Abstract
Both cumulative adversity, an individual’s lifetime exposure to stressors, and insufficient exercise are associated with poor health outcomes. The purpose of this study was to ascertain whether exercise buffers the association of cumulative adverse life events (CALE) with health in a community-wide sample of healthy adults (ages 18–50 years; women: n = 219, 29.5 ± 9.2 years; men: n = 176, 29.4 ± 8.7 years, mean ± standard deviation). Participants underwent the Cumulative Adversity Interview, which divides life events into three subsets: major life events (MLE), recent life events (RLE) and traumatic experiences (TLE). These individuals also completed the Cornell Medical Index and a short assessment for moderate or greater intensity exercise behavior, modified from the Nurses’ Health Study. Results indicated that higher CALE was associated with greater total health problems (r = 0.431, p < 0.001). Interactions between stress and exercise were not apparent for RLE and TLE. However, at low levels of MLE, greater exercise was related to fewer total, physical, cardiovascular and psychological health problems (p value <0.05). Conversely, at high levels of MLE, the benefits of exercise appear to be absent. Three-way interactions were observed between sex, exercise and stress. Increased levels of exercise were related to better physical health in men, at all levels of CALE. Only women who reported both low levels of CALE and high levels of exercise had more favorable physical health outcomes. A similar pattern of results emerged for RLE. Together, these data suggest that increased exercise is related to better health, but these effects may vary by cumulative stress exposure and sex.
Introduction
Stress can be a result of physical or emotional challenges, which may be transient and rather innocuous (e.g. final examinations, exercise), resulting in positive adaptations (McEwen, Citation2007). However, the experience of high, uncontrollable stress may produce excessive wear and tear, resulting in lasting and harmful insults to an individual’s physical and mental health (Chrousos & Gold, Citation1992; McEwen, Citation2007; Thoits, Citation2010). For example, numerous disease states and processes have been associated with high stress, including the pathogenesis of coronary heart disease (Rozanski et al., Citation1999), incidence of acute myocardial infarctions (Rosengren et al., Citation2004) and worse survival from cardiac events (Kivimaki et al., Citation2002). Alterations in the immune and nervous systems by high, uncontrollable stress are well-established (Segerstrom & Miller, Citation2004). Finally, stress is related to musculoskeletal problems and a decline in physical functioning over time (Kjellberg & Wadman, Citation2007). Together, these effects may result from a variety of different stressors, including work stress (Kivimaki et al., 2002), care giving (Lee et al., Citation2003) and childhood adverse experiences (Korkeila et al., Citation2010). Indeed, the accumulation of multiple uncontrollable stressful experiences across the lifetime, or cumulative adversity, may have a particularly pernicious and long-lasting impact on an individual’s physical, psychological and social functioning (Kuh et al., Citation2003; Seo et al., Citation2013). Moreover, repeated stress or cumulative adversity has a stronger association with these deficits than stress exposure identified at any one time point in the life course or with more proximal and isolated experiences of stress (Turrell et al., Citation2007).
In contrast to the deleterious effects of stress or cumulative adversity, the role of physical activity (PA) in the prevention of morbidity and premature mortality is widely recognized (Wei et al., Citation2000). Exercise, a form of PA, is “planned, structured, and repetitive and has as a final or intermediate objective the improvement or maintenance of physical fitness” (Garber et al., Citation2011). Those who exercise have a lower incidence of type II diabetes mellitus and coronary heart disease, among other health outcomes (Stampfer et al., Citation2000). Furthermore, interventions designed to increase exercise have resulted in profound reductions in physical ailments, such as hypertension (Whelton et al., Citation2002). However, there appears to be a minimum level of PA needed to achieve such effects: 150 min of moderate-to-vigorous PA per week has been shown to optimize physical health (Garber et al., 2011). Thus, public health experts have adopted this threshold as the recommended dosage to improve health outcomes, and many interventions are designed to foster exercise at this level of PA (Wadden et al., Citation2006).
