Abstract
Academic examination is a major stressor for students in China. Investigation of stress-sensitive endocrine responses to major examination stress serves as a good model of naturalistic chronic psychological stress in an otherwise healthy population. The cortisol awakening response (CAR) is an endocrine marker of the hypothalamic-pituitary-adrenocortical (HPA) axis in response to stress. However, it remains unknown how chronic examination stress impacts the CAR in a young healthy population To exclude the influence of sex effects on hormone level, the CAR and psychological stress responses were assessed on two consecutive workdays in 42 male participants during their preparations for the Chinese National Postgraduate Entrance Exam (NPEE) and 21 non-exam, age-matched male comparisons. On each day, four saliva samples were collected immediately after awakening, 15 minutes, 30 minutes and 60 minutes after awakening. The waking level (S1), the increase within 30 minutes after awakening (R30), the area under the curve with respect to ground (AUCg), and the area under the curve with respect to increase (AUCi) were used to quantify the CAR. Psychological stress and anxiety were assessed by the Perceived Stress Scale and the Spielberger State-Trait Anxiety Inventory, respectively. Male participants in the exam group had greater perceived stress and anxiety scores relatibe to the non-exam group. Both R30 and AUCi in the exam group were significantly lower than the comparison group and this effect was most pronounced for participants with high levels of perceived stress in the exam group. Perceived stress and anxiety levels were negatively correlated with both R30 and AUCi. Chronic examination stress can lead to the decrease of CAR in healthy young men, possibly due to reduced HPA axis activity under long-term sustained stress.
Introduction
In humans cortisol levels increase rapidly within a 30 minutes interval after awakening then return to baseline levels around an hour later. This phenomenon is known as the cortisol awakening response (CAR) (Pruessner et al., Citation1997a). This response is consistent across consecutive days, showing relatively high intraindividual stability in human adults (Wust et al., Citation2000b). Because of its non-invasiveness and convenience, numerous studies have suggested the CAR as a standard tool for assessment of the integrity of the hypothalamic-pituitary-adrenocortical (HPA) axis even in ambulatory settings (for reviews, see Clow et al., Citation2004; Fries et al., Citation2009). Although it occurs within the normal diurnal rhythm, which includes several secretory episodes of short duration and high amplitude, the CAR appears to have a distinct regulatory mechanism which is different from the diurnal cortisol secretion pattern (Schmidt-Reinwald et al., Citation1999; Wilhelm et al., Citation2007).
Situational factors are key moderators of the CAR (Hellhammer et al., Citation2007). Rohleder and colleagues found that dancers had a greater CAR on a tournament day compared to a non-competition day (Rohleder et al., Citation2007). Furthermore, CAR profiles show a steeper rise on weekdays compared to workfree weekends (Schlotz et al., Citation2004; Thorn et al., Citation2006). These results suggest that the CAR is modulated by anticipated demands of the upcoming day and may serve to provide the necessary energy for shifting from a resting to an active state (Pruessner et al., Citation1997a; Rohleder et al., Citation2007; Stalder et al., Citation2011).
In addition to situational factors, chronic stress also plays an important role in the CAR. Studies examining how chronic stress modulates the CAR have reported heterogeneous results (for reviews, see Miller et al., Citation2007; Fries et al., Citation2009). Some studies report an increased CAR in individuals exposed to temporary-employment related stress (Gustafsson et al., Citation2012), chronic work overload and worrying (Schlotz et al., Citation2004), financial strain (Steptoe et al., Citation2005) or social stress (Wust et al., Citation2000a). There are a few studies, however, showing reduced CAR associated with chronic stress indices, such as in caregivers of chronically ill family members (Barker et al., Citation2012; Buchanan et al., Citation2004) or in individuals exposed to early life adversity (Meinlschmidt & Heim, Citation2005; Quevedo et al., Citation2012).
Several factors have been proposed to explain these mixed results, such as physical/psychiatric conditions, seasonal influence, the timing of cortisol sampling, participant adherence to protocol, and participant attributes such as sex, age, smoking status, and awakening time (for reviews, see Clow et al., Citation2004; Fries et al., Citation2009). Fries and colleagues hypothesized that the duration of stressful conditions may play an important role in the direction of stress influences on the CAR (Fries et al., Citation2009). This proposal was echoed by the results of a meta-analysis, which suggested that “timing is an especially critical element, as hormonal activity is elevated at stressor onset but reduces as time passes” (Miller et al., Citation2007).
According to the extant literature, long-term exposure to major life stressors might be related to an increased or decreased CAR. However, much of the evidence comes from older participants (Barker et al., Citation2012; Buchanan et al., Citation2004) or those with chronic diseases (De Kloet et al., Citation2007; Nater et al., Citation2008; Roberts et al., Citation2004; Sonnenschein et al., Citation2007). The present study focuses on the effect of a chronic stressor, namely long-term preparation for an important examination, on the CAR in young, healthy male students.
