Abstract
Maternal dissociative symptoms which can be comorbid with interpersonal violence-related post-traumatic stress disorder (IPV-PTSD) have been linked to decreased sensitivity and responsiveness to children’s emotional communication. This study examined the influence of dissociation on neural activation independently of IPV-PTSD symptom severity when mothers watch video-stimuli of their children during stressful and non-stressful mother–child interactions. Based on previous observations in related fields, we hypothesized that more severe comorbid dissociation in IPV-PTSD would be associated with lower limbic system activation and greater neural activity in regions of the emotion regulation circuit such as the medial prefrontal cortex and dorsolateral prefrontal cortex (dlPFC). Twenty mothers (of children aged 12–42 months), with and without IPV-PTSD watched epochs showing their child during separation and play while undergoing functional magnetic resonance imaging (fMRI). Multiple regression indicated that when mothers diagnosed with IPV-PTSD watched their children during separation compared to play, dissociative symptom severity was indeed linked to lowered activation within the limbic system, while greater IPV-PTSD symptom severity was associated with heightened limbic activity. Concerning emotion regulation areas, there was activation associated to dissociation in the right dlPFC. Our results are likely a neural correlate of affected mothers' reduced capacity for sensitive responsiveness to their young child following exposure to interpersonal stress, situations that are common in day-to-day parenting.
Introduction
Post-traumatic stress disorder (PTSD) is an impairing psychiatric condition that can result from exposure to psychologically traumatic events. PTSD is characterized according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, Citation2000) by the presence of re-experiencing, avoidance and hyperarousal symptoms that persist following trauma. Dissociation is commonly described as one form of a psychological reaction to stress which, when generalized beyond the acute peritraumatic period, can itself become an impairing form of psychopathology that is distinct from, yet related to PTSD. PTSD and dissociation share an overlap in the avoidance symptom cluster and, in particular, in the symptoms of “emotional numbing” and amnesia. In a more general sense, dissociation refers to “a structured separation of mental processes (e.g. thoughts, emotions, cognition, memory and identity) that are ordinarily integrated” and which can be comorbid with PTSD (Spiegel & Cardena, Citation1991). Importantly, dissociation is neither required for the diagnosis of PTSD nor specific to that clinical diagnosis.
Interpersonal violence is associated with substantially elevated rates of PTSD (Breslau et al., Citation2006). The resulting “interpersonal violence-related PTSD” (IPV-PTSD) has been shown to impact negatively on significant dyadic interactions that are not directly related to the trauma (Schechter et al., Citation2010). Children strongly depend on their parents, emotionally as well as physically. Hence, disturbed parent–child interactions involving parents with PTSD and their potential for deleterious consequences regarding the child’s subsequent social-emotional development (Lyons-Ruth & Block, Citation1996; Schechter et al., Citation2010) merit particular attention. Specifically, parental IPV-PTSD related to avoidance of child distress and aggression can pose significant problems for the parent–child relationship and for the young child’s sense of safety and development of emotional regulation (Cohen et al., Citation2008; Schechter et al., Citation2008). Maternal PTSD severity and interpersonal violence have also been associated with reduced availability for joint attention with their young child upon reunion following separation (Schechter et al., Citation2010; Sturge-Apple et al., Citation2012).
However, IPV-PTSD is often accompanied by other comorbid forms of psychopathology that include dissociative symptoms, in particular, when the violence has been chronic (Breslau et al., Citation2006). Therefore, it is unclear which part of the links reported above can be attributed to PTSD symptom severity and which to comorbid symptom severity. Among mothers with PTSD, the severity of maternal dissociative symptoms has been associated with disturbances of the mother–child relationship and very young children's increased exposure to violent media (Schechter et al., Citation2009). In addition, maternal dissociation was related to decreased maternal sensitivity and responsiveness to children’s emotional communication (Bailey et al., Citation2007; Benjamin et al., Citation1996). Moreover, longitudinal research has shown that maternal behavior that is characterized by a low level of sensitivity to the child’s signals and mental states is highly predictive of the development of dissociative symptoms in the child that generalize and persist into young adulthood so as to affect those individuals’ parenting. As such, maternal dissociation may contribute to the intergenerational transmission of trauma and its effects (Dutra et al., Citation2009).
One way to obtain new insights about the mechanisms underlying dissociation and its concrete function in PTSD is offered by neuroimaging. Neuroimaging studies that did not control for levels of dissociation indicate that PTSD is characterized by difficulties in emotion regulation when new experiences remind patients of aspects of their trauma (New et al., Citation2009). Brain regions that are important for emotion regulation and experience and that were repeatedly linked to PTSD include the amygdala, insula, hippocampus, orbitofrontal cortex (OFC) and cingulate cortex (Etkin & Wager, Citation2007). Activity in these areas has been noted in response to negative emotional stimuli independent of whether these stimuli were relevant to the participants’ traumatic experiences (Etkin & Wager, Citation2007). Overall, PTSD has thus been characterized by hyperarousal in the limbic areas, and by reduced activation in regions that are essential to the regulation of emotion and arousal.
