Abstract
Chronic stress increases anxiety and encourages intake of palatable foods as “comfort foods”. This effect seems to be mediated by altered function of the hypothalamic–pituitary–adrenal axis. In the current study, litters of Wistar rats were subjected to limited access to nesting material (Early-Life Stress group – ELS) or standard care (Control group) from postnatal day 2 to 9. In adult life, anxiety was assessed using the novelty-suppressed feeding test (NSFT), and acute stress responsivity by measurement of plasma corticosterone and ACTH levels. Preference for palatable foods was monitored by a computerized system (BioDAQ, Research Diets®) in rats receiving only regular chow or given the choice of regular and palatable diet for 30 days. ELS-augmented adulthood anxiety in the NSFT (increased latency to eat in a new environment; decreased chow intake upon return to the home cage) and increased corticosterone (but not ACTH) secretion in response to stress. Despite being lighter and consuming less rat chow, ELS animals ate more palatable foods during chronic exposure compared with controls. During preference testing, controls receiving long-term access to palatable diet exhibited reduced preference for the diet relative to controls exposed to regular chow only, whereas ELS rats demonstrated no such reduction in preference after prolonged palatable diet exposure. The increased preference for palatable foods showed by ELS animals may result from a habit of using this type of food to ameliorate anxiety.
Introduction
Acute exposure to stress increases intake of highly palatable foods in humans and animals (Epel et al., Citation2001; Foster et al., Citation2009; La Fleur et al., Citation2005; Oliver et al., Citation2000), even in the absence of homeostatic needs for increased caloric intake or hunger (Foster et al., Citation2009; Rutters et al., Citation2009). Given the choice (Foster et al., Citation2009; La Fleur et al., Citation2005), subjects typically favor foods with high fat and/or sugar content – so-called “comfort foods” – during times of exposure to stress (Dallman et al., Citation2005). Elevated stress hormone levels, palatable food intake and the consequent accretion of abdominal fat may serve as feedback signals that reduce perceived stress (Pecoraro et al., Citation2006), thus reinforcing the stress-induced feeding behavior.
Chronic exposure to stressful environmental conditions can also promote ongoing intake of palatable foods in rodents and humans (Ely et al., Citation1997; Teegarden & Bale, Citation2008; Wardle et al., Citation2000). In rats, chronic repeated restraint stress increases sweet food intake without altering consumption of standard chow (Ely et al., Citation1997). Under chronic variable stress, mice select more of their daily caloric intake from high-fat than from high-protein or high-carbohydrate dietary options (Teegarden & Bale, Citation2008). In humans, periods of increased workload are accompanied by higher fat and sugar intake (Wardle et al., Citation2000).
Furthermore, anxiety has been implicated in eating disorders (Grucza et al., Citation2007) and in the development of obesity (Davis et al., 2008; Scott et al., Citation2008). The prevalence and severity of depression and anxiety in binge eaters suggest that negative emotional states can trigger binging behavior (Womble et al., Citation2001).
While the effect of acute and chronic stress on feeding behavior has been studied widely, very few investigations have explored the possible persistent effects of neonatal stress or environmental variations on the feeding preferences of offspring later in life (da Silva Benetti et al., Citation2007; Silveira et al., Citation2008, Citation2010). Exposure to various adverse experiences during the perinatal period (a critical developmental time window) affects the development of systems involved in stress responses in part by persistent alterations in expression of key genes by epigenetic marking, leading to differences in behavior, neuroendocrine parameters and stress responsivity in later life (review in Meaney, Citation2010; Murgatroyd & Spengler, Citation2011). For instance, exposure to daily maternal separation during the neonatal period in rodents increases anxiety in adulthood (Jahng, Citation2011; Maniam & Morris, Citation2010), as well as corticosterone secretion in response to acute stress later in life (Plotsky & Meaney, Citation1993; Plotsky et al., Citation2005). Interestingly, this effect is reversed by provision of a high-fat diet (Maniam & Morris, Citation2010). Additionally, it remains relatively poorly explored whether early adverse living conditions leading to adult anxiety and increased stress responses also alter spontaneous preference for palatable (comfort) foods, especially in females. Our hypothesis is that neonatal stress, due to its known property of persistent programming of HPA axis hyper-responsiveness, will specifically affect preference for comfort foods in adult life. As women are more prone both to eating disorders (Striegel-Moore & Bulik, Citation2007) and to the effects of stress on feeding (Mikolajczyk et al., Citation2009), we explored this hypothesis in females.