The rationale to focus on an academic examination as a chronic stressor is that academic performance from a series of two- or three-day written exams is the primary factor determining success in many disciplines in China (Siegel (Time.com), Citation2007). Among them the NPEE is one of the most important and highly competitive exams within the Chinese educational system and is the primary factor determining a student’s admission to graduate school. Thus, elucidating the pathways linking academic stress and physiological responses in this group is of substantial importance. For this purpose, we collected saliva samples from male students participating in the Chinese National Postgraduate Entrance Exam (NPEE) and non-exam comparison male students (to limit possible sex effects). Based on previous research, we hypothesized that long-term stress exposure, defined as preparation for the NPEE, would result in higher levels of perceived stress and a blunted CAR compared to the non-exam students.
Methods
Participants and quasi-experimental variables
Only male graduating were recruited for this study. Females were not used in this report due to sex differences in brain structures that modulate HPA axis activity and differences in corticosteroid binding globulin levels that impact basal and stress-induced activation of the HPA axis (for a review, see Kudielka & Kirschbaum, Citation2005). A total of 63 young healthy male college students were recruited through advertisements in Wannan Medical College, of whom 42 took part in the NPEE while the other 21 students did not participate in any academic exams or interviews within one month before or after the experiment.
The NPEE is one of the most important and highly competitive exams within the Chinese educational system. NPEE performance is the primary factor determining a student’s admission to graduate school. It is a written examination conducted on two consecutive days for 6 hours each day. This examination is generally nationwide, consisting of tests of English, political science, and another two speciality tests. Normally, students spend at least 6 months to effortfully prepare for this exam, and the acceptance rate into a graduate program following this exam is less than about 33% over the last ten years (Adminutes (Freekaoyan.com), Citation2012).
Due to the potential influence on the HPA axis, the following exclusion criteria were employed: any medication use within two days of participation in the study; chronic use of any psychiatric, neurological, or endocrine medicine; any history of psychiatric or neurological disorder; current periodontitis; any history of major chronic physiological disorders; current acute inflammation or allergy; current acute episodes of chronic disease; overnight shift work or irregular circadian rhythm; excessive alcohol consumption (more than two alcoholic drinks daily) and nicotine consumption (more than five cigarettes a day); any history of serious head trauma. In addition, presence of other major stressors during the past month assessed by the Life Events Scale (LES) (Tennant & Andrews, Citation1976) (the version used here was translated into Chinese (Zhang et al., Citation1987)) served as an exclusion criteria.
Participants were first screened based on these inclusion/exclusion criteria at the time of recruitment by self-report questionnaire, and further confirmed by a telephone interview. All participants gave written informed consent and were paid for their participation. This experiment was approved by the Ethics Committee of Human Experimentation at the Institute of Psychology, Chinese Academy of Sciences.
Procedure
Between 12 and 27 December 2011, which was 11–25 days before the NPEE, all qualified male participants came to the laboratory and completed questionnaires. Then participants received a detailed instruction packet describing the method of saliva collection over the next two days. They were told the importance of adherence to the sampling instruction and requested to abide by the instructions. The participants were also provided with sleep-related questionnaires which they should complete at their dormitory after saliva collection in the morning. Saliva samples were collected immediately after awakening in the morning on two consecutive workdays and participants were asked to return the saliva samples to the lab as soon as possible (see further for full description of saliva sampling protocol).
Questionnaires
Chronic stress was assessed with the Perceived Stress Scale (10-item version) (Cohen & Williamson, Citation1988). The PSS is a valid and reliable measure that has been used frequently as an index of the perception of chronic stress (such as Liston et al., Citation2009; Tomiyama et al., Citation2011) and many studies with different Chinese sample populations have also demonstrated the PSS10 to be a very useful scale to measure psychological stress among Chinese (Wang et al., Citation2011; Yu & Ho, Citation2010). The State-Trait Anxiety Inventory (Spielberger, Citation1983), which is one of the most commonly used scales to measure anxiety in student populations (Gotlib & Cane, Citation1989), was also administrated. A sleep questionnaire, which assessed sleep duration on the night before sampling and waking time in the morning, was completed after saliva collection. We also collected information on the duration of the time they began effortfully preparing for this exam to the time that they participated in the experiment (not including the time spent on review material purchase and school application) and the review intensity (the time they spent on review everyday). Forty-one of the students in the exam group were contacted after the exam to collect information on performance. Considering the variability of specialty tests taken among participants, we report only the total public test scores of English and political science (see “Results” section).
