Abstract
Both chronic stress conditions and hyperergic reaction to environmental stress are known to enhance cancer susceptibility. We described two mouse lines that displayed high (HA) and low (LA) swim stress-induced analgesia (SSIA) to investigate the relationship between inherited differences in sensitivity to stress and proneness to an increased growth rate of subcutaneously inoculated melanoma. These lines display several genetic and physiological differences, among which distinct sensitivity to mutagens and susceptibility to cancer are especially noticeable. High analgesic mice display high proneness both to stress and a rapid local spread of B16F0 melanoma. However, stress-resistant LA mice do not develop melanoma tumors after inoculation, or if so, tumors regress spontaneously. We found that the chronic mild stress (CMS) procedure leads to enhanced interlinear differences in melanoma susceptibility. Tumors developed faster in stress conditions in both lines. However, LA mice still displayed a tendency for spontaneous regression, and 50% of LA mice did not develop a tumor, even under stressed conditions. Moreover, we showed that chronic stress, but not tumor progression, induces depressive behavior, which may be an important clue in cancer therapy. Our results clearly indicate how the interaction between genetic susceptibility to stress and environmental stress determine the risk and progression of melanoma. To our knowledge, HA/LA mouse lines are the first animal models of distinct melanoma progression mediated by inherited differences in stress reactivity.
Introduction
The intensity of stress-induced analgesia (SIA) is strongly correlated with stress proneness (Butler & Finn, Citation2009; Panocka et al., Citation1986b). Divergent selection for high (HA-high analgesia) and low (LA-low analgesia) stress-induced analgesia induced by 3 min of swimming in 20 °C water (SSIA-swim stress-induced analgesia) established the basis of a murine model for studies on genetic, physiological and pathological aspects of stress (Panocka et al., Citation1986a).
The magnitude of SSIA correlates with differences in opioid system activity in the HA and LA lines. For example, HA mice are more sensitive to morphine analgesia than LA mice (Lutfy et al., Citation1994; Mogil et al., Citation1996a; Panocka et al., Citation1991; Sadowski & Panocka, Citation1993). Their high SSIA is partially reversed by naloxone, revealing a mixed opioid/non-opioid character. Conversely, low analgesia in the LA line is insensitive to this prototypic opioid receptor antagonist (Marek et al., Citation1992; Mogil et al., Citation1996b). Additional studies have revealed the differential binding of exogenous ligands to specific brain regions (Kest et al., Citation1999; Mogil et al., Citation1994). Another factor that contributes to the HA/LA divergence in opioid analgesia may be linked to a C320T transition in exon 2 of the δ-opioid receptor gene (Sacharczuk et al., Citation2010a), or to increased blood-brain barrier (BBB) permeability in HA mice, as mentioned in our previous publication (Gajkowska et al., Citation2011).
High SSIA in HA mice is accompanied by several other characteristics. HA mice appear to be more susceptible to the mutagenic effect of whole-body γ-radiation and mitomycin-C injection. Both treatments cause higher frequencies of chromosomal aberrations and micronuclei in bone marrow and white blood cells in the HA than in the LA line (Sacharczuk et al., Citation2003b). Those tests demonstrated a malfunction in the DNA repair system in HA mice, especially with respect to nucleolar organizer region (NOR) activity, which was lower in HA mice (Sacharczuk et al., Citation2003a). It was proposed that the breeding strategy, along with differentiation of stress-related phenomena, had altered the activity of genes coding rRNA and that this activity is important for controlling DNA repair in both lines (Antoni et al., Citation2006; Antonova et al., Citation2011; Sood et al., Citation2010).
Recent results showed that a subcutaneous inoculation of B16F0 melanoma cells into the hindpaw resulted in rapid tumorigenesis in the HA line, while mice from the LA line were melanoma-resistant or developed medium-sized tumors with a tendency for complete spontaneous regression. Moreover, tumor development in the LA line was significantly delayed in comparison to HA mice. The magnitude of cancer hyperalgesia was similar in both lines and was apparent immediately after the first symptoms of melanoma invasion. However, relatively short-lived hyperalgesia in HA mice was restored by naltrexone, which proved to be ineffective in LA mice that display much more prolonged hyperalgesia. The results suggested that genetically determined opioid system activity participates in both the intensity and persistence of cancer pain (Sacharczuk et al., Citation2012).
In the present study, we established a model of chronic mild stress (CMS) to enhance interlinear divergence in the stress response. The main goal of this study was to determine whether a melanoma-resistant LA line would retain the tendency for tumor regression in stress conditions. We showed that CMS conditions enhance tumor growth, which becomes rapid in both stress-prone HA mice and stress-resistant LA mice. Nevertheless, spontaneous regression in LA mice occurred, even in stress conditions. Moreover, 50% of LA mice did not develop a tumor at any observation point. We also showed that there were no depressive responses to melanoma tumor growth in mice of either line. However, CMS-induced increases in depression-related behavior were observed in the HA line but not in the LA line.
Although the specific mechanisms underlying the differences in HA and LA responses to inoculated melanoma and sensitivity to cancer pain have not yet been defined at the cellular/molecular level, our results clearly demonstrate that the expression of genetically determined stress-related phenomena may be a key factor in the epidemiology of melanoma.
Materials and methods
Animals
Male Swiss-Webster mice were obtained from the colony maintained at the Institute of Genetics and Animal Breeding of the Polish Academy of Sciences in Jastrzebiec. The animals were selectively bred for 78 generations for high (the HA line) and low (the LA line) SSIA. The selection protocol for the HA and LA lines was described previously (Panocka et al., Citation1986a,Citationb). Briefly, outbred Swiss-Webster mice of either sex, 2 min after completion of 3 min swimming in 20 °C water, were screened for the latency of a nociceptive reflex on a hot plate at 56 °C. Those displaying the longest (50–60 s) and the shortest (<10 s) post-swim latencies of the hindpaw flick or lick response (whichever occurred first) were selected as progenitors of the HA and the LA lines. A similar procedure was repeated in each offspring generation, but only subjects displaying the longest and the shortest post-swim hot plate latencies were mated to maintain the lines.