In a recent functional magnetic resonance imaging (fMRI) study, we were interested in how IPV-PTSD would affect mothers’ neural response to seeing their young children in an interpersonally stressful experimental condition such as mother–child separation as compared to a non-stressful condition such as mother–child play (Schechter et al., Citation2012). When watching videos of their children during separation versus play, mothers with PTSD lacked medial prefrontal cortex (mPFC) activation that healthy control (HC) mothers showed. The mPFC is a region implicated in emotion regulation and has been reported to show diminished activation in PTSD patients (Gilboa et al., Citation2004; Schechter et al., Citation2012). In addition, our data demonstrated heightened limbic activation and similarly heightened stress responses among mothers with PTSD.
Only a few neuroimaging studies have investigated the role of dissociation in PTSD. Lanius et al. (Citation2002) found that PTSD patients who have clinically significant comorbid dissociative symptoms, showed greater activation in the middle temporal gyrus (MTG), mPFC and anterior cingulate cortex (ACC) compared to a group of healthy women when they were exposed to trauma-related scripts.
Lanius et al. (Citation2010) interpreted the currently available data as evidence for a neural model of PTSD that has dissociative and non-dissociative subtypes as had been previously hypothesized by Bremner (Citation1999). They hypothesize that dissociative symptoms that overlap with PTSD symptoms, unlike symptoms that are specific to PTSD, are associated with the downregulation of the amygdala and other limbic areas in response to trauma-associated stimuli (e.g. trauma scripts) and this is mediated by an increase of activation in the mPFC. Other researchers have posited that those who are able to dissociate may use this capacity to cope with extreme arousal in the face of trauma via inhibition of the limbic system (i.e. amygdala) via ACC and mPFC activation (Felmingham et al., Citation2008). With only one exception (Felmingham et al., Citation2008), we are not aware of other studies that investigated neural activation in participants with comorbid dissociation and PTSD in the absence of stimuli directly related to their trauma. We are also unaware of studies focusing on the significance of this comorbidity as related to a mother’s response to her child in an interpersonally stressful situation.
Imaging studies that investigated neural activity among healthy parents in response to recorded infant cries and still-images of facial expressions showed both ventral anterior cingulate (vACC) and amygdala activation that was related to the mothers’ reported emotional responses toward their own child and to unfamiliar children (Barrett et al., Citation2011; Strathearn et al., Citation2008). While our previous analysis (Schechter et al., Citation2012) showed that mothers with IPV-PTSD responded differently from controls, in terms of their perception of emotion, we did not investigate the impact of comorbid dissociation. Thus, the question of how maternal dissociation might impact the coordination of neural activity in response to viewing a child in a stressful situation remains. The present study aimed to tease apart the respective effects of dissociative versus PTSD symptom severity among mothers suffering from IPV-PTSD. We expected that comorbid dissociative symptoms would decrease mothers’ awareness of their young child’s distress. This would result in greater disturbance in parent–child relationships because mothers with comorbid dissociative symptoms are even less able to engage in mutual emotion regulation than mothers with IPV-PTSD without dissociation (Schechter et al., Citation2009). If so we would envisage that this is due to dampening of mothers’ post-traumatic hyperarousal, as an effect of the comorbid dissociation. PTSD and dissociative symptoms might thus have contrasting, if not opposing effects, on neural activation that subserves these behaviors. Because, dissociation has been associated with decreased sensitivity and responsiveness to children’s emotional communication (Bailey et al., Citation2007; Benjamin et al., Citation1996), dissociation might be linked to a dampening of limbic activation.
Earlier studies examining the effect of dissociation on PTSD-related brain activation, in contrast to our own study, employed directly trauma-relevant scripts (Hopper et al., Citation2007; Lanius et al., Citation2005; Ludascher et al., Citation2010) or non-interactive situations such as the display of emotional faces (Felmingham et al., Citation2008). Furthermore, these studies used an approach that divided PTSD participants into two groups, those with and those without dissociative disorder. Such an approach is clinically valid, yet has two important shortcomings: First, it does not account for the finding that participants with more severe PTSD may also tend to have higher levels of dissociation (Bremner et al., Citation1992). Second, it is limited in its capacity to assess the relationship between dissociation and PTSD-associated hyperarousal. Our study improved upon these shortcomings by using a multiple regression analysis that uses symptom severity of both dissociation and PTSD as predictors of neural activity.