While exercise may directly influence health through a variety of mechanisms (e.g. weight management and improved insulin sensitivity, less atherosclerosis), it is possible that exercise may indirectly influence physical health by dampening or buffering the deleterious effects of stress or adversity (Gerber et al., Citation2010, 2013b; Holtermann et al., Citation2010; Sigfusdottir et al., Citation2011). There are several sources of evidence to support the notion of an interaction between cumulative adversity and exercise. First, those who exercise exhibit fewer stress symptoms, such as mood impairment, anxiety, and depression, and moreover, exercise interventions have been effective in the effort to lessen the impact of stress (Gerber et al., 2013a; Milani & Lavie, Citation2009; Norris et al., Citation1992). Second, many biological processes adversely affected by psychosocial stress (e.g. inflammation, cardiovascular functioning) are, on the contrary, benefited by exercise (McEwen, Citation2007). Exercise is associated with attenuated sympathetic and cardiovascular stress reactivity when exposed to stressful stimuli (Forcier et al., Citation2006; Klaperski et al., Citation2013; Rimmele et al., Citation2007, Citation2009). In a randomized controlled trial, Blumenthal et al. (Citation2005) demonstrated that both exercise training and stress management significantly improved flow-mediated dilation of the brachial artery and other clinical outcomes compared to a usual care group. Kobasa et al. (Citation1982) pioneered research investigating the interaction between stress and exercise as those factors affected health and mortality, showing that non-exercisers fared worse than exercisers in the face of stress (Krueger & Chang, Citation2008; Puterman et al., Citation2010). For instance, among older adults, physical health symptoms are highest in those with both little exercise and high perceived stress (Rueggeberg et al., Citation2012).
While this line of research is promising and has great relevance for interventions, some investigations have failed to detect a stress buffering effect of exercise (Gerber & Pühse, Citation2009). Inconsistencies may be explained by sex differences, as women may be more sensitive to stress and thus vulnerable to the adverse effects of stress (Oldehinkel & Bouma, Citation2011; Vitaliano et al., Citation2003). Women also tend to report more stress symptoms (Mirowsky & Ross, Citation1995). Recent evidence suggests that women are less protected by exercise from stress-associated declines in physical functioning (Unger et al., Citation1997) and upper respiratory tract infections at high levels of stress (Fondell et al., Citation2011). However, a recent review suggests that gender differences are equivocal, with some studies reporting a protective effect of exercise for women and others for men (Gerber & Pühse, Citation2009). This same review notes, however, that many studies do not analyze data with sex as a covariate. Indeed, gender has been missing from analyses exploring health and cumulative adversity (Ansell et al., 2012b; Seo et al., 2013). Issues relating to sex aside, another potential confound relates to the measurement of stress, with most researchers utilizing short-term measures of self-reported subjective stress over the previous few weeks (Gerber et al., 2010; Rueggeberg et al., 2012). Notably, few studies have investigated whether the effects of cumulative adversity, which has a strong inverse relationship with physical health, may be mitigated by exercise.
The purpose of the present study was to investigate whether exercise buffers the effect of cumulative adversity on physical health in a community sample of non-clinical men and women. We hypothesized that cumulative adverse life events (CALE) would be positively associated with self-reported physical health problems (Seo et al., 2013). As this stress measure contains an indicator of major life events (MLE), which alone have been widely associated with health issues (Kruk, Citation2012), this factor was also proposed to relate to health problems. The second general hypothesis was that exercise would buffer the effects of CALE and MLE on health problems (Gerber & Pühse, Citation2009). Hence, it was expected that at high levels of stress, higher levels of exercise would be related to fewer self-reported health problems. Finally, given that women are more vulnerable to the effects of stress (Oldehinkel & Bouma, 2011; Unger et al., 1997; Vitaliano et al., 2003), it was anticipated that there would be a sex difference between men and women with women, but not men, demonstrating the buffering effect of exercise.