After weaning, the mice were housed in groups, 4–5 siblings per cage, at an ambient temperature of 22 ± 2 °C and 55 ± 5% relative humidity and in a 12-h light/dark cycle (lights on at 07:00 a.m.). They were given free access to tap water and pellet food (rodent block chow) provided by LABOFEED H (Kcynia, Poland): 22% proteins (with 1.5% of lysine), 5% crude fiber, 4% crude fat, 6.5% crude ash, and 13.4 kcal/g of energy). Twenty-one days before B16F0 melanoma cell inoculation (seven days before the CMS-chronic mild stress procedure), the animals from all groups (non-stressed (NS) and CMS groups) were transferred to individual polycarbonate shoebox cages and remained there throughout the entire experiment (). When entering the experiment, HA mice weighed 32–34 g and LA mice weighed 33–35 g.
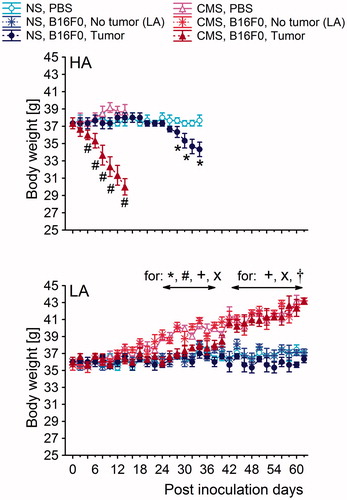
Figure 2. Two daily average body weights in high analgesia (HA) and low analgesia (LA) mice. Body weights of non-stressed (NS) and chronically stressed (CMS) mice from PBS- and B16F0-inoculated groups were measured to monitor the overall health status. Statistically significant differences in body weight were observed between the NS and CMS groups from the HA line across time. Post-hoc comparisons within each line. *: B16F0-inoculated group versus PBS-inoculated group from NS conditions; #: B16F0-inoculated group versus PBS-inoculated group from CMS conditions; x: B16F0-inoculated group from CMS conditions versus B16F0-inoculated group from NS conditions. Symbols indicate p < 0.05. In the HA line, each B16F0-inoculated group consisted of 20 mice. In the LA line, the B16F0-inoculated group from NS conditions consisted of 6 mice with tumors and 14 mice without tumors. In the same line, B16F0-inoculated groups from CMS conditions consisted of 10 mice with developed tumors and 10 mice without tumors. Each PBS-inoculated group from the HA line and the LA line consisted of 10 mice.
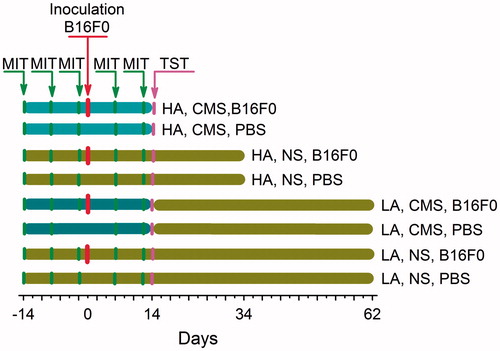
The experiment was performed on 60 mice from each line, with 40 mice inoculated with B16F0 melanoma cells and 20 mice injected with phosphate buffered saline (PBS). To evaluate the effects of line (HA versus LA) and condition (CMS versus NS) on tumor growth and cancer pain, mice from both lines inoculated with B16F0 melanoma cells or PBS were randomly assigned to two equal groups (CMS group and NS group).
Inoculation of B16F0 melanoma cells
B16F0 melanoma cells were cultured in Eagle’s medium (high glucose: 4.5 g/l, Sigma) containing 10% fetal bovine serum (Gibco, Germany). The number of tumor cells was counted with a hematocytometer. The melanoma cells (2 × 105) were suspended in 25 µl of PBS and injected subcutaneously into the plantar region of left paw. Inoculation in HA/LA CMS groups took place after 14 days of the CMS procedure. Inoculation of mice from NS groups was performed at the same time. Control mice were injected with 25 µl of PBS.
Monitoring of tumor growth
The maximum plantar widths of inoculated paws were measured with a manual caliper in 2 day intervals. Photographic documentation of tumor growth was conducted simultaneously.
Behavioral assay
Mice were placed into the test room 30 min before the test. All tests were performed between 7:00–12:00 a.m. in a quiet room. After each test, mice were placed back in their individual cages and returned to the breeding room. Each behavioral test was performed on a different day. The analyses of the behavioral experiments were performed by experimenters who were blind to the mouse groups.
CMS procedure
From day 14 before inoculation, the CMS groups of the HA and LA lines were exposed for over 28 days (14 days before and 14 days after inoculation) to a set of various stressors. Each cycle (7 days) of stress regime consisted of: two periods of food deprivation (8 h), two periods of 45° cage tilt (12 h), two periods of soiled cage (8 h), two periods of low-intensity stroboscopic illumination (8 h), two periods of overnight illumination (12 h), two periods of removed bedding (12 h), two periods of noise emitted by a radio (12 h), and two periods of no stress (12 h). NS groups were maintained under the same baseline conditions. Mice exposed and not exposed to CMS were housed in separate animal rooms. A detailed procedure of CMS was adapted from Willner (1997) with small modifications (to eliminate the unpredictable effect on the tumor growth, the soiled cage period was eliminated after inoculation). To achieve an unpredictable CMS procedure, stressors were applied in a semi-random order.
