a number of pathologies, including obesity in animals exposed to high fat during early development.
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported by a CIHR operating grant (no. 84299 to to C.D.W. and A.G.); CIHR Canada Graduate Scholarship (awarded to L.N.).
Acknowledgements
We would like to thank Ms Hong Long, QuianNi Zhao and Mr Luc Moquin for expert technical help during the course of these experiments.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. (1989). Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52(5):1655–8
- Antonopoulos J, Dori I, Dinopoulos A, Chiotelli M, Parnavelas JG. (2002). Postnatal development of the dopaminergic system of the striatum in the rat. Neuroscience 110(2):245–56
- Benes FM, Taylor JB, Cunningham MC. (2000). Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathologies. Cerebral Cortex 10:1014–27
- Bhatnagar S, Huber R, Nowak N, Trotter P. (2002). Lesions of the posterior paraventricular thalamus block habituation of hypothalamic--pituitary--adrenal responses to repeated restraint. J Neuroendocrinol 14(5):403–10
- Brake WG, Noel MB, Boksa P, Gratton A. (1997). Influence of perinatal factors on the nucleus accumbens dopamine response to repeated stress during adulthood: an electrochemical study in the rat. Neuroscience 77(4):1067–76
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. (2004). Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 19(7):1863–74
- Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM. (2012). Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience 201:331–7
- Buwembo A, Long H, Walker CD. (2012). Participation of endocannabinoids in rapid suppression of stress responses by glucocorticoids in neonates. Neuroscience, epub ahead of print. DOI: 10.1016/j.neuroscience.2012.10.057
- Cabib S, Puglisi-Allegra S. (2012). The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev 36(1):79–89
- Chen Y, Hatalski CG, Brunson KL, Baram TZ. (2001). Rapid phosphorylation of the CRE binding protein precedes stress-induced activation of the corticotropin releasing hormone gene in medial parvocellular hypothalamic neurons of the immature rat. Brain Res Mol Brain Res 96(1–2):39–49
- Copeland BJ, Neff NH, Hadjiconstantinou M. (2005). Enhanced dopamine uptake in the striatum following repeated restraint stress. Synapse 57(3):167–74
- D’Asti E, Long H, Tremblay-Mercier J, Grajzier M, Cunnane S, Di Marzo V, Walker C-D. (2010). Maternal dietary fat determines metabolic profile and the magnitude of endocannabinoid inhibiton of the stress response in neonatal rat offspring. Endocrinology 151(4):1685–94
- Doell RG, Dallman MF, Clayton RB, Gray GD, Levine S. (1981). Dissociation of adrenal corticosteroid production from ACTH in water-restricted female rats. Am J Physiol 241(1):R21–4
- Doherty MD, Gratton A. (1992). High-speed chronoamperometric measurements of mesolimbic and nigrostriatal dopamine release associated with repeated daily stress. Brain Res 586(2):295–302
- Doherty MD, Gratton A. (1996). Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res 15(1–2):86–97
- El-Khodor BF, Boksa P. (2002). Birth insult and stress interact to alter dopamine transporter binding in rat brain. Neuroreport 13(2):201–6
- Fernandes GA, Perks P, Cox NK, Lightman SL, Ingram CD, Shanks N. (2002). Habituation and cross-sensitization of stress-induced hypothalamic--pituitary--adrenal activity: effect of lesions in the paraventricular nucleus of the thalamus or bed nuclei of the stria terminalis. J Neuroendocrinol 14(7):593–602
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. (2002). Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci 22(18):7840–3
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. (1994). Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem 63(2):575–83
- Grissom N, Bhatnagar S. (2009). Habituation to repeated stress: get used to it. Neurobiol Learn Mem 92(2):215–24
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S. (2011). Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152(12):4738–52
- Imperato A, Angelluci L, Casolini P, Zocchi A, Puglisi-Allegra S. (1992). Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res 577(2):194–9
- Jankord R, Herman JP. (2008). Limbic regulation of hypothalamo--pituitary--adrenocortical function during acute and chronic stress. Ann N Y Acad Sci 1148:64–73
- Kovács KJ, Sawchenko PE. (1996). Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci 16(1):262–73