Salivary cortisol sampling
Saliva samples were collected using Salivette collection devices (Sarstedt, Germany). On two consecutive workdays, saliva samples were collected immediately upon awakening (sample 1), and 15 minutes (sample 2), 30 minutes (sample 3), 60 minutes (sample 4) thereafter, resulting in four samples per day and a total of eight samples for each individual. Participants were requested to awake at a constant time between 06:00 h and 08:00 h on both days. To confirm their adherence, participants were asked to record waking and sleeping time as well as the exact time each sample was collected. All participants were asked to stay in bed until all four saliva samples were obtained after awakening, meanwhile they were allowed to read and quietly listen to music as well as to go to bathroom if necessary. To avoid contamination of saliva, participants were asked not to brush teeth, smoke, drink, or eat before completion of saliva sampling. They were also required to refrain from alcohol and nicotine consumption as well as excessive exercise on the day before saliva sampling. Participants were instructed to bring the samples back to the laboratory, where samples were kept frozen (−20 °C) until assay. To capture the morning rise of cortisol, the duration between self-reported wakeup time and sample 1 was less than 15 minutes for all participants (Wolfram et al., Citation2011), and the time between samples had to be >10 and <60 minutes (Gustafsson et al., Citation2012). No samples were reported to exceed these time limits. There were four participants in the exam group and one in the control group missing less than three samples in total. Thus, the missing values were substituted on the basis of a combination of the group mean and standard deviation for the missing cortisol sample, and the mean of the participant’s cortisol samples that were recorded (as described in Booij et al., Citation2013).
Samples were thawed and centrifuged at 3200 rpm for 10 minutes. Cortisol concentration was analyzed by use of electrochemiluminescence immunoassay (Cobas e 601, Roche Diagnostics, Numbrecht, Germany), with following parameters: sensitivity, 0.500 nmol/L (lower limit), and standard range in assay, 0.5–1750 nmol/L. Intra and inter-assay variations were below 10%.
Data analysis
For each of the four time points, cortisol levels were averaged across the 2 days to obtain a single mean level of cortisol. Four scoring methods were used to compute the dynamics of the CAR: (1) cortisol level immediately upon awakening (S1), (2) the change in cortisol level at 30 minutes after awakening (R30) by subtracting the cortisol level at 0 minutes from 30 minutes (Kirschbaum et al., Citation1999), (3) area under the curve with reference to the ground (AUCg = (sample1 + s2)*0.25/2 + (s2 + s3)*0.25/2 + (s3 + s4)*0.5/2) and (4) area under the curve with respect to the increase (AUCi = AUCg-s1*(0.25 + 0.25 + 0.5)) (Pruessner et al., Citation2003). The AUCg is an estimate of the total cortisol secretion over the first hour after awakening, and the AUCi is a measure of the dynamics of the CAR, more related to the sensitivity of the HPA axis, emphasizing changes over time (Hellhammer et al., Citation2007; Pruessner et al., Citation2003).
Comparison between exam and non-exam groups on psychological measures and cortisol were completed by separate independent samples t-tests. To examine the effect of individual differences on psychological stress response, the exam group was further subdivided into high- and low-stress exam groups based on their scores on the PSS10 (median split). An analysis of variance (ANOVA) with the between-subject factor of group (non-exam, low-stress exam group and high-stress exam group) was used to analyze group differences on all psychological measures and CAR parameters: S1, R30, AUCg, AUCi. The distribution of cortisol data was examined by the Kolmogorov–Smirnov test, and a natural log transformation was applied to cortisol data that were not normally distributed. Where ANOVA procedures revealed a significant main effect, post-hoc analyses of Least Square Difference (LSD when test of homogeneity of variances ≥0.05) or Dunnett T3 (when test of homogeneity of variances <0.05) were used to examine the specific effects and significance levels. Measures of effect size were reported using partial eta square (partial η2). Correlational analyses using Pearson’s r were performed between CAR indices, and perceived stress and anxiety scores, examination performance, as well as sleep duration. To clarify the factor of sleep, a partial correlation analysis was computed between perceived stress scores and CAR parameters with waking time and sleep duration controlled. All reported p values are two-tailed.
Results
Demographic and psychosocial characteristics of sample
Characteristics of the study sample and results of psychological measures can be found in . The exam stress group and non-exam control group were matched with respect to age and level of education.
Table 1. Descriptive statistics by group: mean (SD).
Participants in the exam group reported higher levels of perceived stress (t = 2.38, p < 0.05) and anxiety (t = 3.43, p < 0.01) relative to the nonexam group. The exam group was split into high-stress exam group and low-stress exam group by median score of PSS10 (which was 17). Eight participants who had the same score of 17 were classified into the high-stress exam group considering that “the PSS is not a specific-population-dependent instrument” (Wang et al., Citation2011), and this score is higher than that in the community residents of the original norms (Cohen & Williamson, Citation1988). Thus, there were 24 participants in the high-stress exam group (M ± SD: 19.08 ± 2.06) and 18 participants in the low-stress exam group (M ± SD: 15.06 ± 1.06).