Assessment of depressive/sickness behavior
Behavioral response to melanoma tumor growth and CMS was assessed using two widely accepted tests of milk intake (MIT) and tail suspension (TST).
Milk intake test (MIT)
Sweetened solutions, such as milk, are preferred by rodents. Lack of consumption indicates a reluctance to engage in hedonic behavior (Papp et al., Citation1991). MIT was performed three times before inoculation (before, 6 days and 12 days after the start of CMS) and two times after inoculation (20 days and 26 days after the start of CMS) (). Before the first trial, mice were habituated for 3 days to drinking sweetened condensed milk (diluted 1:1 with tap water) from 20 ml glass bottles fitted with metal spouts. Two hours before MIT, all mice, including the NS groups, were deprived of water and food, and all CMS procedures were stopped. Weighed bottles were placed in cages at approximately 10 a.m. for 60 min, then removed and reweighed. All animals consumed 3 g or more of milk during the session on the final day of habituation. Mice resumed eating and drinking freely after MIT.
Figure 1. Experimental protocol. High analgesia (HA) mice from the non-stressing (NS) group and the chronic mild stress (CMS) group were monitored for 14 and 34 post-inoculation days, respectively. Low analgesia (LA) mice from both the NS group and the CMS group were well maintained and monitored for 62 post-inoculation days (PID).
Immobility in the tail suspension test (TST)
TST was performed 28 days after the initiation of the CMS procedure for comparison with the results obtained from the MIT. At this time, decreased milk intake was observed in all CMS mice from both HA and LA lines. Assessment of depression-like behavior in the tail suspension test (TST) was performed as suggested by Steru and coauthors (Citation1985). Animals were observed in a 680 (high) × 365 (wide) × 280 (deep) mm wooden box with the front wall removed. A fabric ribbon (200 × 17 × 1 mm) was attached to the cover. Mice were suspended from the cover by attaching their tail to the ribbon with adhesive tape. Adhesive tape was placed 30 mm from the base of the tail. Suspended mice were 120 mm away from the box walls. The total duration of immobility was scored for 6 min using the EthoVision system (Noldus, Wageningen, the Netherlands). Mouse groups were blinded to the observer that assessed immobility.
Assessment of cancer pain (heat hyperalgesia)
Nociceptive responses to thermally induced pain were measured as hindpaw withdrawal (PW) in the radiant heat anesthesiometer apparatus (Ugo Basile, Italy). Testing was performed during the light phase of the circadian cycle (07:00 a.m.–11:00 a.m.). PW latencies were measured in both paws. During PW, the mouse was held gently by hand in a special glove. The intensity of the radiant heat stimulus was adjusted so that non-selected outbreed Swiss Webster mice (control mice) responded with latencies in the range of 6.0 ± 1 s. The correct response was either a brisk paw flinch or lifting of the paw. The cutoff latency was set at 10 s to avoid tissue damage. PW latencies were measured in triplicate at an interval of 30 s, and the mean value was calculated to determine the PW threshold for each animal and time point. To compare HA and LA mice, the magnitude of hyperalgesia in PW was expressed as the percent of maximum possible effect (%MPC) using a formula:
Right paw – contralateral
Left paw – inoculated
Histology
For examination of the primary hind paw tumor and potential metastasis into lungs, all mice were euthanatized with 100 mg/kg intraperitoneal pentobarbital sodium (Nembutal, Polfa, Poland). Lungs and paws were removed and postfixed in Bouin’s and 1% paraformaldehyde in 0.1 M PBS solution, respectively. Fixed tissues were embedded in paraffin. Sections (5 μm) were mounted onto Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA) and stained with hematoxylin and eosin. Histopathologic examination was performed under a light microscope (Nikon, Tokyo, Japan).
Statistical analysis
To estimate the differences in tumor size between groups and between subsequent time points in one group, nonparametric Mann--Whitney U (2 groups) or Kruskal--Wallis (>2 groups) tests were applied for all group comparisons (with nonmodel outcomes). The data from tumor growth, with inoculation (B16F0 versus PBS), genotype (HA versus LA line) and conditions (NS versus CMS conditions) as independent measures, were analyzed with ANOVA using Statistica® 7.1 Software. Post-hoc comparisons at different time points were made with the Bonferroni test. All results presented in the graphs are the means ± SE. Significance was set at a probability level of p ≤ 0.05.
Ethical note: The general state of the animals' health was monitored by measuring body weight and with everyday observation. The protocol for the experiments on live mice was approved by the State Ethics Commission, in conformity with Polish law. All the procedures are commonly used and considered ethically acceptable in all the European Union countries and North America. They also conform to the International Association for the Study of Pain (Zimmermann, Citation1983).
Results
General features
After inoculation with B16F0 cells, HA and LA mice from the NS groups were maintained for approximately 34 and 62 post-inoculation days PID, respectively. In the HA line, CMS facilitated tumor growth and local changes in the tumor site (e.g. necrosis), accompanied by quicker body weight loss (), earlier deterioration in overall health status (e.g. poor state of fur) and enhanced symptoms of depressive behavior in TST and MIT tests ( and ). Such CMS-induced changes were similar in B16F0 and PBS groups of the HA line ( and ) and were the reason for earlier termination of the experiment in these groups. In contrast, LA mice were in good general health throughout the entire experimental period (). Imposition of CMS similarly increased body weights in both the B16F0 and PBS groups over the entire study period (). Moreover, LA mice chowed less depression-like behavior than HA mice after CMS, as shown in the MIT and TST tests ( and ).