Methods
Pregnant Wistar rats, bred at our animal facility, were single-housed in home cages (40 cm × 40 cm × 30 cm) with a wire mesh bottom (1 cm × 1 cm), kept 2 cm above a removable metal plate used to collect urine and droppings. During pregnancy, the floor was covered with wood chips and dams were kept in a controlled environment (standard dark/light cycle, lights on between 09:00 a.m. and 07:00 p.m.; temperature of 22 ± 2 °C; cage cleaning once a week; food and water provided ad libitum).
The date of birth was considered as day 0. On day 2, dams and pups were randomly allocated to two groups (see below) and kept undisturbed until day 9. On day 10, dams and pups were removed to Plexiglas home cages (46 × 31 × 16 cm) with a wood chip-covered floor and kept in the same controlled environment cited above (Ivy et al., Citation2008).
On postnatal day 21, pups were weaned, separated by sex into groups of two or three per cage and kept in a controlled environment similar to that described above, except for the light cycle (lights on between 07:00 a.m. and 07:00 p.m.). At the time of weekly cage cleaning, body weight was measured using a digital scale with 0.01-g resolution (Marte®, Canoas, Brazil). Males were used in a different study.
Forty-eight adult female rats derived from 17 litters were used in the behavioral tasks started on postnatal day 70. All animal procedures followed international standards and were approved by the Research Ethics Committee of Hospital de Clínicas de Porto Alegre (GPPG/HCPA, project number 11–0182). Tasks were performed in climate-controlled behavioral rooms within our animal research facility (Unidade de Experimentação Animal/HCPA).
Early-life stress model
Early-life stress (ELS) group: as described elsewhere (Ivy et al., Citation2008), the ELS group had limited access to nesting material from postnatal day 2 (PND2) to postnatal day 9 (PND9). In the morning of PND2, wood chips were removed, without touching the animals, and a nesting material consisting of paper towels (approximately 2000 cm3) was provided. This was the only material available for the dam to construct a rudimentary nest area. All litters were left undisturbed, and bedding was not changed during PND2–PND9.
Control group: dams and pups were left undisturbed, during PND2–PND9, in a home cage identical to that of the ELS group, but with abundant nesting material available (approximately 7200 cm3 of wood chips).
Novelty-suppressed feeding test
The novelty-suppressed feeding test consists of an open arena (43 cm × 54 cm) with a sweet food pellet (Kellogg’s® Froot Loops®) placed in the center. Rats were previously habituated to the sweet food by receiving three pellets inside the home cage for 4 days. At 70 days of life, food-deprived rats (16 h) were placed in the arena and the latency to onset of chewing was recorded. Chewing was scored when the rat was sitting on its haunches and biting with the use of forepaws. Immediately after they began eating, rats were transferred to their home cages and the amount of rat chow eaten in 5 min was measured using a digital scale (Marte®, resolution 0.01 g). The arena was cleaned with 70% ethanol between tests. Classically, the inhibition of food intake by exposure to novelty is interpreted as a measure of anxiety (Gross et al., Citation2002; Zhang et al., Citation2010).
Meal pattern analysis
At 90 days of life, rats were transferred into a cage equipped with a BioDAQ® food intake monitoring system (Research Diets). Rats were housed individually and provided access to standard rat chow (composition: 22% protein, 4.5% fat, 54% carbohydrate, 2.95 kcal/g; NUVILAB®) and water ad libitum, with a habituation period of 3 days. The subsequent 4 days were used for analysis. Total food intake and meal pattern were analyzed with the BioDAQ® system as previously described (Farley et al., Citation2003). Briefly, the system uses a food hopper mounted on an electronic strain gauge-based load cell to measure food intake. The food hopper is weighed 50 times per second (accurate to 0.01 g) and the mean and standard deviation (S.D.) of intake over approximately 1 s are calculated by a peripheral computer.
Feeding is signaled by a fluctuation in the food hopper weight (defined as a S.D. > 2000 mg) caused by the animal eating, at which the date, time and hopper weight were recorded. The end of a feeding bout (but not necessarily a meal – see below) is signaled when the hopper is left undisturbed for 2 min (defined as a S.D. < 2000 mg), at which the duration of the feeding event and the amount eaten (initial hopper weight minus the final hopper weight) was calculated. Each feeding event record (cage/animal number, start date and time, feeding duration, final hopper weight and amount eaten) is exported to a central computer and a Microsoft Excel-based spreadsheet (Microsoft, Redmond, WA) is used for the calculation of the desired parameters (see below) and summarization of data. Food spillage was monitored during the study, and no spillage was observed during the test periods. The amount of food eaten, meal size and meal frequency were calculated for the 4-day period as a whole and stratified into nocturnal and diurnal periods. A meal was defined as a difference in hopper weight of >0.1 g, separated from other feeding bouts by >15 min (Eckel et al., Citation1998; Surina-Baumgartner et al., Citation1995), and meal size as the amount in grams that an animal ate divided by the number of meals in a period.