In the present study we thus reanalyzed a previously described data set (Schechter et al., Citation2012), which included both mothers with and without IPV-PTSD who underwent fMRI while watching scenes of their own children during separation and during mutual play. Analyses that included both IPV-PTSD and dissociative symptom severity as predictors of neural activity were performed in order to test the hypothesis that neural responses in brain regions associated with hyperarousal in PTSD would be modulated by the latter as rated on the Dissociative Experiences Scale (DES; Bernstein & Putnam, Citation1986). Based on this hypothesis from the relevant literature (Lanius et al., Citation2010), we expected that, in IPV-PTSD mothers, dissociation would be associated with diminished activation in the limbic system (e.g. amygdala, perirhinal areas, hippocampus) and activation in higher order emotion regulation areas such as the mPFC,and dorsolateral prefrontal cortex (dlPFC), when seeing own children during separation compared to play. We further expected separation to be more sensitive to deficits in maternal emotion perception, due to its socially more stressful nature and the evidence that it affected mother–child joint attention in mothers with PTSD.
Methods
Participants
Recruitment
Participants were recruited via posted advertisements and gave written informed consent for themselves and their child before participation, as prescribed by the Columbia University Medical Center's Institutional Review Board. The study was performed in accordance with the Helsinki Declaration of Human Rights (World Medical Association, Citation1999). This MRI study was nested within a larger study of the relationship of maternal IPV-PTSD to the quality of maternal interactive behavior with her young child, and only began when funded two years after that original study began (see previous description in Schechter & Willheim, 2009). Twenty mothers either with a diagnosis of IPV-PTSD (PTSD-mothers) or HC with no evidence of PTSD who were eligible for an MRI scan participated: 11 IPV-PTSD-mothers (mean age = 29.9 years, SD = 6.38) and 9 HC-mothers (mean age = 30.6, years, SD = 7.07). For reasons of clarity, this paper will concentrate on the results and findings of the PTSD group. Results concerning the HC can be found in the supplementary materials.
Diagnosis
During an initial videotaped interview, PTSD and HC mothers underwent a variety of psychometrics (Schechter & Willheim, Citation2009) including the Clinician Administered PTSD Scale (CAPS; Blake et al., Citation1995; Cronbach's alpha = 0.92) and the Dissociative Experience Scale (DES; Bernstein & Putnam, Citation1986; Cronbach's alpha = 0.89). The CAPS is considered as the gold standard for the assessment of adult PTSD (Weathers et al., Citation2001). Similarly, the DES is a well-established, valid and reliable measure of dissociative symptoms (Dubester & Braun, Citation1995). As one would expect, DES scores differed significantly between HC-mothers and mothers with PTSD (df = 18, t = 2.84, p = 0.011). In both groups the DES correlated positively but not significantly with PTSD symptoms as measured in the CAPS (HC: df = 7, r = 0.53, p = 0.14; PTSD: df = 9, r = 0.49, p = 0.12). Finally, dissociation was positively correlated with the subscale Parent Child Dysfunctional Interaction on the Parenting Stress Index (Abidin, Citation1990) (df = 19, r = 0 65, p = 0.01), but IPV-PTSD symptom severity was not.
Procedure
Stimuli
Two weeks after an initial visit, mothers and their children returned for a videotaped interaction protocol including play and separation sequences that contributed to the fMRI video-stimuli. From these sequences, we created two silent 40-second video excerpts. A first excerpt showed mutual play of mother and child at the moment of greatest joy and mutual transaction; the second the moment of greatest separation distress expressed by the child.
The excerpts were rated independently on a 5-point Separation Distress Scale (SDS) by two graduate-student raters, who were both naïve to group membership (rating scale ranging from 0 = no observable distress to 4 = agitation). Inter-rater reliability was excellent (ICC = 0.95, p < 0.001). Raters found the mean stress on separation to be 2.31 (SD = 1.35; range 1.5–4).
In order to ensure that blood oxygenated level dependent (BOLD) effects associated with dissociation in the mother were not due to higher distress shown by the children, we conducted a Pearson correlation between dissociative symptoms and SDS with the goal to control for the possibility that effects were caused by differences in the video stimuli. The correlation was not significant (df = 10, r = − 0.12, p = 0.70).
The study design consisted of four runs, each lasting 11 minutes 12 seconds, and each containing two blocks during which mothers viewed six 40-second video excerpts per block. Blocks were displayed in a pseudorandom order, counterbalanced within and across runs. For the current research question only two of those six videos were analyzed: each mother saw eight times the scene with her own child playing, and eight times the scene of her own child in the distress of separation.
Image acquisition and pre-processing
All images were acquired on a GE Signa 3 Tesla scanner using a standard GE quadrature head coil operating in transmit and receive mode. After a three-plane localizing image, a T1-weighted, Spoiled Gradient Recall image was acquired in the sagittal plane to prescribe the location of the anterior commissure–posterior commissure (AC/PC) line. Axial functional images positioned parallel to the AC/PC line were obtained using a T2*-weighted gradient-recalled single-shot echoplanar pulse sequence with TR = 2800 ms, TE = 25 ms, 90° flip angle, 24 × 24 cm2 field of view and a 64 × 64 voxel slice matrix. We acquired 43 slices of 3.0 mm thickness with a spacing of 0.5 mm to provide an effective resolution of 3.75 × 3.75 × 3.5 mm3. Slices were acquired in interleaved order and spanned the entire brain. The experimental design included four runs that comprised 240 imaging volumes each. High-resolution fast spoiled gradient recall (FSPGR) anatomical images were also acquired for the normalization of functional images.
