Abstract
Quantification of cortisol in scalp hair seems a promising measurement for long-term cortisol levels, and thereby a biomarker for stress. We examined hair cortisol concentrations (HCC) in children when first entering elementary school. Participants were 42 children (45% boys) with a mean age of 4.2 years (SD = 0.42 months). Hair samples (≥5 cm) were collected 2 months after school entry. Hair analysis was conducted using two 2-cm long segments, reflecting the first 2 months of school attendance (the scalp-near segment) and 2 months prior to school entry. HCC were higher after school entry than before, especially for fearful children. Alterations in HCC were not moderated by experience in group daycare before school entry. Thus, HCC suggest that starting elementary school is accompanied by increased stress hormone levels in young (in particular fearful) children.
Introduction
Cortisol is a well-known stress hormone which in humans is the final product of activation of the hypothalamic–pituitary–adrenal axis. Until recently, cortisol levels have been determined in urine, blood or saliva. To assess cortisol levels over a prolonged period of time, repeated sampling is needed at different daily time points over several days. This sampling method is not only labor intensive, but also challenging, especially for young children. Recently, a new method has been developed to determine long-term cortisol levels: measurement of cortisol in human scalp hair (Russell et al., Citation2011). Because hair grows at an average of 1 cm/month, assessment of hair cortisol can reflect changes over time. Here, we report a study using hair cortisol concentrations (HCC) to examine children’s responses to the transition to elementary school.
Previous studies have shown that HCC correlated significantly with cortisol in 24-h urine (Sauvé et al., Citation2007), and contemporaneously collected salivary cortisol (D’Anna-Hernandez et al., Citation2011; Vanaelst et al., Citation2012). In addition, higher HCC have been shown to be related to higher perceived stress in chronic pain patients (Van Uum et al., Citation2008), and to more days of ventilation in the neonatal intensive care unit for term born infants (Yamada et al., Citation2007). An increasing number of studies supports the validity and reliability of HCC (for reviews, see Meyer & Novak, Citation2012; Stalder & Kirschbaum, Citation2012; Staufenbiel et al., Citation2013).
Because hair analysis allows a retrospective assessment of cortisol exposure, this method seems valuable when studying the effects of major transitions in life. Increases in HCC after relocation from their housing environment were found in rhesus macaques (Davenport et al., Citation2006) and in vervets (Fairbanks et al., Citation2011). Studies in humans have found that HCC of patients with Cushing’s disease were elevated prior to a corrective surgery, and decreased after surgical removal of the pituitary adenoma (Manenschijn et al., Citation2012). HCC in young children have been underexplored.
In the current study, we measure HCC alterations in young children before and after a stress-related transition, that is, their first entry into elementary school. Based on prior studies on salivary cortisol and school entry (Bruce et al., Citation2000), we hypothesize that children’s HCC are higher after school entry than before school entry. Furthermore, we expect that alterations in HCC are moderated by temperament and experience in group daycare before school entry. HCC alterations may be more pronounced in fearful children and less pronounced in children who have experienced many hours in group daycare before starting school.
Method
Subjects
Families were selected from municipality records from a city in the Western region of the Netherlands, and were included if one of the children turned 4 years of age (the legal age of elementary school entry) between February and April 2012. A total of 284 families were invited by postal mail to participate. Registration for the study was closed after agreement from 50 families. Four children were excluded, because their hair was too short (<5 cm), and four children were excluded, because of extremely high cortisol values with (>3SD above the mean). This resulted in a final sample of 42 children (45% boys).
Temperament
The Child Behavior Questionnaire (CBQ) short form was used to measure the child’s temperament (Ahadi et al., Citation1993). Parents were asked to fill out the CBQ, which was sent to them a few weeks before the home visit. The CBQ is a 7-point rating scale for the assessment of different aspects of temperament in children aged 3–7 years. For this study, we used the subscale fearfulness (eight items, Cronbach’s alpha = 0.76), which has been shown to be positively associated with higher salivary cortisol levels (Groeneveld et al., Citation2012; Talge et al., Citation2008; Watamura et al., Citation2003).
Scalp hair collection
Hair was collected during home visits, which were planned ∼2 months after the children had started school. Visits were conducted by the first author or trained (under)graduate students. Hair collection was performed as described previously (Manenschijn et al., Citation2011). A sample containing around 100 hairs was cut from the posterior vertex as close to the scalp as possible. The sample was taped to a piece of paper, the scalp end marked, and stored in an envelope at room temperature until analysis.