Figure 3. Milk intake test (MIT) in the high analgesia (HA) and low analgesia (LA) mice performed at baseline, 7 days, 14 days, 21 days, and 28 days after the start of chronic mild stress (CMS). The intake of milk in the CMS-B16F0 and CMS-PBS groups (as controls for the melanoma group) was significantly lower than in the NS-Melanoma and NS-PBS groups at 7, 14, 21 and 28 days after the start of CMS in the HA line and at 21 and 28 in the LA line. Post-hoc comparisons within in each line: +: (PBS-inoculated group from CMS conditions versus PBS-inoculated group from NS conditions); x: (B16F0-inoculated group from CMS conditions versus B16F0-inoculated group from NS conditions). Symbols indicate p < 0.05. In the HA line, each B16F0-inoculated group consisted of 20 mice. In the LA line, the B16F0-inoculated group from NS conditions consisted of 6 mice with tumors and 14 mice without tumors. In the same line, B16F0-inoculated groups from CMS conditions consisted of 10 mice with developed tumors and 10 mice without tumors. Each PBS-inoculated group from the HA line and the LA line consisted of 10 mice.
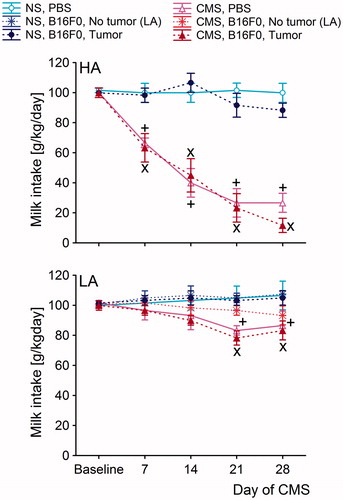
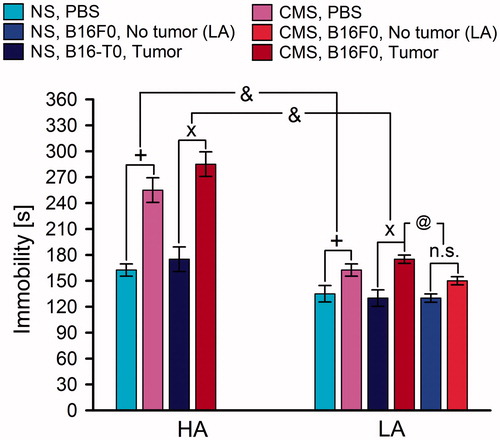
Figure 4. Mean time of immobility (±SE) in the tail suspension test (TST) in high analgesia (HA) and low analgesia (LA) mice. Mice were housed in non-stress conditions (NS) or in chronic mild stress conditions (CMS) and were inoculated with PBS (PBS groups) or B16F0 melanoma cells (B16F0 groups). Post-hoc comparisons within each line: +: (PBS-inoculated group from CMS conditions versus PBS-inoculated group from NS conditions); x: (B16F0-inoculated group from CMS conditions versus B16F0-inoculated group from NS conditions); @: (CMS x Tumor growth). Interaction between CMS interactions between lines: &: Line x CMS (for PBS- and B16F0-inoculated groups). n.s.: p = 0.062. Symbols indicate p < 0.05. In the HA line, each B16F0-inoculated group consisted of 20 mice. In the LA line, the B16F0-inoculated group from NS conditions consisted of 6 mice with tumors and 14 mice without tumors. In the same line, the B16F0-inoculated groups from CMS conditions consisted of 10 mice with developed tumors and 10 mice without tumors. Each PBS-inoculated group from the HA line and the LA line consisted of 10 mice.
Effects of chronic mild stress (CMS) on melanoma tumorigenesis
In the HA line, 100% of mice from the NS and CMS groups inoculated with B16F0 cells developed tumors. However, the first symptoms of melanoma cell invasion occurred earlier in the CMS group than in the NS group (5.1 PID versus 14.2 PID; difference = 9.1 PID; p < 0.001) (, ). Mice from the CMS group developed large-sized tumors (≥400% of the non-tumor paw width) () much earlier than NS mice (13.3 days versus 30.5 days from the first symptoms, difference = 17.2 days, p < 0.001). However, we found that there were no statistically significant differences in maximum tumor size between the groups.
Figure 5. The time course of the change in plantar width of the plantar-inoculated mice of the high analgesia (HA) and low analgesia (LA) lines. Mice were given a subcutaneous injection of B16F0 cells into the plantar region of left hind paw. The values are the mean ± S.E.M. Post-hoc comparisons within each line: *: (B16F0-inoculated group versus PBS-inoculated group from NS conditions); #: (B16F0-inoculated group versus PBS-inoculated group from CMS conditions). Symbols indicate p < 0.05. In the HA line, each B16F0-inoculated group consisted of 20 mice. In the LA line, the B16F0-inoculated group from NS conditions consisted of 6 mice with tumors and 14 mice without tumors. In the same line, B16F0-inoculated groups from CMS conditions consisted of 10 mice with developed tumors and 10 mice without tumors. Each PBS-inoculated group from the HA line and the LA line consisted of 10 mice.
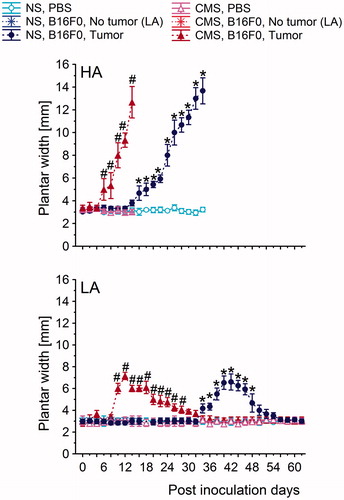
Figure 6. Melanoma tumor processes in the high analgesic (HA) and low analgesic (LA) mice after subcutaneous inoculation with B16F0 melanoma cells into the plantar region of the hindpaw.