In addition, participants in the exam group also reported earlier awakening time and shorter sleep duration (t = −3.59 and t = −4.45 respectively, ps < 0.01). As for the exam performance in the exam group, there was no significant difference between high-stress exam group and low-stress exam group (M ± SD: 113.71 ± 13.77 versus 114.88 ± 16.33, t = −0. 25, p > 0.1).
Cortisol results
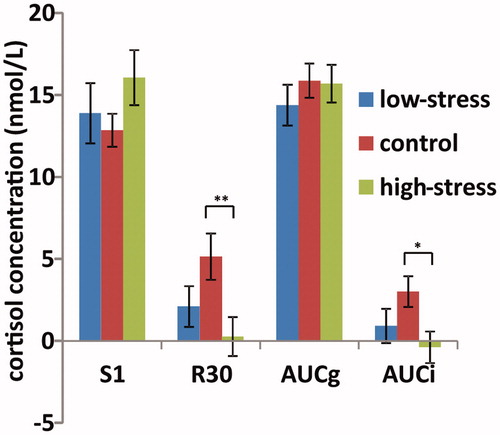
Paired t-tests revealed no significant difference in cortisol levels between the two days (ps > 0.1) and cortisol levels at each sampling point between the two days were significantly correlated (rs = 0.32–0.5, ps < 0.01), indicating high intraindividual stability across day 1 to day 2. Thus, we combined cortisol data from the two days to increase the reliability of CAR for statistical analyses, and all parameters presented below are means of the two consecutive days for each participant. The waking level at the first time point immediately upon awakening (S1) was log transformed (K-S value 1.363, p < 0.05) and compared between groups, while raw data were reported in all figures to allow comparison with other studies. The mean cortisol levels of two days over time after awakening (S1, S2, S3, S4) and CAR parameters in exam and control groups are shown in . Both R30 and AUCi were significantly lower in the exam group as compared to the nonexam control group (t = −2.59 and −2.37 respectively, ps < 0.05), while no such differences were found on S1 or AUCg (t = 1.20 and −0.54 respectively, ps > 0.05).
Table 2. Mean cortisol values over two days by group: mean (SE) nmol/L.
Cortisol data over two days from the high-stress exam group and low-stress exam group according to median score of PSS10, as well as the comparison group are shown in . Separate ANOVAs with the between-subjects factor of group demonstrated a significant main effect of group on R30 (F(2,59) = 4.10, p < 0.05, partial η2 = 0.11) and AUCi (F(2,59) = 3.24, p < 0.05, partial η2 = 0.10), but not for S1 and AUCg (F(2,59) = 1.20 and 0.46 respectively, ps > 0.1). Post-hoc analyses on R30 and AUCi showed that the means of the high-stress exam group were significantly lower than the non-exam group (ps < 0.01), but there was no difference between the low-stress exam and nonexam groups (p > 0.1). These data are shown in .
Figure 1. Mean awakening cortisol levels over two days in high-stress exam (n = 24), low-stress exam (n = 18) and nonexam (n = 21) groups. The x-axis represnets time points of saliva sampling, and the y-axis represent averaged raw cortisol levels across two days. Error bars represent the standard error of the mean.
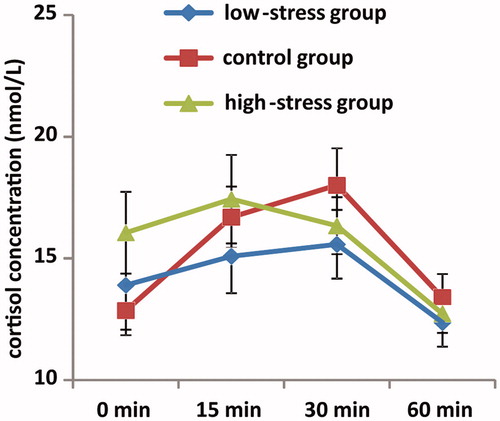
Figure 2. Values of four different CAR measures over two days in the high-stress exam group (n = 24), low-stress exam group (n = 18) and nonexam group (n = 21). The graph shows mean value, with error bars representing the standard error of the mean. *p < 0.05; **p < 0.01.
Previous studies showed that cortisol response changes as examinations get closer in time (Kamezaki et al., Citation2012; Lacey et al., Citation2000), thus we compared the CAR between participants taking part in the experiment of 11–18 days and those of 19–25 days before the examination. There were no significant difference either on psychological stress scores or cortisol concentration (|t|s < 1.65, ps > 0.1). Furthermore, the relationship between the day of cortisol collection prior to the examination and CAR was nonsignificant (|r|s < 0.14, ps > 0.1).