The functional echoplanar images were pre-processed and statistically analyzed using batch programing based on SPM2 under MATLAB. Prior to the analysis, images were visually inspected for major artifacts and signal dropout. Images were slice-time corrected and then realigned to the middle slice of the middle (second) of the four runs of each scan. After motion correction, functional images were co-registered with the FSPGR anatomical image of the same participant prior to being spatially normalized and reformatted into a 2 × 2 × 2 mm resolution space using a template brain from the Montreal Neurological Institute. Finally, normalized images were spatially smoothed using a Gaussian filter with a full-width half-maximum of 8 mm. High-pass filters based on discrete cosine transform implemented in SPM2 with a default cut-off period of 128 s were additionally applied as temporal filters to filter out scanner drift and other types of low-frequency noise.
Hypothesis-driven analyses
The BOLD response in each run was assessed using a General Linear Model containing seven time-dependent functions representing each stimulus type (resting gaze fixation and six videos) and one constant (baseline BOLD signal). For each participant, a contrast specifying “separation versus play” represented the difference in estimated neural activity during the viewing of the child in the separation condition compared with estimated activity when viewing the own-child free-play condition.
A whole-brain multiple regression was performed with CAPS and DES-scores as predictors of BOLD-contrast (separation-play) effects, in order to assess their respective contributions to the variance in neural activations. Only voxels using a two-tailed p value threshold < 0.005 together with the requirement that the correlation occurred in a spatial cluster ≥ 30 adjacent voxels are reported. The combined application of a statistical threshold and a cluster filter substantially reduces the false-positive identification of activated pixels at any given threshold (Forman et al., Citation1995). This threshold is more conservative toward Type I errors than what Lieberman & Cunningham (Citation2009) suggest for the study of affective phenomena where effects are usually weaker than in studies for motor responses. No region of interest analyses were performed.
To determine the origins of these associations, we extracted the beta values of all significant clusters for each of the separation and the play condition, and calculated, within each condition, partial correlations between the BOLD activation and the DES score as well as between the BOLD activation and the CAPS score (df = 8, see ). The DES score was thus controlled for the CAPS score and vice versa.
Table 1. Multiple regression of the BOLD contrast while mothers with interpersonal violence-related post-traumatic stress disorder (IPV-PTSD; n = 11, df = 8) are watching their child during separation as compared to play, including their dissociative and PTSD symptom severity as predictors. Only significant effects are shown. “tDES” stands for the predictive t-value that is related to the Dissociative Experiences Scale (DES), “tCAPS” for the predictive t related to the Clinician Administered PTSD Symptom Scale (CAPS). XYZ describes the coordinates of the peak voxel within the Montreal Neurological Institute (MNI) coordinate system (Ashburner et al., Citation1997). The table also shows the partial correlation of the cluster with the DES-score (as corrected for CAPS values), and for the CAPS-score (as corrected for DES values) followed by the same cluster's partial correlations with separation and play.
Results
In support of our hypothesis, the multiple regression for IPV-PTSD mothers (see and ) showed a positive association of limbic activation and PTSD symptom severity as well as a negative association of limbic activation and dissociative symptom severity (all df = 8, mid-cingulate cortex: partial correlations: rDES = −0.88, pDES = 0.001, rCAPS = 0.89, pCAPS = 0.001, right perirhinal cortex: rDES = −0.96, pDES < 0.001, rCAPS = 0.94, pCAPS < 0.001, and right hippocampus: rDES = −0.92, pDES < 0.001, rCAPS = 0.94, pCAPS < 0.001). This was also true for a cluster including the temporo–parietal junction and the right insula (df = 8, rDES = −0.82, pDES = 0.003, rCAPS = 0.84, pCAPS = 0.002). With respect to the origins, beta extractions for significant clusters showed that activity for these clusters of the limbic system and the right insula was positively associated with PTSD symptom severity and negatively with dissociative symptom severity, in particular, during separation (for more information on origins please refer to the last two columns of ).
Figure 1. Results of a multiple regression of the participants' DES and CAPS scores with BOLD activity as the dependant variable. BOLD activity refers to mothers' viewing excerpts of their own children during separation as compared to play. Code: additional activation associated with higher DES scores: double line perforated arrow (blue); with lower DES scores: double line arrow (yellow); with higher CAPS scores: single line perforated arrow (red); with higher CAPS scores and lower DES scores: single line arrow (orange). Numbers on the left side indicate the z-level within the Montreal Neurological Institute (MNI) coordinate system (Ashburner et al., Citation1997). OFC = Orbitofrontal Cortex, dlPFC = dorsolateral Prefrontal Cortex, MTG = Middle temporal Gyrus, STG = Superior Temporal Gyrus, SMA = Supplementary Motor Area.