Prior to the home-visit, parents received a questionnaire, with questions about age, educational level and birth country of the parents, and the following information concerning the participating child: history in daycare, hair color, hair washing frequency, use (and type) of corticosteroids during last 6 months, other medication use and chronic diseases. All procedures were carried out with adequate understanding and written consent of the parents. Ethical approval for this study was provided by the Research Ethics Committee of the Institute of Education and Child Studies of Leiden University.
Hair processing
From the hair samples, we used two 2-cm sections (). The first section had grown out directly following school entry, reflecting approximately the first 2 months of school attendance. The second section had grown out prior to school entry and was used as a baseline. Between these two sections, we left a gap of 1 cm to avoid overlap of periods (due to differences in hair growth rate). In addition, 11 of the 42 2-cm hair sections that were processed following school entry were suited division into two 1-cm sections. These 1-cm sections were additionally analyzed to explore stability in HCC after school entry.
Figure 1. Retrospective segmental hair analysis in children before and after school entry. Hair fragments of 1 cm correspond approximately to 1 month.

Hair samples were prepared as described by Sauvé et al. (Citation2007) and Manenschijn et al. (Citation2011), and weighed and cut into small pieces in glass vials using surgical scissors. Cortisol was extracted by 16 h incubation in 1 mL methanol at 52 °C while gently shaking. Subsequently, the methanol was transferred to disposable glass vials, and evaporated under a constant stream of nitrogen. The samples were then dissolved in 250 μL phosphate buffered saline, pH 8.0 and vortexed for 1 min. Prior to analysis, samples were vortexed again for 30 s. To account for the relative lower sample weight of 1-cm sections opposed to the 2-cm sections (26.2 and 36.4 mg, respectively) in 11 children, a correction factor was calculated. This correction factor adjusts for a background signal caused by the hair matrix, which results in a slightly elevated hair cortisol measurement in lower sample weight. The correction factor was not applied to the remaining hair samples as the sample weight did not significantly differ between the proximal and distal samples.
Cortisol measurement
Cortisol concentrations were measured using a commercially available salivary cortisol ELISA kit (DRG Instruments GmbH, Marburg, Germany) according to manufacturer’s directions. As stated by the manufacturer, antibody cross reactivity is as follows: corticosterone 29%, cortisone 3%, 11-Deoxycortisol <1%, 17-OHP <0.5%, prednisone <0.1%. The intra-assay coefficient of variation (CV) calculated on the average CV of the duplicate hair sample measurements was 4.7%. The inter-assay CV that was calculated on internal controls in three concentrations (though not in hair matrix, we use internal quality controls that are applied in routine saliva measurements) was 3.7%.
Data analysis
Because the distributions of the cortisol measurements were positively skewed, log10 transformations were used for analysis. After log10 transformation, HCC did not deviate from normality. To test whether HCC differed before and after school entry, we conducted a repeated measure MANCOVA with time as within-subject variable, experience in group care as between-subjects variable, and fearfulness as a covariate. For purposes of analysis, we created a variable “experience in group care” using a median-split procedure (median at 18 hours). This resulted in a group with more experience (N = 20; M = 29.9; SD = 8.8) versus less experience (N = 22; M = 13.9; SD = 4.4). For 11 children, HCC were available at two time points after school entry. We tested whether HCC alterations could be established in this subsample as well.
Results
Descriptives
Mean age of the children was 50.1 months (SD = 0.42 months). Mothers were aged between 27 and 47 years (M = 37.24, SD = 4.15) and fathers were between 27 and 52 years of age (M = 39.48, SD = 5.20). Parents’ educational level was coded as the number of years of education after age 6. Mean educational level across mothers and fathers was 14.8 years (SD = 1.9). The year preceding school entry, children had spent on an average 21.5 h a week (SD = 10.5, range from 3 to 50 h) in any type of group care.
Pearson correlations between HCC before and after school entry showed a high stability across time (r = 0.76, p < 0.001). HCC were not significantly related to any of the child (gender, hair color, hair washing frequency, use of corticosteroids, other medication, hours in group care, fearfulness) or parent characteristics (educational level).