Correlational analysis
For the whole participant sample, bivariate correlations of the variables under study are shown in . Both perceived stress and anxiety levels were negatively related with R30 and AUCi (ps < 0.05). There were no significant relationships between sleep duration or waking time and CAR parameters (|r| = 0.05–0.19, ps > 0.1). Furthermore, perceived stress remained significantly correlated with R30 as well as AUCi while partialling out sleep duration and waking time (partial r = −0.31 and −0.26, respectively, ps < 0.05), and the significant correlations of anxiety level with R30 and AUCi also remained when sleep duration and waking time were controlled (partial r = −0.30 and −0.31, respectively, ps < 0.05). The exam performance did not relate to any parameters of CAR (|r|s < 0.19, ps > 0.1).
Table 3. Bivariate correlations.
Discussion
In the present study, we examined the effects of long-term stress induced by exposure to and preparation for a major examination on awakening cortisol response in healthy young male students. To pass this exam, participants spend an average of 9.6 hours each day for a duration of about 6 months (). We found that self-reported perceived stress and anxiety levels were significantly higher in the exam group. The CAR of the exam group was significantly lower than the non-exam control group, and this effect was most pronounced in participants who reported higher levels of perceived stress. Perceived stress and anxiety were negatively correlated with both the R30 and AUCi for all participants. These results are consistent with our prediction that long-term stress would be associated with a reduced CAR.
To date, several studies have observed a decreased CAR in clinical stress-related disorders including posttraumatic stress disorder (De Kloet et al., Citation2007; Johnson et al., Citation2008; Neylan et al., Citation2005), chronic fatigue syndrome (Nater et al., Citation2008; Roberts et al., Citation2004), and burnout (Kirschbaum et al., Citation1999; Sonnenschein et al., Citation2007). There were also studies that find a decreased CAR in chronically stressed caregivers (Barker et al., Citation2012; Buchanan et al., Citation2004). These studies included a sample of older adults (mean age of 60s) who might have altered endocrine activity compared to a younger group (Kudielka & Kirschbaum, Citation2003; Van Cauter et al., 1996). Similarly, Ranjit et al. (Citation2005) and O’Connor et al. (Citation2009) found that among middle-aged women who had high chronic material stress or job-related stress, the CAR was blunted. Our findings extend the association between chronic stress and decreased CAR into healthy young males who were under chronic examination stress.
The observed reduction of CAR in the present study is in contrast with previous findings from a few studies on exam stressors (Hewig et al., Citation2008; Weekes et al., Citation2008; Weik & Deinzer, Citation2010). Weekes et al. (Citation2008) and Weik & Deinzer (Citation2010) found that the CAR was greater in high exam stress period, whereas participants in Hewig et al. (Citation2008) had no such relationship between the two variables. These three studies included mostly female rather than male participants (as in the current study), and previous studies have shown that females have higher cortisol response to awakening compared to males (Lacey et al., Citation2000; Vreeburg et al., Citation2009). The fact that the higher CAR in Weekes et al. (Citation2008) was only significant in female students rather than males indicates that sex differences in the CAR may explain part of these mixed results. Another important issue to address is the differences in stress intensity as indicated by failure rate among the examinations in previous studies compared to the failure rate of the NPEE in the current investigation. The study by Weik & Deinzer (2010) explored stress in response to an examination in which the failure rate was ∼33%, and this was much lower than the failure rate for the NPEE around 66% (or 33% acceptance rate). Note that participants in our present study spent about 9.6 hours each day for a duration about 6 months in preparation for the exam. The lack of more detailed information about the intensity and duration of exam stressors in these other studies do not allow for a direct comparison of the current results with them, but these characteristics of stressors suggest one possible mechanism to explain this discrepancy in CAR results.
One possible explanation for the decreased CAR in participants under chronic stress may be a relative decrease in HPA axis activity due to the long-term high stress in our study as well as other previous studies. Both Miller et al. (Citation2007) and Fries et al. (Citation2009) proposed that hyperactivity of HPA axis would eventually develop into hypoactivity with the long duration of stress. Evidence from men with vital exhaustion also found that prolonged period of stress leads to the hypoactivity of basal cortisol (Nicolson & van Diest, Citation2000), and the ability of the HPA axis to self-regulate may be compromised resulting in an “exhaustion stage” (Wirth et al., Citation2011). In our study, the impact of long duration and high intensity of exam stress on the CAR supports the above conclusion, i.e., HPA axis is down-regulated by chronic major stress, with this downregulation reflected by a reduction of the CAR.