Methods
Recruitment and sample characteristics
Male and female participants were recruited from the community in the greater New Haven, CT area via advertisements placed online or in local newspapers and community centers. Potential participants completed an initial screening over the telephone to determine eligibility based on inclusion/exclusion criteria. All participants were required to be between the ages of 18 and 50 years and able to read and write in English at a 6th grade level or above. Exclusion criteria included substance dependence other than nicotine or use of prescribed medications or other treatments to control any chronic medical conditions (e.g. hypertension, diabetes, hypothyroidism). Those with current psychiatric illness (e.g. bipolar disorder, major depressive disorder, eating disorders) were also excluded because those presently experiencing mental illness may have altered motivation for healthy behavior change and additional barriers in place for exercise. Individuals who completed exercise behavior assessments (n = 395) are included in this sample. They received monetary compensation for their time invested in study participation at the rate of $20/h. There were no collegiate or professional athletes in the sample. See participant characteristics in .
Table 1. Participant characteristics for demographics, exercise, health, adverse life events and lifetime incidence of psychological disorders.
Procedure
Following initial eligibility screening, participants were scheduled for an evaluation session (within 1–2 weeks) to verify eligibility status and to assess cumulative adversity, health and exercise behavior. A specialist research nurse confirmed all physical health exclusion criteria, and participants underwent breath alcohol and urine toxicology screens in order to confirm freedom from alcohol and illicit drugs. The Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Axis 1, Fourth Edition, Research Version (SCID) was completed to verify that all participants were free from current psychiatric (including substance use) disorders and to quantify lifetime presence of these conditions (First et al., Citation1996). Individuals not meeting eligibility criteria at this time point were excluded. Participants filled out paper/pencil surveys of health and behavior and were given a structured interview to determine cumulative adversity. All participants gave written informed consent and the study was approved by the Human Investigation Committee of Yale University (HIC Protocol No. 0710003159, initial approval date 18 December 2007, last renewal date 14 December 2012). All research was conducted in accordance with the Declaration of Helsinki.
Measures
Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Axis 1, Fourth Edition, Research Version (SCID)
The SCID was utilized to determine current and lifetime psychiatric disorders (First et al., 1996). This is a widely used and well-validated clinical interview based on the DSM-IV (Williams et al., Citation1992). The interview was administered by clinicians with experience in performing structured diagnostic evaluations, trained mental health professionals, or full-time post-baccalaureate research assistants with extensive experience administering the SCID with this community population.
Cumulative Adversity Interview (CAI)
The CAI, adapted from Turner & Wheaton (Citation1995) is a comprehensive measure of cumulative adversity. It assesses the extent to which individuals have been exposed to major and recent stressful life events and life trauma. The CAI is highly predictive of psychiatric disorders over the lifetime (Turner & Lloyd, Citation2004). The measure was administered in a structured interview format by trained interviewers who met the same qualifications as described above. The interview consists of a checklist of discrete stressful events, with response options of either “yes” (have experienced the event) or “no” (have not experienced the event). Prior research supports the use of interviewers to efficiently improve reliability and validity of retrospective reports of stressful life events (Dohrenwend, Citation2006).
Subscales of interest included (i) MLE, (ii) RLE and (iii) Life Traumas (TLE). The 11-item MLE subscale includes 11 items relating to social adversities, not typically violent in nature, but which differ from standard life events due to their severity and potentially long-term consequences (Turner & Lloyd, Citation2003). Examples of items are parental divorce, failing a grade in school and losing a child. The 33-item RLE subscale assesses stressful events which occurred in the previous 12 months. Events are broadly divided into items referring to exits from the social field (e.g. death, divorce, relationships ending), undesirable interpersonal and financial events (e.g. being attacked, financial crises, robberies) and work-related experiences (e.g. being laid off, demotion, going on strike). The TLE subscale consists of 34 items relating to traumatic events, witnessed violence and traumatic news. Such events may include physical, emotional and/or sexual abuse, such as rape or being injured with a weapon. Witnessed violence may involve being present in dangerous or upsetting situations, such as seeing someone get shot or attacked with any weapon. Traumatic news is comprised of items that do not involve being present, but involve hearing news about someone else being killed, abused or injured.