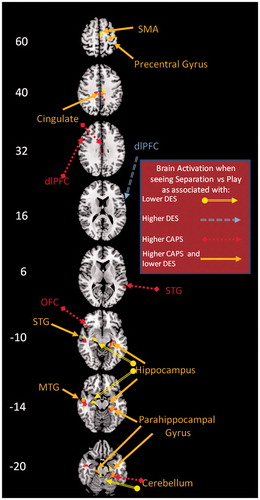
Significantly increased neural activity that was specifically related to higher dissociative symptom severity was further noted in right dlPFC (two clusters; both df = 8, cluster 1: rDES = 0.83, pDES = 0.003; cluster 2: rDES = 0.82, pDES = 0.002). This increased activity originated from decreased activation during play, as correlated with higher dissociative symptom severity.
Deactivations uniquely associated with increasing dissociative symptom severity were noted in other clusters within the limbic system (all df = 8, left hippocampus: rDES = −0.89, pDES = 0.001, and parts of the mid-cingulate cortex: rDES = −0.94, pDES < 0.001, and right entorhinal cortex: rDES = −0.95, pDES < 0.001). These deactivations stemmed from diminished neural activity during separation that was associated with greater dissociative symptom severity.
Higher PTSD symptom-severity further predicted activation in an additional limbic area, the enthorinal cortex (df = 8, rCAPS = 0.84, pCAPS = 0.003), as well as in areas associated with (attempts at) emotional regulation (both df = 8, left dlPFC: rCAPS = 0.83, pCAPS = 0.003, and left OFC: rCAPS = 0.83, pCAPS = 0.003). Activation in the entorhinal cortex originated from a positive correlation of neural activation with PTSD symptom severity during separation. Activation in the OFC originated from a negative correlation between PTSD symptom-severity and play. Activation in the dlPFC originated from both a negative correlation of PTSD symptom severity with play, and a positive correlation of PTSD symptom severity with separation.
Discussion
This study has shed light on the influence of psychological dissociation on traumatized mothers’ responses to videotaped stimuli of their own children in the interpersonally stressful situation of separation as compared to the control condition of free play. Analyses within the IPV-PTSD group of mothers were simultaneously controlled both for PTSD and dissociative symptom severity. In accordance with our hypotheses, dissociative and PTSD symptom severity each exerted a continuous effect in opposite directions on neural activation within the limbic system. PTSD symptom severity as a variable gained predictive power when dissociative symptom severity was entered into the regression model (and vice versa). Importantly, results do not seem to be restricted to high levels of IPV-PTSD because comparable results were obtained when the HC and IPV-PTSD groups were analyzed together (Supplementary Table 1 and Supplementary Figure 2).
This finding of opposite effects of PTSD and dissociation on limbic system activity is consistent with findings of a previous study of PTSD-affected adults among whom dissociative symptoms had an apparent inhibitory effect on limbic activation (Lanius et al., Citation2010). This is remarkable given that this earlier study used trauma-related stimuli. The present research thus clearly extends the relevance of those previous findings to significant situations that mothers of young children face in everyday life, situations that are not directly associated with trauma. Another study that did not use traumatic scripts either (Felmingham et al., Citation2008) found differences within PTSD diagnosed individuals with and without comorbid dissociation in the limbic system only for subliminal presentation of fearful facial expressions, but not for supraliminal presentation. That we found diminished limbic activity associated with dissociation for supraliminally presented stimuli here suggests that dissociation does not affect the processing of all emotional stimuli in the same way, which clearly merits further investigation.
In the clinical literature, greater PTSD symptom severity (i.e. including re-experiencing and hyperarousal clusters) has been associated with greater dissociative symptom severity (Simeon et al., Citation2007). Studies comparing PTSD patients who have also been diagnosed with dissociative disorders (i.e. high levels of dissociative symptoms) to PTSD patients without dissociative disorder (i.e. low to moderate dissociative symptoms) usually do not control for group differences in PTSD symptom severity. Such studies may be underestimating the actual influence of dissociation on neural activation within the limbic regions of the fear circuit, as they do not take into account that PTSD symptom severity is associated with increased neural activity in these very same regions. The same holds for studies investigating the effect of PTSD symptom severity on limbic system activity without simultaneously controlling for dissociation symptom severity. The opposing effects of dissociation compared to IPV-PTSD on the limbic system suggest that dissociation has an important influence on traumatized mothers’ emotional experience and regulation.
The importance of the limbic system for emotions and attention is well documented, and dissociation is traditionally seen as a mechanism that can be used for psychological defense. Less limbic activation in mothers with IPV-PTSD who have greater dissociative symptom severity may represent a specific adaptation by these individuals to a chronically violent and dangerous environment (Schalinski et al., Citation2011). Along these lines, it has been shown that reduced hippocampal volume was associated with PTSD only if patients did not have a comorbid dissociative disorder (Weniger et al., Citation2008). This might mean that functional hyperactivity of the hippocampus that is linked to PTSD may cause structural damage, and that dissociation may be adaptive to prevent this. Our finding of a negative correlation between dissociation and hippocampal activity may also be linked to amnesia or incoherence of narrative memory in relation to a traumatic life-event or trigger, which is one cardinal symptom of psychological dissociation (American Psychiatric Association, Citation2000).