The decreased CAR in individuals with chronic stress might be interpreted as the cortisol overproduction during sleep before awaking. It is worthwhile to mention that the exam group showed higher waking cortisol levels than the nonexam group as shown in S1 (waking level) in , although this difference was not statistically significant. At the same time, S1 was negatively related to the R30 as well as AUCi. Many studies also observed such an inverse relationship between S1 and CAR (Adam et al., Citation2006; Vreeburg et al., Citation2009; Wilhelm et al., Citation2007). Thus there is a possibility that the exam group had a weak postawakening increase of cortisol as a result of the already high waking level (S1). The higher waking cortisol might reflect altered nocturnal patterns of cortisol secretion rather than changes occuring post-awakening, considering that all participants were required to take the first sample immediately upon waking. Evidence from a sleep laboratory study suggests that the S1 level reflects secretory activity in the HPA axis during the late stages of sleep (Born et al., Citation1999; Hucklebridge et al., Citation2000; Wilhelm et al., Citation2007), which might relate to the function of the hippocampus and suprachiasmatic nucleus (SCN) (Buijs et al., Citation1999; Clow et al., Citation2010; Dallman et al., Citation1992, Citation1994). In other words, the cortisol secretion in late sleep stage was gradually altered during the course of long-term stress exposure, leading to the appearance of high cortisol levels at awakening coupled with an insufficient waking response in the following half hour.
Corticosteroid feedback inhibition provides another perspective for interpretation of our results. The HPA axis is very sensitive to inhibition by corticosteroids when they are administered exogenously. However, prior stress exposure causing marked corticosteroid secretion can have a facilitatory effect on subsequent responses of the HPA axis to stress (for reviews, see Dallman, Citation1993; Dallman et al., Citation1992, Citation1994). Considering the characteristics of our examination stressor, which is intense and persistent, it is likely that the repetitive activation of HPA axis will increase its sensitivity to glucocorticoid negative feedback and thus result in an inhibition of the HPA axis response to stimulation (Fries et al., Citation2005; Heim et al., Citation2000).
Although the exam group woke up earlier and had shorter sleep time than control group, CAR was not related to these two variables. Furthermore, when sleep duration and waking time were controlled, the relationship between perceived stress and CAR was still significant, which suggests that the relation between stress and CAR was not confounded by sleep duration and waking time. This finding was also consistent with a body of literature that suggested CAR be predominantly influenced by stress rather than sleep (Pruessner et al., Citation1997b; Schlotz et al., Citation2004; Wust et al., Citation2000b).
Our research has some limitations that should be mentioned. One of the confounding factors that may affect the CAR is light (Figueiro & Rea, Citation2012). However, all participants’ awakening time was controlled between six and eight o’clock, suggesting that light might have little contribution to our results. Second, regarding generalizability, our study focuses on examination stressor on young male students only. Therefore, it is possible that the effects of chronic psychological stress observed in this study might not be generalisable to a female sample, especially given the higher proportion of women with stress-related illnesses (for a review, see Kudielka & Kirschbaum, Citation2005). Third, non-adherence to sampling timing and procedures affects morning cortisol (Broderick et al., Citation2004; Thorn et al., Citation2006). We monitored adherence by self-reported waking and sampling time only. The gold standard for ensuring adherence to sampling timing and procedures involves the use of electronic monitors (Broderick et al., Citation2004; Jacobs et al., Citation2005), which we did not employ. In spite of this, we used several techniques to assure that our participants’ samples were always collected as close to the instructed time as possible: (1) instructions for compliance were given to all participants in written and oral form; (2) participants were instructed to call the laboratory if they had any questions regarding the testing procedure; and (3) all participants wrote down the times at which their samples were collected. Any deviation from this behavior in terms of timing or lack of report led to exclusion of some or all of that participant’s data. Fourth, the cortisol samples per person was collected only in the morning given the physiological meaning and complexity of the diurnal pattern. Future researches should collect more samples over the day which permit to delineate more sophisticated and dynamic HPA axis activity. Finally, the intensity and duration of our examination stress is relatively categorical (in that students are either in the exam group or nonexam group). However, our high-stress versus low-stress exam subgroups demonstrate that continuum of perceived stress plays a role in influencing the dynamics of the CAR. Future studies may more clearly treat these factors as a continuous variable to help elucidate the effects of intensity and duration of stressors on the CAR.
In conclusion, academic examination is an important stressor for students in China. This kind of stressor results in high psychological and physiological tax on students. Specifically, long-term preparation for a major examination leads to a decrease in the CAR, and this decrease is related to the increased psychological stress. The dampened CAR may result from the decreased HPA aixs activity.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported by the NSF China (91124003,90924017,31100734) and the Excellent Young Scientists Fund, IP, CAS (YICX63S03).