Chronic exposure to palatable diet (“comfort foods”)
Two weeks after the end of the BioDAQ® measurements, the two neonatal groups were subdivided into four experimental groups:
Control group exposed to regular diet (3.64 kcal/g, 22% protein, 4% fat, 60% carbohydrate) – maximum number of animals/litter = 2.
ELS group exposed to regular diet; – maximum number of animals/litter = 4.
Control group exposed to regular diet + palatable diet (4.82 kcal/g, 14% protein, 34% fat, 30.2% carbohydrate, 20% of the latter derived from sucrose); – maximum number of animals/litter = 3.
ELS group exposed to regular diet + palatable diet; – maximum number of animals/litter = 4.
To compensate for the neophobia effect among the groups, both regular and palatable diets were new; therefore, rats from the four different groups had not been exposed to the diets previously. These diets were offered for 30 days. Food intake was evaluated weekly during this period and preference for “comfort food” was calculated as the percentage of palatable food ingested in relation to total intake [(palatable diet intake/regular + palatable diet intake) *100] for the groups receiving the two diets. After this chronic exposure, the animals were placed on the BioDAQ® again for preference testing (see below).
Preference testing
Animals were placed on the same system described above (BioDAQ®). However, the two diets (regular + palatable) were made available to all four groups for 24 h to evaluate preference for the palatable diet in animals that were or were not exposed to it chronically. Again, preference for “comfort food” was calculated as the percentage of palatable food ingested in relation to total intake [(palatable diet intake/regular + palatable diet intake) *100].
Abdominal fat weight
At 150 days of life, animals from the four groups were weighed and decapitated, and the two major portions of abdominal fat (gonadal and retroperitoneal adipose tissue depots) were dissected and weighted using a scale with 0.01 g resolution (Marte®, Canoas, Brazil). Results were expressed as % of body weight.
HPA response to acute restraint stress
A subset of adult animals was used solely for this experiment. Pre-stress blood samples were taken from rats within 30 s of removal from the home cage, by cutting of 3 mm of the tip of the tail and drainage of a small amount of blood (0.15 mL) through gentle massage from the base to the tip of the tail. Rats were immediately and individually placed in Plexiglas restrainers (8.5 cm × 21.5 cm), with an open end for air intake, for a 20-min period. Restraint stress was performed during the light cycle, between 09:00 a.m. and 11:00 a.m., with blood sampling from the tail vein at 20, 40, 60 and 90 min after the onset of restraint. Tail blood was collected into microtubes and plasma was separated and frozen at −20 °C until the day of analysis. Plasma corticosterone was measured with a commercially available ELISA kit (Life Sciences Int’l Inc., Plymouth Meeting, PA; intra-assay coefficient of variation: 6.6–8.0, inter-assay coefficient of variation: 7.8–13.1; sensitivity: 26.99 pg/mL) at the Biochemistry Department (Laboratory 37), UFRGS and ACTH was measured by immunoassay under direct chemiluminescence at the Clinical Analysis Laboratory of Hospital de Clínicas de Porto Alegre (intra-assay coefficient of variation: 8.7–9.5, inter-assay coefficient of variation: 6.1–10.0; sensitivity: 500.00 pg/mL).
Statistical analysis
Data were analyzed by Student’s t-test, using neonatal group as a factor (NSFT and meal pattern analysis on the BioDAQ®), or repeated measures ANOVA (body weight, habituation to the BioDAQ®, weight gain, regular diet intake and preference for the palatable diet during the 4 weeks of exposure to the palatable diet) using neonatal group as a factor and adjusting for litter size (body weight, weight gain and preference for the palatable diet during chronic exposure) and for initial weight (preference for the palatable diet during chronic exposure) as needed (i.e. when these covariates influenced the outcome of preliminary analysis). Other food intake data were shown both adjusted and unadjusted by body weight. Generalized Estimating Equation (GEE) analysis was used for the hormonal response to acute stress (corticosterone and ACTH), as GEE is ideally suited for within-subject repeated measures that are likely to be correlated. Two-way ANOVA was used to evaluate abdominal fat and preference testing on the BioDAQ® system (followed by Bonferroni correction). Data were analyzed using the Statistical Package for the Social Sciences (SPSS) 18.0 software (SPSS Inc., Chicago, IL). Significance levels for all measures were set at p < 0.05.