Using responses to the three subscales of interest, a CALE score was computed by summing the subscale scores. In all cases, a higher score relates to a higher number of stressful events. Reliability of the CAI subscales and CALE is good (Ansell et al., 2012a,b; Seo et al., 2013). Intra-class correlations (ICCs) for MLE, RLE, TLE and CALE were 0.91, 0.83, 0.94 and 0.92, respectively. In the current sample, test–retest ICCs over 3 months with 273 participants were 0.90 (MLE), 0.70 (RLE), 0.86 (TLE) and 0.86 (CALE). Among 121 participants interviewed by a different rater each time, these were: 0.91, 0.67, 0.86 and 0.88. These were similar to ICCs reported (0.79–0.91) in a previous study (Ansell et al., 2012b), and exceed values reported for self-administered life event checklists (Dohrenwend, Citation2006). Pearson product correlations for (i) 121 participants interviewed by a different rater and (ii) 152 participants interviewed by the same rater at both times were moderate to high for MLE (r = 0.85, 0.78), RLE (r = 0.65, 0.54), TLE (r = 0.80, 0.79) and CALE (r = 0.82, 0.79).
Cornell Medical Index (CMI)
The CMI is a widely used instrument which queries about past and current health problems (Brodman et al., Citation1949). It has 195 items and is grouped into 18 subscales (e.g. respiratory, cardiovascular, musculoskeletal, psychological). The first 12 subscales (138 items), considered physical in nature, were assessed as a composite separately from psychological subscales. Furthermore, 2 of these 12 subscales (cardiovascular, 13 items; musculoskeletal, 8 items), were also separately assessed as an outcome variable because of enduring work demonstrating a strong link between stress, cardiovascular disease and muscle function (Kjellberg & Wadman, 2007; Korkeila et al., 2010; Stults-Kolehmainen & Bartholomew, Citation2012). Example items from these subscales include: “Has a doctor ever said your blood pressure was too high?”, “Are you often bothered by a thumping of the heart?”, “Do pains in the back make it hard for you to keep up with your work?” and “Do your muscles and joints constantly feel stiff?” The composite psychological scale was also utilized (57 items). Therefore, there was a total of five outcome variables of interest from the CMI (total, physical, cardiovascular, musculoskeletal and psychological). The CMI is highly associated with actual health status, predicting 89–99% of specific medical conditions (Pendleton et al., Citation2004).
Exercise assessment
Minutes of moderate-to-vigorous exercise (leisure activity above three metabolic equivalents) were assessed with items modified from the Nurses’ Health Study (Wolf et al., Citation1994). In line with recommendations from Sallis (Citation2009), participants completed two items inquiring about average frequency and duration of moderate (or greater) exercise. For frequency of moderate-to-vigorous exercise, participants were asked “How often do you typically exercise?” Participants were referred to a list of exercises with intensities in the moderate-to-vigorous range from the Nurses’ Health Study (Wolf et al., 1994). Response options were: never, monthly or less, 2–3 times/month, once/week, 2–3 times/week, and ≥4 times/week. If respondents selected the last category, they were instructed to provide a specific value. Other categories were transformed into average days of exercise per week. For average exercise duration per session, they were asked, “How long do you typically exercise?” Responses for this question included: not applicable, <15 min, 15–29 min, 30–44 min, 45–59 min, ≥60 min. These were transformed into average minutes per session, except for the last category in which respondents were asked to provide a specific value. Participants were instructed to rate their duration based on actual exercise time and not time spent preparing for exercise (e.g. time changing into exercise apparel), in transport to exercise facilities or socializing in an exercise setting. The two items were multiplied to calculate a continuous variable of minutes per week of moderate or greater intensity exercise. In a separate sample of 97 participants from a similar population, the correlation between these values and the Godin Leisure-Time Exercise Questionnaire was moderate to strong (r = 0.78) (Godin et al., Citation1986). To validate the current measure against an objective measure of PA, a similar sample of 10 adults was objectively monitored with an Omron HJ-720 Dual-Axis Pedometer for a 1-month period (Silcott et al., Citation2011). Mean daily steps calculated from this instrument were moderately correlated with values derived from the current exercise instrument (r = 0.68). Test–retest reliability of the composite score of exercise minutes was good (r = 0.82) over this same time period.