- Levin BE. (2006). Metabolic imprinting: critical impact of the perinatal environment on the regulation of energy homeostasis. Philos Trans R Soc Lond B Biol Sci 361(1471):1107–21
- Liu D, Diorio J, Tannenbaum B, Caldgi C, Francis D, Freedman A, Sharma S, et al. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic--pituitary--adrenal responses to stress. Science 277(5332):1659–62
- Lupinsky D, Moquin L, Gratton A. (2010). Interhemispheric regulation of the medial prefrontal cortical glutamate stress response in rats. J Neurosci 30(22):7624–33
- Mansi JA, Rivest S, Drolet G. (1998). Effect of immobilization stress on transcriptional activity of inducible immediate-early genes, corticotropin-releasing factor, its type 1 receptor, and enkephalin in the hypothalamus of borderline hypertensive rats. J Neurochem 70(4):1556–66
- Naef L, Moquin L, Gratton A, Walker CD. (2012). Reduced anticipatory dopamine responses to food in rats exposed to high-fat during early development. Int J Obes, epub ahead of print. DOI: 10.1038/ijo.2012.153
- Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD. (2011). Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 176:225–36
- Naef L, Srivastava L, Gratton A, Hendrickson H, Owens SM, Walker CD. (2008). Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: reduction on the behavioral responses to repeated amphetamine administration. Psychopharmacology 197:83–94
- Nesse RM, Bhatnagar S, Young E. (2007). The evolutionary origins and functions of the stress response. In: Fink G, editor, The encyclopedia of stress. 2nd ed. New York: Academic Press
- Nicniocaill B, Gratton A. (2007). Medial prefrontal cortical alpha1 adrenoreceptor modulation of the nucleus accumbens dopamine response to stress in Long-Evans rats. Psychopharmacology 191(3):835–42
- Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S. (2007). The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cereb Cortex 17(12):2796–804
- Patel S, Hillard CJ. (2008). Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur J Neurosci 27(11):2821–9
- Paxinos G, Watson C. (1998). The rat brain in stereotaxic coordinates, 4th ed. New York: Academic Press
- Pierce RC, Bari AA. (2001). The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci 12(2):95–110
- Proulx K, Clavel S, Nault G, Richard D, Walker CD. (2001). High neonatal leptin exposure enhances brain GR expression and feedback efficacy on the adrenocortical axis of developing rats. Endocrinology 142(11):4607–16
- Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S. (1991). Acute stress induces time-dependent responses in dopamine mesolimbic system. Brain Res 554(1–2):217–22
- Rougé-Pont F, Deroche V, Le Moal M, Piazza PV. (1998). Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci 10(12):3903–7
- Sullivan RM, Gratton A. (2002). Prefrontal cortical regulation of hypothalamic--pituitary--adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology 27(1–2):99–114
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. (1997). High-fat feeding alters both basal and stress-induced hypothalamic--pituitary--adrenal activity in the rat. Am J Physiol 3(6 Pt 1):E1168–77
- Teegarden SL, Scott AN, Bale TL. (2009). Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 162(4):924–32
- Tozuka Y, Kumon M, Wada E, Onodera M, Mochizuki H, Wada K. (2010). Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem Int 57(3):235–47
- Trottier G, Koski KG, Brun T, Toufexis DJ, Richard D, Walker C-D. (1998). Increased fat intake during lactation modifies hypothalamic--pituitary--adrenal responsiveness in developing rat pups: a possible role for leptin. Endocrinology 139(9):3704–11
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. (2010). Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 1(10):4756–64
- Walker C-D, Anand KJS, Plotsky PM. (2001). Ontogeny of the hypothalamus--pituitary--adrenal axis and the stress response. In: McEwen BS, Goddman HM, editors. Handbook of Physiology section 7: the endocrine system: coping with the environment: neural and endocrine mechanisms. New York: Oxford Press. p 237
- Walker C-D, Naef L, d’Asti E, Long H, Xu Z, Moreau A, Azeddine B. (2008). Perinatal maternal fat intake affects metabolism and hippocampal function in the offspring: a potential role for leptin. Annals NY Acad Sciences 1144:189–202
- Weinberg MS, Girotti M, Spencer RL. (2010). Medial prefrontal cortex activity can disrupt the expression of stress response habituation. Neuroscience 168(3):744–56
- Zhang TY, Chretien P, Meaney MJ, Gratton A. (2005). Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J Neurosci 25(6):1493–502