Results
Body weight
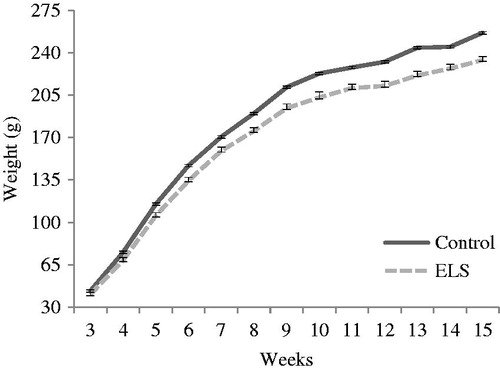
Repeated measures ANOVA, considering the 13 weeks after weaning and adjusting for litter size, demonstrated an interaction between time and group [F(12, 324) = 3.321, p < 0.0001], in which ELS rats gained less weight with the passage of time. Isolated effects of time [F(12, 324) = 167.591, p < 0.0001] and group [F(1, 27) = 15.064, p = 0.001] were also observed ().
Novelty-suppressed feeding test
Student’s t-test demonstrated that ELS females had increased latency to begin chewing Froot Loops® as compared with controls (controls, 111.30 ± 50.99 s; ELS, 194.25 ± 97.68 s; p = 0.005). Furthermore, ELS females exhibited decreased intake of rat chow upon return to their home cages (controls, 0.17 ± 0.11 g; ELS, 0.08 ± 0.04 g; p = 0.045).
Meal pattern analysis
During habituation to the BioDAQ® (days 1–3), there was an effect of time [F(2, 32) = 4.273; p = 0.023], in which the animals ate more rat chow as days went by; however, there was no effect of the neonatal group [F(1, 16) = 3.927; p = 0.065], nor any interaction between group and time [F(2, 32) = 0.774; p = 0.470]. When adjusting for body weight, the effect of the time is no longer seen [F(2, 30) =2.142; p = 0.135]. In addition, there was no effect of group [F(1, 15) = 1.815; p = 0.198] or any group by time interaction [F(2, 30) = 1.124; p = 0.338].
During the period of meal pattern analysis (days 4–7), there was no effect of time [F(3, 48) = 1.217; p = 0.314], demonstrating that animals maintained the same pattern of chow intake during the evaluation period and suggesting that they were habituated to the equipment. There was neither isolated group effect [F(1, 16) = 1.632; p = 0.220] nor interaction [F(3, 48) = 0.197; p = 0.898]. When adjusting for body weight, there were no effects of time ([F(3, 45) = 1.450; p = 0.241] or group [F(1, 15) = 1.132; p = 0.304] and no group by time interaction [F(3, 45) = 0.201; p = 0.895].
shows the data from meal pattern analysis. Meal pattern characterization revealed a difference in nocturnal meal intake (controls, 14.13 ± 0.61 g; ELS, 11.93 ± 0.40 g; p = 0.007) and in nocturnal meal size (controls, 13.90 ±0.64 g; ELS, 12.04 ± 0.41 g; p = 0.022), with ELS females eating less and showing a smaller nocturnal meal size compared to controls. The other patterns analyzed were statistically similar between the groups (). These results persisted when adjusting for body weight, except that the nocturnal meal size difference between the groups does not reach statistical significance (p = 0.058).
Table 1. Comparison between the Control and ELS groups related to meal pattern.
Chronic exposure to palatable “comfort foods”
During the 4 weeks of exposure to “comfort food”, ELS continued to gain less weight than controls, regardless of whether they were receiving the palatable diet [F(1, 25) =6.532, p = 0.0017]. There was no effect of time [F(3, 75) =0.967, p = 0.413] and no interactions ().
Figure 2. (A) Body weight during chronic exposure to the palatable diet. An effect of the neonatal group was detected (p = 0.0017), without interactions. (B) Regular diet intake during chronic exposure to the palatable diet. There was an interaction between dietary group and time (p = 0.008), as well as an effect of the neonatal group (p = 0.026) and the dietary group, as expected (p < 0.0001). Control (regular diet and 2 choices n = 5), ELS (regular diet and 2 choices n = 10). (C) Preference for the palatable diet during chronic exposure. There was an interaction between time and the neonatal group (p = 0.008), as well as isolated effects of the group (p = 0.006) and time (p = 0.016). Control group (n = 5) and ELS group (n = 9). Data are expressed as mean ± SEM.