Statistical analysis
Simple Pearson product moment correlations were conducted to determine possible threats from multicollinearity and to test the association of exercise with CMI outcomes and with CALE. Health outcome variables were examined for normality with Kolmogorov–Smirnov tests, which determined that these outcomes were not normal, and thus were log-transformed. In order to test the hypotheses that (i) adversity is associated with health and (ii) exercise buffers the adversity–health relationship, linear multiple regression analyses were conducted. Adversity measures (CALE and MLE) were the predictors, and health indicators (CMI total, physical, cardiovascular, musculoskeletal and psychological) were the outcomes with average exercise minutes per week as the moderator of the relationship. Because sex may influence these relationships (Gerber & Pühse, Citation2009), three-way interaction terms were created for these models (stress ×exercise × sex). Exercise, sex and stress were entered into Step 1, two-way interactions were entered into Step 2 and the three-way interaction was entered into Step 3 of the regression models. Because the statistical models included interactions of exercise and stress, the main effect of stress was statistically assessed holding the mean of exercise constant at 0. Likewise, the main effect of exercise was assessed controlling for a standardized score of stress. Non-significant interactions were trimmed from the final models, except when three-way interaction terms were significant. Stress scores and the exercise continuous measure were standardized and sex was centered on 0 to facilitate interpretation (Aiken & West, Citation1991). To test hypothesis 1, models were examined for main effects of stress in Step 1 of the model. Each model was then adjusted for eight covariates: age, ethnicity (Caucasian, non-Caucasian), body mass index (BMI), years of education, marital status (married, not married), cigarettes smoked per day, household income and employment status (employed, not employed) (Ansell et al., 2012a). Non-significant covariates were trimmed from the models. p Values were considered significant at the 0.05 level. Data are expressed as means (±standard deviation, SD).
Results
The majority of the sample reported some exercise at a moderate or greater intensity (91.6%), but 33 participants reported none at all; 102 reported an average of 1–59 min, 99 reported 60–149 min, 100 reported 150–219 min and 61 reported ≥220 min. Three subjects were eliminated for extreme scores (>3 SD from the group mean). Consequently, 40.8% reported ≥150 min of moderate or greater intensity aerobic exercise per week. Incidence of lifetime psychiatric disorders was very low. A small proportion of the sample reported an anxiety disorder over their entire lifetime (15.8%). Only five participants (three women, two men) reported an eating disorder in their past history. See for participant characteristics.
The first step in the analysis was to determine whether exercise was linearly related with stress (). Exercise was not correlated with CALE, r(395) = −0.076, p > 0.10, or with MLE, r(395) = −0.096, p = 0.057. However, CMI (total) was correlated with CALE, r(388) = 0.431, p < 0.001, and MLE, r(388) = 0.331, p < 0.001. CMI (physical) was correlated with CALE, r(388) = 0.402, p < 0.001 and MLE, r(388) = 0.289, p < 0.001. A similar pattern of results was observed for RLE and TLE ().
Table 2. Correlations between exercise, BMI, adverse life events and health problems.
Separate regression models relating different dimensions of health to stress (either the MLE or CALE score), exercise, and their interaction, revealed main effects of each stress variable ( and and Supplementary Tables 1 and 2). MLE was associated with total, t(387) = 6.21, p < 0.001, physical, t(387) = 5.49, p < 0.001, cardiovascular, t(387) = 2.10, p = 0.037 and musculoskeletal problems, t(387) = 2.61, p = 0.010, in addition to psychological health problems, t(387) = 5.21, p < 0.001 (). CALE was associated with total, t(387) = 7.70, p < 0.001, physical, t(387) = 7.49, p < 0.001, cardiovascular, t(387) = 3.42, p = 0.001, musculoskeletal problems, t(387) = 4.04, p < 0.001, and psychological health problems, t(387) = 6.63, p < 0.001 (). RLE and TLE also related to every health variable (p values all <0.01). In each case, higher stress levels were related to more health problems. When models were adjusted for covariates, MLE failed to associate with cardiovascular and musculoskeletal problems and TLE was not associated with cardiovascular problems (p > 0.10), but all other associations were similar (p < 0.01).
Table 3. Moderation of the MLE-health problems (complaints) relationship by exercise and gendera.
Table 4. Moderation of the CALE-health problems (complaints) relationship by exercise and gendera.