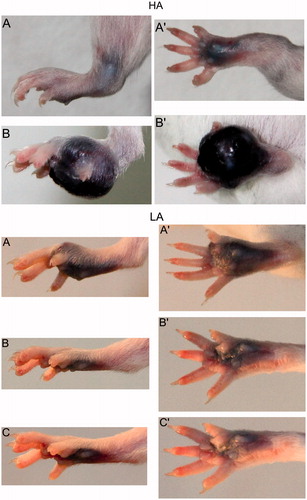
Table 1. Summary of the results. ↑ – increase or ↔ – no changes in tumor growth, cancer pain or depression-like behavior in comparison to the Control or Melanoma groups.
Similar to the HA line, differences in tumor incidence between the stressed and the nonstressed LA mice were not statistically significant (50% versus 30%; χ2 = 1.67, df = 1, p = 0.20). The median time to onset of the first symptoms of melanoma cell invasion was shorter in the CMS group than in the NS group (8.2 PID versus 34.1 PID, difference = 25.9 PID, p < 0.001) (, ). However, the median time from the first symptoms of melanoma cell invasion to developed tumors (≥200% of the non-tumor paw width) () was similar in the CMS and NS groups of the LA line (2.8 versus 3.9, difference = 1.1, p = 0.79). There were also no significant differences in maximum tumor size between the CMS and NS groups. Furthermore, by 38.3 PID and 60.6 PID, the CMS and NS tumor-bearing mice showed complete tumor regression and no new tumors ( and ). The elapsed time from the first signs of melanoma invasion to complete tumor regression was similar in the CMS group and the NS group (30.1 versus 26.5, difference = 3.6, p = 0.052).
Effect of CMS conditions on cancer pain
Baseline nociception
Baseline nociceptive latencies obtained from all HA and LA mice before inoculations and CMS procedures are shown in . Two-way ANOVA between lines showed that compared with LA mice, HA mice displayed longer PW latencies in both paws [F(1,116) = 10.14; p < 0.001]. Post-hoc analysis within lines showed the lack of differences between the right and left paws in the HA line and in the LA line.
Figure 7. Baseline nociceptive latencies (±SE) in high analgesia (HA) and low analgesia (LA) mouse lines. Nociception was measured with using a heat analgesiometer. Each line consisted of 40 mice. # indicate p < 0.05 (HA line versus LA line).
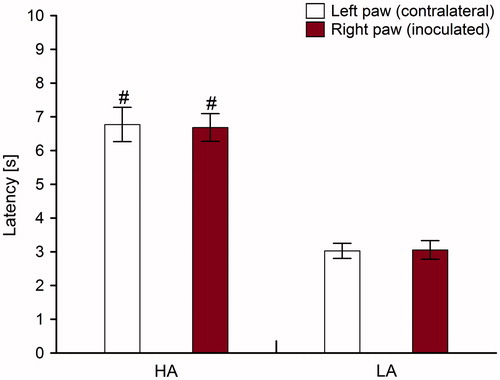
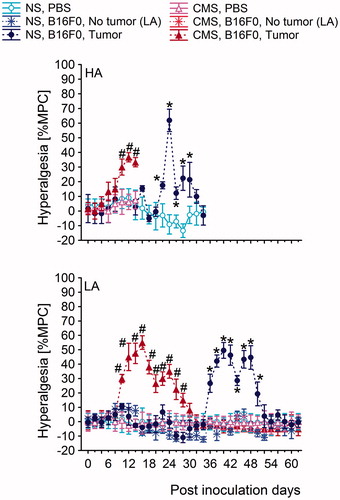
Figure 8. Time course of the development of thermal hyperalgesia in high analgesia (HA) and low analgesia (LA) mice. The pain threshold was measured in the ipsilateral paw and the contralateral paw stimulated with radiant heat. The values are the mean ± S.E.M. Post-hoc comparisons within each line: *: (B16F0-inoculated group versus PBS-inoculated group from NS conditions); #: (B16F0-inoculated group versus PBS-inoculated group from CMS conditions). Symbols indicate p < 0.05. In the HA line, each B16F0-inoculated group consisted of 20 mice. In the LA line, the B16F0-inoculated group from NS conditions consisted of 6 mice with tumors and 14 mice without tumors. In the same line, B16F0-inoculated groups from CMS conditions consisted of 10 mice with developed tumors and 10 mice without tumors. Each PBS-inoculated group from the HA line and the LA line consisted of 10 mice. %MPC: (maximum possible change).
Thermal hyperalgesia in melanoma-bearing mice
In the HA line, CMS accelerated the onset of thermal hyperalgesia from 8.1 days in non-stressed mice to 4.9 days in mice subjected to CMS; difference = 3.2; p < 0.05). Moreover, stressed HA mice reached the maximum magnitude of tumor-induced hyperalgesia 3 days earlier than non-stressed mice (7.2 days versus 10.2 days from the first signs of tumor growth; difference = 3.0; p < 0.05). Interestingly, we found that thermal hyperalgesia was more profound in mice from the NS group than in the CMS group [F(1,38) = 7.93; p < 0.01] (, Figure 8).
In the LA line, the first signs of hyperalgesia in NS conditions and CMS conditions occurred concurrently with the first symptoms of tumor growth (Figure 8). The maximum magnitude of tumor-induced hyperalgesia was also similar and occurred at similar times under CMS and NS conditions (7.7 days versus 6.2 days from the first symptoms of tumor progression).
Effects of melanoma and CMS on body weight
Inoculation with B16F0 melanoma cells decreased body weight in HA, but not in LA mice, as confirmed by 3-way ANOVA [F(1,112) = 4.03; p < 0.05 – B16F0 × line interaction]. This finding is supported by the significant main effect of B16F0 within the HA line [F(1,56) = 7.96; p < 0.01]. Moreover, a significant B16F0 × CMS interaction within the HA line [F(1,56) = 4.12; p < 0.05] indicates that body weight loss was more pronounced under CMS than in NS conditions.