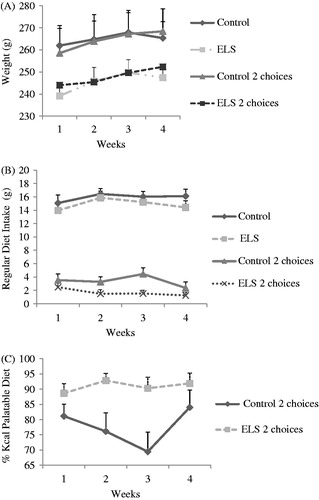
Regular diet intake was also decreased in ELS rats during this period [F(1, 26) = 5.596, p = 0.026] and in rats (both controls and ELS) given the choice of the palatable diet, as expected [F(1, 26) = 0.326, p < 0.001]. There was a interaction between time and dietary group [F(3, 78) = 4.244, p = 0.008], without effects between time and neonatal group [F(3, 78) = 0.491, p = 0.690] (). Repeated measures ANOVA using initial body weight as a co-variable also shows an effect of the diet and the time versus dietary group interaction, but the effect of ELS decreasing the food consumption is no longer significant (p = 0.202).
An interaction between time and group was observed with respect to “comfort food” preference (percentage of the palatable diet ingested in relation to total food intake), in which ELS animals ate more palatable food than controls as days went by [F(3, 30) = 4.764, p = 0.008]. There were also isolated effects of group [F(1, 10) = 12.167, p = 0.006] and time [F(3, 30) = 4.001, p = 0.016] ().
Preference testing
Isolated effects of the neonatal group [F(1, 27) = 4.859, p = 0.037] and of the dietary group [F(1, 27) = 4.897, p = 0.037] on preference testing were detected. In addition, there was an interaction between the neonatal group and the dietary group with regard to preference for the palatable diet [F(1, 27) = 13.718, p = 0.001]. In controls, the chronic exposure to the palatable diet was associated with a decreased preference for this type of food, as expected, but no such decrease was seen in ELS animals (). The interaction remains significant when adjusting the analysis by body weight [F(1, 27) = 13.790, p = 0.001].
Table 2. Preference test at BioDaq® system during 24 hours.
Abdominal fat after 4 weeks of exposure to “comfort food”
There was no effect of the neonatal group on abdominal fat (gonadal and retroperitoneal adipose tissue depots) expressed as % of body weight. Mean (SD): control + regular diet, 2.44 (0.43); ELS + regular diet, 2.05 (0.66); control + regular +palatable diets, 3.36 (0.97); ELS + regular + palatable diets, 3.87 (0.99); F(1, 26) = 0.038; p = 0.846]. Chronic palatable diet exposure increased the abdominal fat [F(1, 26) = 18.761; p < 0.0001], but there were no significant interactions between the neonatal group and dietary group [F(1, 26) = 2.031, p = 0.166] on this parameter.
HPA response to acute restraint stress
There was an interaction between the neonatal group and time on corticosterone levels in response to acute stress [GEE, 3, 691: 6, p = 0.02], in which the ELS group demonstrated an exacerbation of corticosterone secretion across the time points of analysis. There was also an isolated effect of time [GEE, 6370, 111: 6, p < 0.0001], but not of group [GEE, 0, 058: 1, p = 0.255] ().
Figure 3. HPA response to acute stress. The dark line on the x-axis represents time of exposure to restraint stress. Data are expressed as mean ± SEM. (A) Analysis of corticosterone secretion in control (n = 8) and ELS (n = 8) females revealed an interaction between time and group, indicating that ELS females increased secretion in response to stress (p = 0.02), and an isolated effect of time (p < 0.0001). (B) Analysis of ACTH secretion in the same samples had no group effect (p = 0.467) and no interaction between time and group (p = 0.282), only an isolated effect of time (p < 0.0001).
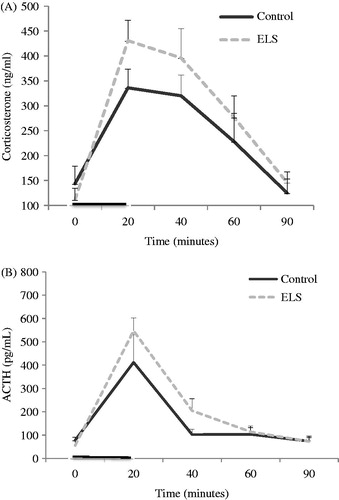
There was neither effect of the group [GEE, 0, 0528: 1, p = 0.467] nor any interaction between time and group [GEE, 5, 057: 4, p = 0.282] on ACTH levels in response to acute stress, only an isolated effect of time [GEE, 103, 843; 4, p < 0.0001] ().