Change in HCC
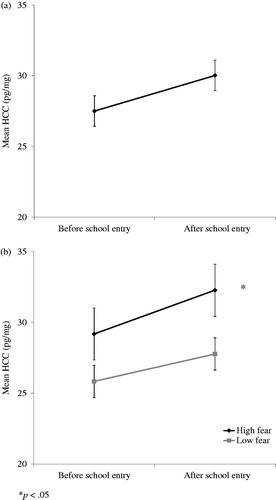
A repeated measures MANCOVA on children’s HCC yielded a significant main effect of time (Pillais, F[1, 38] = 9.90, p = 0.003, = 0.21). Children’s HCC were significantly lower prior to the start at school (M = 27.50 pg/mg, SD = 13.94) than after the start at school (M = 30.02 pg/mg, SD = 14.05), d = 0.52 (). Furthermore, we found a significant 3-way interaction of time by experience in group care by fearfulness (Pillais, F[1, 38] = 6.85, p = 0.01,
= 0.15). Separate analyses for children scoring high or low on fearfulness (n = 21 in each group; median split at 2.53), showed that HCC significantly increased after school entry, but only in children scoring high on fearfulness, (Pillais, F[1, 19] = 4.67, p = 0.04,
= 0.20) (). Although within this subsample the 2-way interaction time by experience in group care was not significant, there was a trend showing that the rise in cortisol was especially evident in high fearful children with ample experience in group care (Pillais, F[1, 19] = 2.84, p = 0.11,
= 0.13).
Figure 2. Mean untransformed HCC (pg/mg ± SEM) before and after school entry for the total sample (a; N = 42), and for high (N = 21) and low fearful (N = 21) children (b).
In a subsample of 11 children for whom HCC were available at three time points (reflecting 2 months before school entry, the first month after school entry and the second month after school entry, respectively), a repeated measures MANOVA with HCC at the three time points also showed a significant main effect of time (Pillais, F[2, 9] = 11.65, p = 0.003, = 0.71). Paired t-tests showed that HCC 2 months before school entry (M = 21.44 pg/mg, SD = 12.31) were significantly lower than HCC in the first month after school entry (M = 32.20 pg/mg, SD = 21.38), t (10) = −5.06, p < 0.001, d = 2.18 and HCC in the second month after school entry, (M = 32.60 pg/mg, SD = 19.02), t (10) = −4.21, p = 0.002, d = 1.71. A paired t-test showed that HCC in the first month after school entry did not differ from HCC in the second month after school entry, t (10) = −0.47, p = 0.65, as was expected.
The same analyses were performed after adjusting for hair sample weight (see Method). These analyses yielded similar results (data not shown).
Discussion
Four-year-old children’s HCC were higher after school entry than before school entry. Within a subsample, we found that HCC remained stable in two consecutive periods of a month after school entry. This finding supports our hypothesis that a rise in HCC can be specifically linked to a stress-related transition, the first entry into elementary school. As in adults, HCC in children were not affected by hair color or hair washing frequency (Dettenborn et al., Citation2012; Manenschijn et al., Citation2011; Sauvé et al., Citation2007).
As hypothesized, high fearful children showed the highest rise in HCC after school entry. These results suggest that rises in HCC in reaction to stressful changes in the environment may have differential predictive value as a function of fearful temperament. Furthermore, our data illustrate a trend of high fearful children with ample experience in group daycare showing the highest rise in HCC after school entry. Possibly, for high fearful children with ample experience in group care the transition to a new setting may be particularly stressful, because of the separation from a familiar environment, with familiar caregivers and peers. Future studies with larger sample sizes may shed more light on these individual differences in reaction to the transition to elementary school.
Because HCC have rarely been examined in children, normative values within this age group are not documented. In a Canadian sample of healthy preschoolers in child care, Vaghri et al. (Citation2012) reported HCC of 0.024 ng/mg (=24 pg/mg) within the youngest age group (41–51 months; N = 85). In the study reported here, HCC both before school entry (M = 27.50 pg/mg) and after school entry (M = 30.02 pg/mg) were slightly higher, which may have been caused by the (anticipation of the) start of elementary school or, more likely, is due to the known assay variation between laboratories. In our analysis, we excluded outlying cases with cortisol values >3SD above the mean (all >100 pg/mg). The main focus of this study was to examine intra-individual differences in children’s HCC, using their own HCC before school entry as a baseline.