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. (2006). Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci USA 103:17058–63
- Adminutes. Number of candidates for graduate school has increased year by year and admission ratio is about 3:1. Freekaoyan [Internet]. 2012 Jan 13. Available at http://www.freekaoyan.com/news/20120113/1326386061205799.shtml (accessed 3 May 2013)
- Barker ET, Greenberg JS, Seltzer MM, Almeida DM. (2012). Daily stress and cortisol patterns in parents of adult children with a serious mental illness. Health Psychol 31:130–4
- Booij SH, Bouma EM, de Jonge P, Ormel J, Oldehinkel AJ. (2013). Chronicity of depressive problems and the cortisol response to psychosocial stress in adolescents: the TRAILS study. Psychoneuroendocrinology 38:659–66
- Born J, Hansen K, Marshall L, Mölle M, Fehm HL. (1999). Timing the end of nocturnal sleep. Nature 397:29–30
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. (2004). Salivary cortisol sampling compliance: comparison of patients and healthy volunteers. Psychoneuroendocrinology 29:636–50
- Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C. (2004). Circadian regulation of cortisol after hippocampal damage in humans. Biol Psychiatry 56:651–6
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. (1999). Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 11:1535–44
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. (2010). The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev 35:97–103
- Clow A, Thorn L, Evans P, Hucklebridge F. (2004). The awakening cortisol response: methodological issues and significance. Stress 7:29–37
- Cohen S, Williamson G. (1988). Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont symposium on applied social psychology. Newbury ParkCA: Sage Publications, Inc. p 31–67
- Dallman MF. (1993). Stress update: adaptation of the hypothalamic–pituitary–adrenal axis to chronic stress. Trends Endocrinol Metab 4:62–9
- Dallman MF, Akana SF, Bradbury MJ, Strack AM, Hanson ES, Scribner KA. (1994). Regulation of the hypothalamo–pituitary–adrenal axis during stress: feedback, facilitation and feeding. Seminutes Neurosci 6:205–13
- Dallman MF, Akana SF, Scribner KA, Bradbury MJ, Walker CD, Strack AM, Cascio CS. (1992). Stress, feedback and facilitation in the hypothalamo-pituitary-adrenal axis. J Neuroendocrinol 4:517–26
- De Kloet C, Vermetten E, Heijnen C, Geuze E, Lentjes E, Westenberg H. (2007). Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology 32:215–26
- Figueiro MG, Rea MS. (2012). Short-wavelength light enhances cortisol awakening response in sleep-restricted adolescents. Int J Endocrinol 2012:301935–42
- Fries E, Dettenborn L, Kirschbaum C. (2009). The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 72:67–73
- Fries E, Hesse J, Hellhammer J, Hellhammer D. (2005). A new view on hypocortisolism. Psychoneuroendocrinology 30:1010–16
- Gotlib IH, Cane DB. (1989). Self-report assessment of depression and anxiety. In: Kendall PC, Watson D, editors. Anxiety and depression: distinctive and overlapping features. Personality, psychopathology, and psychotherapy. San Diego, CA, US: Academic Press. p 131–69
- Gustafsson PE, Janlert U, Virtanen P, Hammarström A. (2012). The association between long-term accumulation of temporary employment, the cortisol awakening response and circadian cortisol levels. Psychoneuroendocrinology 37:789–800
- Heim C, Ehlert U, Hellhammer DH. (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25:1–35
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. (2007). Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology 32:80–6
- Hewig J, Schlotz W, Gerhards F, Breitenstein C, Lürken A, Naumann E. (2008). Associations of the cortisol awakening response (CAR) with cortical activation asymmetry during the course of an exam stress period. Psychoneuroendocrinology 33:83–91
- Hucklebridge F, Clow A, Rahman H, Evans P. (2000). Cortisol response to normal and nocturnal awakening. J Psychophysiol 14:24–8
- Jacobs N, Nicolson NA, Derom C, Delespaul P, van Os J, Myin-Germeys I. (2005). Electronic monitoring of salivary cortisol sampling compliance in daily life. Life Sci 76:2431–43
- Johnson DM, Delahanty DL, Pinna K. (2008). The cortisol awakening response as a function of PTSD severity and abuse chronicity in sheltered battered women. J Anxiety Disord 22:793–800
- Kamezaki Y, Katsuura S, Kuwano Y, Tanahashi T, Rokutan K. (2012). Circulating cytokine signatures in healthy medical students exposed to academic examination stress. Psychophysiology 49:991–7
- Kirschbaum C, Pruessner JC, Hellhammer DH. (1999). Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med 61:197–204
- Kudielka BM, Kirschbaum C. (2003). Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology 28:35–48
- Kudielka BM, Kirschbaum C. (2005). Sex differences in HPA axis responses to stress: a review. Biol Psychol 69:113–32
- Lacey K, Zaharia MD, Griffiths J, Ravindran AV, Merali Z, Anisman H. (2000). A prospective study of neuroendocrine and immune alterations associated with the stress of an oral academic examination among graduate students. Psychoneuroendocrinology 25:339–56
- Liston C, McEwen BS, Casey BJ. (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA 106:912–17