Discussion
In this study, females subjected to early life stress (induced by the limitation of nesting material) exhibited adult anxiety and HPA axis hyper-reactivity to acute stress, decreased body weight, reduced consumption of chow and increased intake of a palatable diet under conditions of free choice.
A relationship has been demonstrated between HPA axis activity and increased consumption of palatable food in adult rats. Dallman et al. (Citation2005) showed that adrenalectomized rats treated with various levels of corticosterone did not eat more chow when only chow was available. However, when sucrose and/or fat were available, rats increased their intake of these foods in proportion to circulating glucocorticoids levels. Another study showed that CRF2 deficient mice, which exhibit an exaggerated HPA-axis response to stress, increase their intake of high-fat food following chronic variable stress to a greater degree than do wild-type controls (Teegarden & Bale, Citation2008).
Most studies conducted in animals demonstrate that exposure to stress in adult life reduces food intake, unless access to palatable food is secured during the stress period (Dallman et al., Citation2005; Pecoraro et al., Citation2004). In our study, although a stressful stimulus was applied during neonatal life, and animals were not exposed to other stimuli thereafter, an increased consumption of palatable food was observed in adulthood. In general, other early life stress models are associated with increased food intake and obesity risk in adult life (Panagiotaropoulos et al., Citation2004; Ryu et al., Citation2008, Citation2009). However, we did not observe any difference between ELS and control rats regarding abdominal fat content after 4 weeks of exposure to the palatable food, even though the ELS rats showed an increased consumption of this type of food. Additionally these animals continued to gain less weight than controls regardless of whether they were receiving the palatable diet. One possibility to explain these findings could be a metabolic programing effect of this type of early life stress that would protect ELS animals against increased abdominal adiposity. More studies are warranted to verify if this pattern remains or changes with a longer exposure to the palatable diet.
When food intake was continuously monitored, we showed that ELS females had decreased intake of standard rat chow. Additionally, using the BioDAQ® system, we observed that these animals had a decreased nocturnal meal intake as well as nocturnal meal size when compared to controls. This was observed during the dark phase of the light cycle, which means that ELS animals ate less during their active phase. It is possible that ELS females were less physically active, and therefore consumed fewer calories. It remains to be explored whether differences in physical activity or energy expenditure in these animals explain these differences.
Programing of HPA axis sensitivity is an important mechanism that is suggested to explain how fetal/perinatal stress affects food preferences later in life (Portella et al., Citation2012; Silveira et al., Citation2008). Our results support this idea, as we demonstrated that ELS animals had increased corticosterone levels compared to controls after exposure to acute stress and showed increased consumption of palatable food. Others have demonstrated that limitation of nesting material availability leads to changes in the HPA axis at day 9 of life, through an increase in peripheral corticosterone levels and adrenal weight (Avishai-Eliner et al., Citation2001; Brunson et al., Citation2005; Rice et al., Citation2008), as well as a marked reduction in CRH mRNA levels in the paraventricular nucleus of the hypothalamus compared with controls (Rice et al., Citation2008). Persistence of these changes was seen in adult mice exposed to the same ELS model (Rice et al., Citation2008) (however, this was not sustained at 12 months (Brunson et al., Citation2005)). The HPA axis programing in this animal model is related to altered maternal care when nest-building demands overcome the dams’ ability to spend time caring for her pups. Earlier studies from our group showed that while control animals have a normal distribution regarding maternal care (licking and grooming score), ELS dams had a condensed distribution suggesting a “stereotyped” maternal behavior (Dalle Molle et al., Citation2012). Others, using the same model of limited nesting material availability, report that both the quantity and the quality of the dams’ care toward their pups were influenced by the early life stress. Licking and grooming activities were decreased and the frequency of being off the nest increased, resulting in fragmented interactions between dams and pups (Ivy et al., Citation2008). One limitation in this study was the fact that we did not restrict the number of animals used per litter, and the use of multiple animals/litter is a potential confound due to the close genetic relationship of the siblings. However, to minimize this effect, when the litter size was influencing the outcome the analysis was adjusted for this variable.