Our data support the idea that dissociation and its neural correlates represent the organism’s efforts to protect itself from overwhelming hyperarousal and its adverse neurophysiological consequences (Sapolsky, Citation1990). While this diminished activation may originally be adaptive for the mother's downregulation of physiological arousal (i.e. so as to protect her from toxicity of chronic severe stress when in danger of actual violent victimization by an adult), it can subsequently become maladaptive if it generalizes to another context such as the mother–child relationship. Dissociation-related downregulation of physiological arousal could then, for example, be triggered by maternal trauma-associated memory traces such as the effect of a toddler's helplessness or distress. In such a moment, the negative impact of that mother's dissociation on her maternal sensitive responsiveness to her child and her joint attention with her child (and thus more generally on her relationship with her child and her child's social-emotional development) can be disorganizing for the mother and the child, and their relationship together (Schechter et al., 2013). Thus, this kind of psychological defense may diminish maternal attention available for the emotional needs of the child, particularly in the context of a background of domestic violence in which the child can trigger maternal traumatic memory traces (Schechter et al., 2013).
Consistent with this view, we found that greater dissociative symptom severity was associated with lower activity in the insula; while PTSD predicted additional activation when mothers were watching separation compared to play. The fact that we found insular activity to be associated with dissociative symptom severity emphasizes the importance of dissociation on brain regions involved in empathic resonance (Singer et al., Citation2009), which is a key component of parental sensitivity (Noriuchi et al., Citation2008).
Our data further suggest that, as hypothesized by us, dissociative symptom severity was more predictive of (diminished) activation in the limbic system when IPV-PTSD participants watched scenes of separation than when they watched scenes of play. While brief separations can be stressful to the dyad, they are common day-to-day stressors. Therefore, they may easily and frequently provoke limbic hyperarousal.
Parent–child dysfunction, a subscale of the Parenting Stress Index, was significantly correlated with dissociative but not IPV-PTSD symptom severity in this fMRI study sample. Parent–child dysfunction has previously been reported to be negatively correlated to joint attention between mothers and their children; and mothers with IPV-PTSD as compared to non-PTSD controls were significantly less available for joint attention following brief separation than during free-play before separation (Schechter et al., Citation2010). It is possible that such a reduction in joint-attention after separation is related to the reduction of limbic activity by dissociation.
Our findings in the right dlPFC suggest that the impact of dissociation within IPV-PTSD on mother–child interactions may not be restricted to stressful contexts. The dlPFC has been linked to emotion regulation of both positively and negatively valenced stimuli (Viinikainen et al., Citation2010), and we observed activation in that area to be associated with significantly greater dissociative symptom severity when mothers watched their own children during separation compared to mutual play. In contradiction to our hypothesis, however, the effect did not originate in the separation, but rather in the play condition, where dissociation was negatively associated with neural activity in the dlPFC. Our finding may thus suggest that mothers with IPV-PTSD and comorbid dissociation have an altered regulation of positive social interactions and are at a particular risk of failing to (re-) establish a positive interaction with their child in non-stressful contexts. The failure to do so should then complicate the experience of warmth and safety by the child in the absence of direct danger. Interestingly, the dlPFC has been shown to be implicated in both natural viewing and reappraisal of social interaction scenes in adults with avoidant attachment tendencies (Vrticka et al., Citation2012). Therefore, comorbid dissociation in the parenting context here may be linked to distinctive attachment style and associated maternal emotion regulation. However, clearly more research is needed to determine the concrete function of dissociation-related dlPFC activation in non-stressful and stressful contexts. Moreover, our observation that IPV-PTSD symptom severity was positively associated with left dlPFC activation in the separation condition, but negatively in the play condition needs further clarification as well.
It has been hypothesized that participants with PTSD can be divided into two subtypes: those with comorbid dissociative pathology and those without (Bremner, Citation1999). Lanius et al. (Citation2010) posited that particularly activation in the dorsal anterior cingulate cortex (dACC) differentiates these two subtypes: a “re-experiencing/hyperarousal” subtype is associated with activation of the dACC; and a “dissociative” subtype that shows the deactivation of the amygdala and right insula. Our data are partially consistent with this theory in which we did find that the predicted deactivation of the hippocampus and insula was associated with dissociative symptom severity; however, amygdala deactivation fell short of statistical significance.
Our study did not replicate the previously reported association of higher mPFC activation and dACC deactivation with dissociative symptom severity either (Felmingham et al., Citation2008). This can possibly be explained by the fact that we did not examine the more direct effect of trauma-related reminders on neural activation among the mothers with PTSD. We rather investigated how the affective communication of their young children during the stressful interpersonal situation of separation, by virtue of the child’s helpless state of mind, served to activate traumatic memory traces. As such, we did not expect mothers’ neural activity to show exactly the same patterns as one observes among PTSD patients exposed to directly related trauma-scripts, images or other stimuli.