CMS increased body weight in a line-dependent manner [F(1,56) = 4.83; p < 0.05 – line × CMS interaction]. Subsequent two-way ANOVA confirmed that the CMS effect was significant in LA (F(1,56) = 5.12; p < 0.05) mice, but not in HA mice. This effect was similar in the B16F0-inoculated and PBS-inoculated groups of the LA line, as shown by the non-significant CMS × B16F0 interaction.
Effect of CMS and tumor growth on depression-like behavior
Milk intake test
As depicted in , there were no significant differences in milk intake between the HA and LA lines under NS conditions. We also showed that melanoma growth did not alter milk intake in either of the lines. In contrast, a significantly lower consumption of milk was observed in CMS conditions, as confirmed by three-way ANOVA [F(1,112) = 5.45, p < 0.05 – main effect of CMS]. CMS decreased milk intake more markedly in HA mice than in LA mice [F(1,112) = 7.02; p < 0.01 – line × CMS interaction].
Two-way ANOVA of the data from the HA line showed that CMS significantly decreased milk intake [F(1,56) = 9.19; p < 0.01] (). The anhedonic effect of CMS was similar in PBS-inoculated and B16F0-inoculated animals (no significant CMS × melanoma interaction). Furthermore, post hoc Fisher’s PLSD test showed that milk intake in CMS groups (PBS-inoculated and B16F0-inoculated) was significantly lower than in corresponding groups under NS conditions at 7 (p < 0.01), 14 (p < 0.001), 21 (p < 0.001) and 28 days (p < 0.001).
In the LA line, individuals housed in CMS conditions displayed lower milk intake than individuals from the NS groups [F(1,56) = 4.15; p < 0.05] (). The non-significant interaction between CMS and B16F0 mainly reflects the lower impact of CMS in the LA line. Post hoc Fisher’s PLSD test showed that milk intake was significantly lower in the CMS group of PBS-inoculated individuals at 21 days (p < 0.05) and 28 days (p < 0.01) after the start of CMS. Interestingly, animals resistant to inoculated melanoma were simultaneously less susceptible to the anhedonic effect of CMS than tumor-bearing mice, as was shown by a significantly lower decrease in milk intake at the 21st day of the CMS procedure (p < 0.05).
Tail suspension test (TST)
CMS significantly prolonged the time of immobility in the TST [F(1,112) = 4.03; p < 0.05 – three-way ANOVA] and was more effective in HA mice than in LA mice, as shown by line × CMS interaction [F(1,112) = 7.34; p < 0.01]. Analysis within lines confirmed the higher effect of CMS within the HA line [F(1,56) = 17.02; p < 0.001] than within the LA line [F(1,56) = 7.56; p < 0.05]. The effect of CMS was similar in PBS-inoculated and B16F0-inoculated animals, as indicated by the non-significant line × B16F0 × CMS interaction as well as the non-significant effects of B16F0 and B16F0 × CMS interaction within both lines.
Histology
During CMS, histological changes in the paws with developed tumors were highly correlated with tumor progression and were similar to those described previously for tumor progression in NS conditions (Sacharczuk et al., Citation2012). Only the intensity of vascularization in the largest tumors in HA mice was increased in CMS conditions. In LA mice, regressed tumors displayed normal structure, with remnant, extracellular melanin deposits. In both the HA and LA lines housed in NS or CMS groups, histological analysis of the inner organs did not confirm any metastases.
Discussion
Numerous studies have found that long-lasting stress increases individual vulnerability to cancer (Dhabhar et al., Citation2012; Frick et al., Citation2009; Kruk, Citation2012; Peters, Citation2012; Saul et al., Citation2005). However, biological factors that contribute to the stress-enhanced development of cancer are not completely known. Because of its multifaceted character, cancer-induced pain also remains inadequately controlled, thus posing a challenge for modern medicine (Christo & Mazloomdoost, Citation2008). In the field of melanoma, studies on the genetic- and stress-dependent proneness to cancer progression have recently become a deeply discussed topic (Sanzo et al., Citation2010).
In our research, we have found one of the putative links between chronic stress, genetic background, proneness to inoculated melanoma and cancer pain. Using two mouse lines selected for distinct stress susceptibility, we created a murine model of melanoma B16F0, which was recently studied in the context of CMS.
First, we showed that inoculation with B16F0 melanoma cells caused rapid tumorigenesis in the HA line. The mice from the LA line were melanoma-resistant or developed medium-sized tumors with a tendency for complete, spontaneous regression. Moreover, tumor development in the LA line was significantly delayed. Cancer pain induced by tumor growth and measured as thermal hyperalgesia was apparent at a similar time after the first symptoms of melanoma invasion in both lines, and was manifested by long-lasting hyperalgesia in LA mice, which rapidly declined in HA mice. In the HA line, loss of hyperalgesia was observed together with tumor growth, whereas in the LA line, this process was linked with tumor regression (Sacharczuk et al., Citation2012). We concluded that high proneness to inoculated melanoma in HA mice may be caused by the prooncogenic effects of enhanced opioid system activity in this line. We also hypothesize that the pathological leakage of the BBB for peripheral endogenous opioids may be responsible for the rapid termination of hyperalgesia in the HA mice (Gajkowska et al., Citation2011).
In the present study, we showed that CMS conditions enhance tumor growth, which becomes rapid in both stress-prone HA mice and stress-resistant LA mice. In NS conditions, tumor growth was considerably delayed in LA mice in comparison to HA mice, but the first symptoms of melanoma cell invasion in CMS conditions occurred at a similar time rate. However, later tumorigenesis was strongly differentiated among lines. In HA mice, tumor growth was rapid, while LA mice showed delayed tumorigenesis. The majority of HA specimens developed large-sized tumors, while LA mice in general developed small tumors. Moreover, while HA mice did not display regression, LA mice experienced this phenomenon. Additionally, the general health of LA animals was markedly better than the condition of the HA line. Thus, it can be stated that the LA line is rather melanoma-resistant, while the HA line is melanoma-prone.