In our study, ELS induced increased anxiety as measured by the NSFT, which is consistent with other models of early life adversity, such as maternal separation (Jahng, Citation2011; Liu et al., Citation2000). Additionally, a previous study from our group showed that adult ELS females had a lower frequency of head dips when compared to controls in the elevated plus-maze test (Dalle Molle et al., Citation2012), a parameter consistent with anxiety-like behavior. One could argue that the increased latency to eat Froot Loops® on the NSFT could be due to decreased motivation to obtain food. We cannot rule out this possibility, but considering our results on plus maze test (Dalle Molle et al., Citation2012), we suggest that ELS females demonstrate anxiety-like behavior in tasks involving food intake or not. Acute depletion of serotonin reduces anxiety-related behaviors in a setting similar to the NSFT in males (Bechtholt et al., Citation2007), suggesting that changes in this neurotransmitter system may explain the findings of our animal model. Classically, it has been proposed that serotonergic systems facilitate anxiety-related behavior (Wise et al., Citation1972), what was confirmed in more recent studies (Bechtholt et al., Citation2007; Graeff et al., Citation1996; Kahn et al., Citation1988). The consumption of food rich in fat and carbohydrate has been used to cheer people up, make them feel and function better as well as an anxiety relief (Dallman et al., Citation2003). It is possible that the ELS animals use this type of food to accomplish this goal.
In conclusion, we observed that ELS induced by limitation of nesting material led to adult anxiety and altered HPA axis sensitivity to acute stress in females, with decreased body weight as well as persistent increases in intake of a palatable diet when provided. In these animals, the preference for palatable, high-calorie, sugar/fat-enriched foods may result from a habit of using this type of food to ameliorate anxiety on a daily basis. It is also possible that these animals use this strategy to limit negative effects of chronic stress exposure. Knowledge on early-life programing of the inappropriate use of food as a relief mechanism may shed light on future studies designed to address this issue in humans. Females are usually less favored as experimental subjects, but are of extreme importance to studies of increased susceptibility to eating disorders and potential transgenerational effects of early life events. More studies on long-term effects of early-life stress in females are warranted.
Declaration of interest
The authors declare no conflict of interest. Financial support from: PRONEX 2009, FAPERGS/CNPq 10/0018.3, Projeto IVAPSA – Impacto das Variações do Ambiente Perinatal sobre a Saúde do Adulto; Brazilian National Council for Technological and Scientific Development (CNPq) – 14/2009; Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE/HCPA). Machado TD received an MSc supporting grant from the Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil.
References
- Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-el Y, Baram TZ. (2001). Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol 13:799–807
- Bechtholt AJ, Hill TE, Lucki I. (2007). Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology 190:531–40
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, et al. (2005). Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci 25:9328–38
- da SilvaBenetti C, Silveira PP, Portella AK, Diehl LA, Nunes E, De Oliveira VS, Dalmaz C, Goldani MZ. (2007). Could preference for palatable foods in neonatally handled rats alter metabolic patterns in adult life? Pediatr Res 62:405–11
- Dalle Molle R, Portella AK, Goldani MZ, Kapczinski FP, Leistner-Segala S, Salum GA, Manfro GG, Silveira PP. (2012). Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry 2:E195--203
- Dallman MF, Pecoraro NC, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, et al. (2003). Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA 100:11696–701
- Dallman MF, Pecoraro NC, La Fleur SE. (2005). Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun 19:275–80
- Davis C, Levitan RD, Carter J, Kaplan AS, Reid C, Curtis C, Patte K, Kennedy JL. (2008). Personality and eating behaviors: a case-control study of binge eating disorder. Int J Eat Disord 41:243–50
- Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. (1998). Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol 275:186–93
- Ely DR, Dapper V, Marasca J, Correa JB, Gamaro GD, Xavier MH, Michalowski MB, et al. (1997). Effect of restraint stress on feeding behavior of rats. Physiol Behav 61:395–8
- Epel E, Lapidus R, McEwen B, Brownell K. (2001). Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26:37–49
- Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. (2003). Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11:845–51
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. (2009). Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology 150:2325–33
- Graeff FG, Guimarães FS, Andrade TGCSD, Deakin JFW. (1996). Role of 5-ht in stress, anxiety, and depression. Pharmacol Biochem Behav 54:129–41