- Meinlschmidt G, Heim C. (2005). Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology 30:568–76
- Miller GE, Chen E, Zhou ES. (2007). If It goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 133:25–45
- Nater UM, Maloney E, Boneva RS, Gurbaxani BM, Lin JM, Jones JF, Reeves WC, Heim C. (2008). Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. J Clin Endocr Metab 93:703–9
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, Marmar CR. (2005). PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology 30:373–81
- Nicolson NA, van Diest R. (2000). Salivary cortisol patterns in vital exhaustion. J Psychosom Res 49:335–42
- O’Connor DB, Hendrickx H, Dadd T, Elliman TD, Willis TA, Talbot D, Mayes AE, et al. (2009). Cortisol awakening rise in middle-aged women in relation to psychological stress. Psychoneuroendocrinology 34:1486–94
- Pruessner J, Wolf O, Hellhammer D, Buske-Kirschbaum A, Von Auer K, Jobst S, Kaspers F, Kirschbaum C. (1997a). Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61:2539–49
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. (1997b). Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61:2539–49
- Quevedo K, Johnson AE, Loman ML, LaFavor TL, Gunnar M. (2012). The confluence of adverse early experience and puberty on the cortisol awakening response. Int J Behav Dev 36:19–28
- Ranjit N, Young EA, Kaplan GA. (2005). Material hardship alters the diurnal rhythm of salivary cortisol. Int J Epidemiol 34:1138–43
- Roberts AD, Wessely S, Chalder T, Papadopoulos A, Cleare AJ. (2004). Salivary cortisol response to awakening in chronic fatigue syndrome. Br J Psychiatry 184:136–41
- Rohleder N, Beulen SE, Chen E, Wolf JM, Kirschbaum C. (2007). Stress on the dance floor: the cortisol stress response to social-evaluative threat in competitive ballroom dancers. Pers Soc Psychol Bull 33:69–84
- Schlotz W, Hellhammer J, Schulz P, Stone AA. (2004). Perceived work overload and chronic worrying predict weekend–weekday differences in the cortisol awakening response. Psychosom Med 66:207–14
- Schmidt-Reinwald A, Pruessner J, Hellhammer D, Federenko I, Rohleder N, Schürmeyer T, Kirschbaum C. (1999). The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci 64:1653–60
- Siegel B. Stressful times for Chinese Students. Time [Internet]. 12 Jun 2007. Available at http://www.time.com/time/world/article/0,8599,1631854,00.html#ixzz21TdlgTeQ (accessed 3 May 2013)
- Sonnenschein M, Mommersteeg P, Houtveen JH, Sorbi MJ, Schaufeli WB, van Doornen LJ. (2007). Exhaustion and endocrine functioning in clinical burnout: an in-depth study using the experience sampling method. Biol Psychol 75:176–84
- Spielberger CD. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press
- Stalder T, Evans P, Hucklebridge F, Clow A. (2011). Associations between the cortisol awakening response and heart rate variability. Psychoneuroendocrinology 36:454–62
- Steptoe A, Brydon L, Kunz-Ebrecht S. (2005). Changes in financial strain over three years, ambulatory blood pressure, and cortisol responses to awakening. Psychosom Med 67:281–7
- Tennant C, Andrews G. (1976). A scale to measure the stress of life events. Aust N Z J Psychiatry 10:27–32
- Thorn L, Hucklebridge F, Evans P, Clow A. (2006). Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology 31:1009–18
- Tomiyama AJ, Dallman MF, Epel ES. (2011). Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology 36:1513–19
- Van Cauter E, Leproult R, Kupfer DJ. (1996). Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab 81:2468--73
- Vreeburg SA, Kruijtzer BP, van Pelt J, van Dyck R, DeRijk RH, Hoogendijk WJ, Smit JH, et al. (2009). Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology 34:1109–20
- Wang Z, Chen J, Boyd JE, Zhang H, Jia X, Qiu J, Xiao Z. (2011). Psychometric properties of the Chinese version of the Perceived Stress Scale in policewomen. PLoS One 6:e28610
- Weekes NY, Lewis RS, Goto SG, Garrison-Jakel J, Patel F, Lupien S. (2008). The effect of an environmental stressor on gender differences on the awakening cortisol response. Psychoneuroendocrinology 33:766–72
- Weik U, Deinzer R. (2010). Alterations of postawakening cortisol parameters during a prolonged stress period: results of a prospective controlled study. Horm Behav 58:405–9
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. (2007). Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology 32:358–66
- Wirth M, Burch J, Violanti J, Burchfiel C, Fekedulegn D, Andrew M, Zhang H, et al. (2011). Shiftwork duration and the awakening cortisol response among police officers. Chronobiol Int 28:446–57
- Wolfram M, Bellingrath S, Kudielka BM. (2011). The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology 36:905–12
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. (2000a). Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology 25:707–20
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. (2000b). The cortisol awakening response-normal values and confounds. Noise Health 2:79–88
- Yu R, Ho SC. (2010). Psychometric evaluation of the perceived stress scale in early postmenopausal Chinese women. Psychology 1:1–8
- Zhang M, Fan B, Cai G, Chi Y, Wu W, Jin H. (1987). Life event scale: norm result. Chin J Nerv Ment Dis 13:70–3