Acceleration of tumor growth by stress conditions in HA and LA mice indicate that stress is crucial in melanoma growth. Despite the fact that LA mice developed more tumors in stress conditions than in NS conditions, visible melanoma tumor growth was more moderate in those specimens. Moreover, LA mice preserved a tendency for spontaneous regression, even under CMS conditions. Different sensitivities to stress-induced changes in tumor growth between HA and LA mice imply a critical role of interactions between endophenotypes (high versus low sensitivity to stress) and environmental stress in the risk of melanoma development and therapy prognosis.
Such distinctions in susceptibility to inoculated melanoma in CMS conditions between HA and LA mice can be linked to specific features of these lines. HA mice are more sensitive not only to the swim stress, but also display higher sensitivity to development of depression-like behaviors following CMS (Sacharczuk et al., Citation2009), which was confirmed in the present study. Interestingly, tumor growth did not promote depression-like behavior in either HA and LA mice. The effect of CMS was also similar in the PBS and B16F0 groups of HA and LA lines. These findings suggest that progressed melanoma growth does not potentiate the severity of depression-like behavior and anhedonia evoked by CMS, even in the terminal stage in HA mice. LA mice resistant to locally implanted melanoma were simultaneously less susceptible to the anhedonic effect of CMS than tumor-bearing HA mice.
Our results on the animal model argue against the preventive use of antidepressants in cancer patients and are supported by the findings of Kubera et al. (Citation2009), who documented an association between antidepressants use and the higher risk of enhanced melanoma promotion and metastasis formation. On the other hand, the lack of depressogenic effects of tumor growth in HA and LA mice may be associated with decreased expression of neuroactive cytokines, such as interleukin (IL)-1β, IL-6, TNF-α and IFN-α, which display depressogenic properties while being simultaneously associated with endogenous antineoplastic response processes (Anisman, Citation2011).
In the HA and LA lines, thermal hyperalgesia was strongly linked with tumor development. The stressed HA mice developed hyperalgesia that reached the maximum magnitude 3 days earlier than non-stressed mice. Acceleration of thermal hyperalgesia was determined by increased total sensitivity to cancer pain. Interestingly, we found that the maximum magnitude of thermal hyperalgesia was higher in HA mice from the NS group than in the CMS group. The fast increase of hyperalgesia in the NS group of the HA line preceded its rapid decline. In CMS conditions, the loss of hyperalgesia in HA mice was not monitored because of the rapid deterioration of the animals' condition. In the LA line, the first signs of hyperalgesia in both the NS and CMS conditions occurred concurrently with the first symptoms of tumor growth. The maximum magnitude of tumor-induced hyperalgesia was also similar in NS and CMS conditions and occurred at a similar time from the first symptoms of tumor progression. The loss of hyperalgesia was also observed in LA mice, but in contrast to HA mice, this process was linked to tumor regression.
The rapid loss of hyperalgesia in the HA line was most likely determined by genetic differences in the activity of opioid systems, which participate in both the intensity and persistence of cancer pain. Similar to tumor progression, between-line differences in the persistence of cancer pain may be enhanced by the increased permeability of the BBB in HA mice and the increased influx of peripheral opioids to the CNS. It may be concluded that, if high resistance to inoculated melanoma may be a specific feature of the stock of Swiss Webster mice, the pathological sensitivity to stress in HA mice could be the primary reason for high susceptibility to cancer.
It would be quite interesting to perform additional studies to evaluate the potential role of growth promoting neurotrophic factors (Berking et al., Citation2004) in enhanced melanoma progression in HA mice, in association with the pathological leakage of BBB.
In summary, the present results enriched our knowledge about the role of the interaction between genetically determined sensitivity to stress and the environment in the final predisposition to cancer and the intensity of cancer pain. We showed that chronic stress, but not tumor progression, induces depressive behavior, which may be of relevance to cancer therapy. Further tests leading to the inhibition of the enhanced opioid system activity in HA mice by opioid antagonists and its stimulation by opioid agonists in LA mice may bring more substantial evidence of the scope of the involvement of the opioid system in the phenomenon of tumor regression.
Ethical note: The State Ethics Commission, in conformity with Polish law, approved the experimental protocol. All the procedures are commonly used and are considered to be ethically acceptable in all the European Union countries and North America. They also conform to the International Association for the Study of Pain (Zimmermann, Citation1983).
Declaration of interest
The authors have no competing financial interests to declare. A NormoLife Project supported this work. The selective breeding program was financed by the Institute of Genetics and Animal Breeding at the Polish Academy of Science (Project No. S.I.1.3).