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, et al. (2002). Serotonin 1a receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416:396–400
- Grucza RA, Przybeck TR, Cloninger CR. (2007). Prevalence and correlates of binge eating disorder in a community sample. Compr Psychiatry 48:124–31
- Ivy AS, Brunson KL, Sandman C, Baram TZ. (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154:1132–42
- Jahng JW. (2011). An animal model of eating disorders associated with stressful experience in early life. Horm Behav 59:213–20
- Kahn RS, Van Praag HM, Wetzler S, Asnis GM, Barr G. (1988). Serotonin and anxiety revisited. Biological Psychiatr 23:189–208
- La Fleur SE, Houshyar H, Roy M, Dallman MF. (2005). Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology 146:2193–9
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. (2000). Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol 12:5–12
- Maniam J, Morris MJ. (2010). Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology 35:717–28
- Meaney MJ. (2010). Epigenetics and the biological definition of gene × environment interactions. Child Dev 81:41–79
- Mikolajczyk RT, El Ansari W, Maxwell AE. (2009). Food consumption frequency and perceived stress and depressive symptoms among students in three European countries. Nutr J 8:31--8
- Murgatroyd C, Spengler D. (2011). Epigenetic programming of the HPA axis: early life decides. Stress 14:581–9
- Oliver G, Wardle J, Gibson EL. (2000). Stress and food choice: a laboratory study. Psychosom Med 62:853–65
- Panagiotaropoulos T, Papaioannou A, Pondiki S, Prokopiou A, Stylianopoulou F, Gerozissis K. (2004). Effect of neonatal handling and sex on basal and chronic stress-induced corticosterone and leptin secretion. Neuroendocrinology 79:109–18
- Pecoraro N, Dallman MF, Warne JP, Ginsberg AB, Laugero KD, La Fleur SE, Houshyar H, et al. (2006). From malthus to motive: how the hpa axis engineers the phenotype, yoking needs to wants. Prog Neurobiol 79:247–340
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. (2004). Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology 145:3754–62
- Plotsky PM, Meaney MJ. (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (crf) MRNA, median eminence crf content and stress-induced release in adult rats. Brain Res Mol Brain Res 18:195–200
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. (2005). Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 30:2192–204
- Portella AK, Kajantie E, Hovi P, Desai M, Ross MG, Goldani MZ, Roseboom TJ, Silveira PP. (2012). Effects of in utero conditions on adult feeding preferences. J Dev Orig Health Dis 3:140–52
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. (2008). A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149:4892–900
- Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-plantenga MS. (2009). Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring) 17:72–7
- Ryu V, Lee JH, Yoo SB, Gu XF, Moon YW, Jahng JW. (2008). Sustained hyperphagia in adolescent rats that experienced neonatal maternal separation. Int J Obes 32:1355–62
- Ryu V, Yoo SB, Kang DW, Lee JH, Jahng JW. (2009). Post-weaning isolation promotes food intake and body weight gain in rats that experienced neonatal maternal separation. Brain Res 1295:127–34
- Scott KM, Bruffaerts R, Simon GE, Alonso J, Angermeyer M, de Girolamo G, Demyttenaere K, et al. (2008). Obesity and mental disorders in the general population: results from the world mental health surveys. Int J Obes 32:192–200
- Silveira PP, Portella AK, Assis SA, Nieto FB, Diehl La, Crema LM, Peres W, et al. (2010). Early life experience alters behavioral responses to sweet food and accumbal dopamine metabolism. Int J Dev Neurosci 28:111–18
- Silveira PP, Portella AK, Crema L, Correa M, Nieto FB, Diehl L, Lucion AB, Dalmaz C. (2008). Both infantile stimulation and exposure to sweet food lead to an increased sweet food ingestion in adult life. Physiol Behav 93:877–82
- Striegel-Moore RH, Bulik CM. (2007). Risk factors for eating disorders. Am Psychol 62:181–98
- Surina-Baumgartner DM, Langhans W, Geary N. (1995). Hepatic portal insulin antibody infusion increases, but insulin does not alter, spontaneous meal size in rats. Am J Physiol 269:978–82
- Teegarden SL, Bale TL. (2008). Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol Behav 93:713–23
- Wardle J, Steptoe A, Oliver G, Lipsey Z. (2000). Stress, dietary restraint and food intake. J Psychosom Res 48:195–202
- Wise CD, Berger BD, Stein L. (1972). Benzodiazepines: anxiety-reducing activity by reduction of serotonin turnover in the brain. Science 177:180–3
- Womble LG, Williamson DA, Martin CK, Zucker NL, Thaw JM, Netemeyer R, Lovejoy JC, Greenway FL. (2001). Psychosocial variables associated with binge eating in obese males and females. Int J Eat Disord 30:217–21
- Zhang J, Huang XY, Ye ML, Luo CX, Wu HY, Hu Y, Zhou QG, et al. (2010). Neuronal nitric oxide synthase alteration accounts for the role of 5-ht1a receptor in modulating anxiety-related behaviors. J Neurosci 30:2433–41