The following limitations apply to our study: as to whether the BOLD-effects of dissociative symptoms and comorbid PTSD would be linear or not, we note that this study only tested for linear effects. In addition, the within-PTSD group analysis had less statistical power than if all participants had been included (see Supplementary materials). Due to inherent difficulties in recruiting this high-risk, inner-city sample for an MRI study, the number of participants remained relatively small. Thus, the generalizability of findings and interpretation of results with application to other groups of IPV-PTSD affected parents would require replication with more participants.
Finally, despite our efforts to standardize the stimuli, the naturalistic video clips of participants’ own children, while having the advantage of providing more ecologically valid stimuli that were individualized for each mother, contained slightly differing levels of distress and movement across the individual children.
Conclusions
This study examined closely how IPV-PTSD and dissociative symptom severity impact neural activation in response to parent–child interactions. We demonstrated limbic activation in IPV-PTSD mothers to be positively associated with IPV-PTSD symptom severity and negatively associated with dissociative symptom severity. Our data indicate that the functional interaction which IPV-PTSD and dissociation exert on emotion regulation has a neurobiological basis.
Dissociative symptoms may thus serve to protect traumatized participants from chronic hyperarousal (i.e. limbic activation) within a chronically unpredictable and dangerous environment involving interpersonal violence. Some studies have suggested that while the capacity to dissociate is constitutionally derived, dissociation can be used in the service of psychological defense (Butler et al., Citation1996). Our study supports the idea that the presence of comorbid dissociative symptoms may signal an endophenotypic variant of IPV-PTSD. Dissociative symptoms, in any case, can protect the traumatized mother against profound dysregulation of arousal, emotion and aggression, but can also compromise her ability to engage in mutual emotional regulation with her young child. Finally, our study showed dissociative symptom severity to be linked to neural activation in response to stressful situations that are common in day-to-day parenting (i.e. mother–child separation). In order to address interpersonal dysfunction so as to develop better targeted clinical interventions, it is important to separate PTSD-related and dissociation-related effects on mental and neurobiological functioning.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
The authors gratefully acknowledge the following sources of funding that made this paper possible: NCCR “SYNAPSY” of the Swiss National Science Foundation, NIH K23 MH068405, International Psychoanalytical Association Research Advisory Board, the Sackler Institute for Developmental Psychobiology at Columbia University and the Bender-Fishbein Fund.
Supplementary Material
Download PDF (330.5 KB)Acknowledgements
The authors would like to thank the following individuals for their support of the work described in this paper: Hana Kutlikova, Rachel Marsh, XueJun Hao, Yunsuo Duan, Shan Yu, Benjamin Gunter, David Murphy, Jaime McCaw, Alayar Kangarlu, Erica Willheim, Michael M. Myers, Myron A. Hofer, Francesca Suardi.
Notes
*Maps of the t-tests of separation versus rest, and play versus rest without inclusion of dissociation or PTSD symptom severity have been previously published (Schechter et al., Citation2012). Maps of neural activation showing a multiple regression that includes PTSD and dissociative symptom severity for both groups together, are given in the supplementary material (Supplementary Table 1).
References
- Abidin R. (1990). Parenting stress index test manual. Charlottesville, VA: Pediatric Psychology Press
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: APA
- Ashburner J, Neelin P, Collins DL, Evans A, Friston K. 1997. Incorporating prior knowledge into image registration. Neuroimage 6:344–52
- Bailey HN, Moran G, Pederson DR. (2007). Childhood maltreatment, complex trauma symptoms, and unresolved attachment in an at-risk sample of adolescent mothers. Attach Hum Dev 9:139–61
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS. (2011). Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc Neurosci 7:252–68
- Benjamin LR, Benjamin R, Rind B. (1996). Dissociative mothers' subjective experience of parenting. Child Abuse Negl 20:933–42
- Bernstein EM, Putnam FW. (1986). Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 174:727–35
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. (1995). The development of a Clinician-Administered PTSD Scale. J Trauma Stress 8(1):75–90
- Bremner JD. (1999). Acute and chronic responses to psychological trauma: where do we go from here? Am J Psychiatry 156:349–51
- Bremner JD, Southwick S, Brett E, Fontana A, Rosenheck R, Charney DS. (1992). Dissociation and posttraumatic stress disorder in Vietnam combat veterans. Am J Psychiatry 149:328–32
- Breslau N, Lucia VC, Alvarado GF. (2006). Intelligence and other predisposing factors in exposure to trauma and posttraumatic stress disorder: a follow-up study at age 17 years. Arch Gen Psychiatry 63:1238–45