Although several studies show that HCC can be reliably determined measured over quite an extensive historical time span, there is still controversy as to the exact time frame in which cortisol levels can be reliably measured. Some studies showed that HCC are on average stable over time, and can be measured up to several years (depending on the length of the hair) in retrospect (Manenschijn et al., Citation2011, Citation2012; Thomson et al., Citation2010). Other studies, using a slightly different hair cortisol preparation and extraction method, show that a decline in cortisol levels is detected over time (Gao et al., Citation2010; Kirschbaum et al., Citation2009). At present this “wash-out” effect is not well understood, but might be due to external factors affecting hair integrity and the structure of hair keratin in the older (distal) hair segments (perming, dyeing, bleaching, ultraviolet irradiation in prolonged air exposure; Gao et al., Citation2010) or via passive diffusion of cortisol over the hair shaft. In general, mild damage to the distal hair segments may differentially influence the measurements depending on the used techniques of processing hair samples and measuring cortisol levels. However, our study comprises a timeframe of 3 months, and in the literature there is agreement that the wash-out decline occurs only after a time span of 3–6 months (corresponding to 3–6 cm of hair). Moreover, in the current study we used a method of which we showed that it yields reliable cortisol levels over prolonged periods of time (even years) (Manenschijn et al., Citation2011, Citation2012; Thomson et al., Citation2010). Therefore, the increase in hair cortisol after school entry is not likely attributable to a technical artifact.
In summary, measurement of cortisol in hair seems a promising and nonintrusive method of detecting individual differences in young children’s reactions to stressful changes in the environment, such as the transition to elementary school.
Declaration of interest
Marinus H. van IJzendoorn was supported by the SPINOZA prize from the Netherlands Organization for Scientific Research.
Acknowledgements
We are grateful to the children, parents and teachers who participated as well as the students who assisted in the study. In addition, we thank J. H. Groeneveld-Blom who put forward the idea of offering a professional hair styling to all children and their siblings, as a thank you for participating in the study.
References
- Ahadi SA, Rothbart MK, Ye R. (1993). Children’s temperament in the U.S. and China: similarities and differences. Eur J Personality 7:359–78
- Bruce J, Poggi Davis E, Gunnar MR. (2000). Individual differences in children’s cortisol response to the beginning of a new school year. Psychoneuroendocrinology 27:635–50
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. (2006). Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147:255–61
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. (2011). Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav 104:348–53
- Dettenborn L, Tietze A, Kirschbaum C, Stalder T. (2012). The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress 15:578–88
- Fairbanks LA, Jorgensen MJ, Bailey JN, Breidenthal SE, Grzywa R, Laudenslager ML. (2011). Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology 36:1201–8
- Gao W, Xie Q, Jin J, Qiao T, Wang H, Chen L, Deng H, Lu Z. (2010). HPLC-FLU detection of cortisol distribution in human hair. Clin Biochem 43:677–82
- Groeneveld MG, Vermeer HJ, van IJzendoorn MH, Linting M. (2012). Stress, cortisol and well-being of caregivers and children in home-based child care: a case for differential susceptibility. Child Care Health Dev 38:251–60
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. (2009). Hair as a retrospective calendar of cortisol production-increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34:32–7
- Manenschijn L, Koper JW, Lamberts SWJ, van Rossum EFC. (2011). Evaluation of a method to measure long term cortisol levels. Steroids 76:1031–6
- Manenschijn L, Koper JW, van den Akker ELT, De Heide LJM, Geerdink EAM, De Jong FH, Feelders RA, van Rossum EFC. (2012). A novel tool in the diagnosis and follow-up of (cyclic) Cushing’s syndrome: measurement of long-term cortisol in scalp hair. J Clin Endocrinol Metab 97:1836–43
- Meyer JS, Novak MA. (2012). Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocorticol activity. Endocrinology 153:1–8
- Russell E, Koren G, Rieder M, Van Uum S. (2011). Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37:589–601
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SH. (2007). Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 30:183–91
- Stalder T, Kirschbaum C. (2012). Analysis of cortisol in hair – state of the art and future directions. Brain Behav Immun 26:1019–29
- Staufenbiel S, Penninx BW, Spijker AT, Elzinga B, van Rossum EF. (2013). Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 38:1220--38
- Talge NM, Donzella B, Gunnar, MR. (2008). Fearful temperament and stress reactivity among preschool-aged children. Infant Child Dev 17:427–45
- Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SHM. (2010). Hair analysis provides a historical record of cortisol levels in Cushing’s syndrome. Exp Clin Endocrinol Diabetes 118:133–8
- Vaghri Z, Guhn M, Weinburg J, Grunau RE, Yu W, Hertzman C. (2012). Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology 38:331–40
- Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. (2008). Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress 11:483–8
- Vanaelst B, Huybrechts I, Bammann K, Michels N, De Vriendt T, Vyncke K, Sioen I, et al. (2012). Intercorrelations between serum, salivary and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology 49:1072–81
- Watamura SE, Donzella B, Alwin J, Gunnar MR. (2003). Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: age differences and behavioral correlates. Child Dev 74:1006–20
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. (2007). Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology 92:42–9