Next, in the same regression models, we consistently detected a main effect of exercise when participants were statistically equated at average levels of stress. In each model, higher levels of exercise were associated with less total (MLE model, p = 0.025, CALE model, p = 0.017), physical (MLE model, p = 0.009, CALE model, p = 0.003), cardiovascular (both models, p = 0.005) and musculoskeletal (p = 0.038 for both models) health problems, but not psychological problems (p > 0.10 for both models). When MLE and CALE models were adjusted for covariates, exercise was not associated with cardiovascular problems (MLE model, p = 0.089, CALE model, p = 0.055) and not with musculoskeletal or psychological problems (p > 0.10 for both models). Exercise was associated with total and physical health problems in the remaining adjusted models ( and ).
With regards to interactions between exercise and stress, minutes of moderate-to-vigorous exercise per week moderated all MLE-health problem relationships (p < 0.05) except in the case of musculoskeletal health problems (). Exercise did not moderate CALE, RLE and TLE and health relationships (p > 0.05). At low levels of MLE stress, higher levels of exercise were associated with fewer total health problems. This finding was supported when the analysis was rerun with MLE recentered at 1 SD below the mean; the main effect of exercise was significant, t(387) = −3.30, p = 0.001. At high levels of stress, visual inspection of results suggested that exercise has little or no effect on physical health. When the analysis was rerun with MLE recentered at 1 SD above the mean, exercise did not have a main effect, t(387) = 0.271, p > 0.10, indicating that at high levels of stress, exercise at any level is not protective for total health problems.
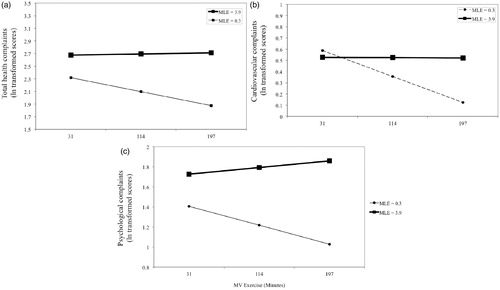
Figure 1. Moderation by moderate or vigorous exercise (MV exercise) of the MLE and self-reported health relationships for (a) total, (b) cardiovascular and (c) psychological problems as measured by the Cornell Medical Index. Higher levels of stress (MLE score) were associated with poorer health for both total and psychological problems in both adjusted and non-adjusted models (p < 0.001). Stress was associated with cardiovascular health in non-adjusted models (p = 0.037) but there was no association in adjusted models (p = 0.083). Interactions between exercise and stress emerged such that at increasing levels of exercise this linear effect became more marked (p values = 0.017, 0.013 and 0.037, respectively). Associations were tested by hierarchical multiple linear regression (n = 395). Stress is shown at 1 SD below and above the mean MLE score. MV exercise is shown at the mean (114 min) and 1 SD below and above the mean (31 and 197 min). For cardiovascular complaints, stress was not associated with exercise at 31 and 114 min (p > 0.05). Values for health outcomes are log transformed.
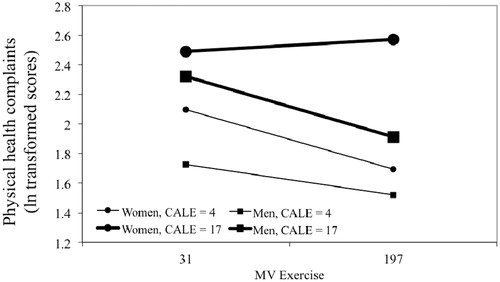
Three-way interactions (gender × stress × exercise) were not significant for any MLE or TLE model. However, CALE models examining physical health complaints revealed significant three-way interactions, even when adjusted for significant covariates (e.g. age, BMI, ethnicity) (unadjusted model, p = 0.029; adjusted model, p = 0.026) (). A visual examination of trends revealed that for men, exercising at a high level (1 SD above the mean) was associated with fewer physical health problems, and the benefits of exercise on health emerged at low or high levels of stress. However, this was not true for women. Sedentary or low-activity women at any level of stress, or active women at high levels of stress, reported increased physical health problems; only low-stress, active women reported good physical health. Two-way interactions in these models, however, were not significant and thus are not interpretable. A similar pattern was observed for RLE (p = 0.045) but this failed to reach significance when adjusted for covariates (p = 0.088). Multicollinearity was not detected as a potential problem in any analysis, as determined by tolerance coefficients (TC) and variance inflation factors (VIF). The final models are in and .