- Butler LD, Duran RE, Jasiukaitis P, Koopman C, Spiegel D. (1996). Hypnotizability and traumatic experience: a diathesis-stress model of dissociative symptomatology. Am J Psychiatry 153:42–63
- Cohen LR, Hien DA, Batchelder S. (2008). The impact of cumulative maternal trauma and diagnosis on parenting behavior. Child Maltreat 13(1):27–38
- Dubester KA, Braun BG. (1995). Psychometric properties of the Dissociative Experiences Scale. J Nerv Ment Dis 183:231–5
- Dutra L, Bureau JF, Holmes B, Lyubchik A, Lyons-Ruth K. (2009). Quality of early care and childhood trauma: a prospective study of developmental pathways to dissociation. J Nerv Ment Dis 197:383–90
- Etkin A, Wager TD. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–88
- Felmingham K, Kemp AH, Williams L, Falconer E, Olivieri G, Peduto A, Bryant R. (2008). Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol Med 38:1771–80
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–47
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. (2004). Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry 55:263–72
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. (2007). Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress 20:713–25
- Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D. (2010). Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry 167:640–7
- Lanius RA, Williamson PC, Bluhm RL, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. (2005). Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol Psychiatry 57:873–84
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. (2002). Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry 52:305–11
- Lieberman MD, Cunningham WA. (2009). Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci 4:423–8
- Ludascher P, Valerius G, Stiglmayr C, Mauchnik J, Lanius RA, Bohus M, Schmahl C. (2010). Pain sensitivity and neural processing during dissociative states in patients with borderline personality disorder with and without comorbid posttraumatic stress disorder: a pilot study. J Psychiatry Neurosci 35:177–84
- Lyons-Ruth K, Block D. (1996). The disturbed caregiving system: relations among childhood trauma, maternal caregiving, and infant affect and attachment. Infant Mental Health J 17:257–75
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Tang CY, Charney DS. (2009). A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry 66:656–64
- Noriuchi M, Kikuchi Y, Senoo A. (2008). The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biol Psychiatry 63:415–23
- Sapolsky RM. (1990). Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog Brain Res 86:13–23
- Schalinski I, Elbert T, Schauer M. (2011). Female dissociative responding to extreme sexual violence in a chronic crisis setting: the case of Eastern Congo. J Trauma Stress 24:235–8
- Schechter DS, Coates S, Kaminer T, Coots T, Zeanah C, Davies M, Schonfeld IS, et al. (2008). Distorted maternal mental representations and atypical behavior in a clinical sample of violence-exposed mothers and their toddlers. J Trauma Dissociation 9(2):123–47
- Schechter DS, Gross A, Willheim E, McCaw J, Turner JB, Myers MM, Zeanah CH, Gleason MM. (2009). Is maternal PTSD associated with greater exposure of very young children to violent media? J Trauma Stress 22:658–62
- Schechter DS, Moser DA, McCaw J, Myers MM. (2013). Autonomic functioning in mothers with interpersonal violence-related posttraumatic stress disorder in response to separation--reunion. Dev Psychobiol. [Epub ahead of print]. doi: 10.1002/dev.21144
- Schechter DS, Moser DA, Wang Z, Marsh R, Hao X, Duan Y, Yu S, et al. (2012). An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Soc Cogn Affect Neurosci 7:969–79
- Schechter DS, Willheim E. (2009). Disturbances of attachment and parental psychopathology in early childhood. Child Adolesc Psychiatr Clin N Am 18:665–86
- Schechter DS, Willheim E, Hinojosa C, Scholfield-Kleinman K, Turner JB, McCaw J, Zeanah CH, Myers MM. (2010). Subjective and objective measures of parent–child relationship dysfunction, child separation distress, and joint attention. Psychiatry 73:130–44
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. (2007). Hypothalamic--pituitary--adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biol Psychiatry 61:966–73
- Singer T, Critchley HD, Preuschoff K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends Cogn 13:334–40
- Spiegel D, Cardena E. (1991). Disintegrated experience: the dissociative disorders revisited. J Abnorm Psychol 100:366–78
- Strathearn L, Li J, Fonagy P, Montague PR. (2008). What's in a smile? Maternal brain responses to infant facial cues. Pediatrics 122:40–51
- Sturge-Apple ML, Davies PT, Cicchetti D, Manning LG. (2012). Interparental violence, maternal emotional unavailability and children's cortisol functioning in family contexts. Dev Psychol 48:237–49
- Viinikainen M, Jaaskelainen IP, Alexandrov Y, Balk MH, Autti T, Sams M. (2010). Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Hum Brain Mapp 31:1030–40
- Vrticka P, Bondolfi G, Sander D, Vuilleumier P. (2012). The neural substrates of social emotion perception and regulation are modulated by adult attachment style. Soc Neurosci 7:473–93
- Weathers FW, Keane TM, Davidson JR. (2001). Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 13:132–56
- Weniger G, Lange C, Sachsse U, Irle E. (2008). Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatr Scand 118:281–90
- World Medical Association. (1999). Proposed revision of the Declaration of Helsinki. BME 147:18–22