References
- Anisman H. (2011). Inflaming depression. J Psychiatry Neurosci 36:291–5
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, et al. (2006). The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 6:240–8
- Antonova L, Aronson K, Mueller CR. (2011). Stress and breast cancer: from epidemiology to molecular biology. Breast Cancer Res 13:208--23
- Berking C, Takemoto R, Satyamoorthy K, Shirakawa T, Eskandarpour M, Hansson J, VanBelle PA, et al. (2004). Induction of melanoma phenotypes in human skin by growth factors and ultraviolet. Cancer Res 64:807–11
- Butler RK, Finn DP. (2009). Stress induces analgesia. Prog Neurobiol 88:184–202
- Christo PJ, Mazloomdoost D. (2008). Cancer pain and analgesia. Ann NY Acad Sci 1138:278–98
- Dhabhar FS, Saul AN, Holmes TH, Daugherty C, Neri E, Tillie JM, Kusewitt D, Oberyszyn TM. (2012). High-anxious individuals show increased chronic stress burden, decreased protective immunity, and increased cancer progression in a mouse model of squamous cell carcinoma. PLoS One 7:e33069
- Frick LR, Arcos ML, Rapanelli M, Zappia MP, Brocco M, Mongini C, Genaro AM, Cremaschi GA. (2009). Chronic restraint stress impairs T-cell immunity and promotes tumor progression in mice. Stress 12:134–43
- Gajkowska B, Kosson A, Sacharczuk M, Kosson P, Lipkowski AW. (2011). Blood-brain barrier permeability differentiates two mouse lines divergently bred for high (HA) and low (LA) swim stress-induced analgesia: electron microscopy analysis. (Primary: Blood-brain barrier permeability differentiates Sadowski Mouse lines of high and low stress-induced analgesia. Electron microscopy analysis.). Folia Neuropathol 49:311–18
- Kest B, Jenab S, Brodsky M, Sadowski B, Belknap JK, Mogil JS, Inturrisi CE. (1999). Mu and delta opioid receptor analgesia, binding density, and mRNA levels in mice selectively bred for high and low analgesia. Brain Res 816:381–9
- Kruk J. (2012). Self-reported psychological stress and the risk of breast cancer: a case-control study. Stress 15:162–71
- Kubera M, Grygier B, Arteta B, Urbańska K, Basta-Kaim A, Budziszewska B, Leśkiewicz M. (2009). Age-dependent stimulatory effect of desipramine and fluoxetine pretreatment on metastasis formation by B16F10 melanoma in male C57BL/6 mice. Pharmacol Rep 61:1113–26
- Lutfy K, Sadowski B, Kwon IS, Weber E. (1994). Morphine analgesia and tolerance in mice selectively bred for divergent swim stress-induced analgesia. Eur J Pharmacol 265:171–4
- Marek P, Mogil JS, Sternberg WF, Panocka I, Liebeskind JC. (1992). N-Methyl-D-aspartic acid (NMDA) receptor antagonist MK-801 blocks non-opioid stress-induced analgesia. II. Studies using three different water temperature paradigms and selectively bred mice. Brain Res 578:197–203
- Mogil JS, Kest B, Sadowski B, Belknap JK. (1996a). Differential genetic mediation of sensitivity to morphine in genetic models of opiate antinociception: influence of nociceptive assay. J Pharmacol Exp Ther 276:532–44
- Mogil JS, Marek P, O'Toole LA, Helms ML, Sadowski B, Liebeskind JC, Belknap JK. (1994). Mu-opiate receptor binding is up-regulated in mice selectively bred for high stress-induced analgesia. Brain Res 653:16–22
- Mogil JS, Sternberg WF, Balian H, Liebeskind JC, Sadowski B. (1996b). Opioid and non-opioid swim stress-induced analgesia: a parametric analysis in mice. Physiol Behav 59:123–32
- Panocka I, Marek P, Sadowski B. (1986a). Differentiation of neurochemical basis of stress-induced analgesia in mice by selective breeding. Brain Res 397:156–60
- Panocka I, Marek P, Sadowski B. (1986b). Inheritance of stress-induced analgesia in mice. Selective breeding study. Brain Res 397:152–5
- Panocka I, Marek P, Sadowski B. (1991). Tolerance and cross-tolerance with morphine in mice selectively bred for high and low stress-induced analgesia. Pharmacol Biochem Behav 40:283–6
- Papp M, Willner P, Muscat R. (1991). An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 104:255–9
- Peters EM. (2012). Psychological support of skin cancer patients. Br J Dermatol 167:105–10
- Sacharczuk M, Jaszczak K, Sadowski B. (2003a). Chromosomal NOR activity in mice selected for high and low swim stress-induced analgesia. Behav Genet 33:435–41
- Sacharczuk M, Jaszczak K, Sadowski B. (2003b). Cytogenetic comparison of the sensitivity to mutagens in mice selected for high (HA) and low (LA) swim stress-induced analgesia. Mutat Res 535:95–102
- Sacharczuk M, Juszczak G, Swiergiel AH, Jaszczak K, Lipkowski AW, Sadowski B. (2009). Alcohol reverses depressive and pronociceptive effects of chronic stress in mice with enhanced activity of the opioid system. Acta Neurobiol Exp (Wars) 69:459–68
- Sacharczuk M, Lesniak A, Korostynski M, Przewlocki R, Lipkowski A, Jaszczak K, Sadowski B. (2010a). A polymorphism in exon 2 of the delta-opioid receptor affects nociception in response to specific agonists and antagonists in mice selectively bred for high and low analgesia. Pain 149:506–13
- Sacharczuk M, Ragan AR, Szymanska H, Lesniak A, Sadowski B, Lipkowski AW. (2012). Distinct susceptibility to inoculated melanoma and sensitivity to cancer pain in mouse lines of high and low sensitivity to stress. J Environ Pathol Toxicol Oncol 31:167–77
- Sadowski B, Panocka I. (1993). Cross-tolerance between morphine and swim analgesia in mice selectively bred for high and low stress-induced analgesia. Pharmacol Biochem Behav 45:527–31
- Sanzo M, Colucci R, Arunachalam M, Berti S, Moretti S. (2010). Stress as a possible mechanism in melanoma progression. Dermatol Res Pract 2010:483–93
- Saul AN, Oberyszyn TM, Daugherty C, Kusewitt D, Jones S, Jewell S, Malarkey WB. (2005). Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst 97:1760–7
- Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, Carroll AR, et al. (2010). Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest 120:1515–23
- Steru L, Chermat R, Thierry B, Simon P. (1985). The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–70
- Willner P. (1997). Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134:319–29
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–10