Figure 2. Moderation by moderate or vigorous exercise (MV exercise) of relationships between CALE score with self-reported physical health as measured by the Cornell Medical Index. The three-way interaction between stress, exercise and sex was significant in both non-adjusted and adjusted models (p values = 0.029 and 0.026, respectively). Associations were tested by hierarchical multiple linear regression (n = 381). All slopes were significantly different from 0 (p < 0.05), except the slope for women at high stress (simple slopes tests). This same slope was different from all other slopes, which did not differ from each other (slopes differences tests). Stress (CALE score) is shown at 1 SD below and above the mean as is MV exercise (31 and 197 min). Values for health problems are log transformed.
Discussion
The purpose of this study was to determine whether exercise behavior modulates the relationship between cumulative stress and self-reported health problems in a community sample of adults free from current physical or mental health disorders. First, it was hypothesized that CALE and MLE would be associated with self-reported health problems (i.e. total, physical, cardiovascular, musculoskeletal and psychological problems). Second, a buffering effect of exercise was hypothesized, such that exercise would decrease the negative effects of CALE on health problems. Hence, it was predicted that at high levels of stress, greater exercise would be associated with fewer health problems. Third and finally, it was predicted that gender would further interact with the effects of exercise and stress on health problems. The present results yielded support for the first hypothesis as those individuals reporting high levels of stress, assessed via CALE or MLE, reported more health problems in multiple domains.
Exercise influenced the relationships between MLE and health, but not as anticipated in our second hypothesis. Across the sample, at low levels of stress, higher levels of exercise were related to fewer health problems. This was found even when statistical models were adjusted for significant covariates, such as age, smoking, and education level. Nevertheless, at high levels of stress (MLE), exercise provided no protection. Therefore, moderate-to-vigorous exercise does not appear to buffer the association between cumulative adversity and health complaints. Notably, in within-sex analyses, a similar pattern of moderation emerged for women: increased exercise was related to better health for low-stress women, but not high-stress women. In contrast, for men, an additive benefit of exercise emerged such that increased levels of exercise were associated with fewer physical health problems at any given level of stress. Together, these results indicate that exercise moderates the relationship between stress and health outcomes. Exercise improves health for low-stress individuals, but it may also protect health outcomes in the face of high cumulative stress for some individuals (e.g. men). Finally, it should be noted that other models associating health problems with CALE generally did not find interactions between stress and exercise.
These data support several other studies which associate lifetime adverse event experiences to poor health outcomes (Korkeila et al., 2010; Stein et al., Citation2010) and psychiatric disorders, including depression (Turner & Lloyd, 2004). Cumulative adversity is also related to a lifetime of wear and tear, or allostatic load (Evans et al., Citation2007). Notwithstanding this previous body of work, few studies relate indices of CALE with health problems in a community-dwelling adult population free from major physical health problems (Thoits, Citation2010). Consequently, these data provide a novel contribution to this area of investigation.
Despite the pernicious effects of a lifetime of adversity on health, these data indicate that exercise behavior modulates the association. Interestingly, at high levels of stress, exercise may fail to protect health. At low levels of adversity, however, exercise appears to have stronger influence on health outcomes, with greater volume of exercise behavior being related to lower levels of health problems. This finding is contrary to the several studies which have found that exercise had little impact on health symptoms at low levels of stress (Fondell et al., 2011; Rueggeberg et al., 2012). Instead, exercise only made a difference when an individual was high in perceived stress. Certainly this difference may be related to the fact that cumulative adversity and perceived stress, while moderately associated, are distinctly different conceptualizations of stress. Nevertheless, Stults-Kolehmainen and Bartholomew (Citation2012) found that both objective and subjective measures of mental stress predicted trajectories of physical recovery from exercise, with higher levels of stress associated with worse recovery. This study suggests that the combination of strenuous exercise and high levels of stress may result in a maladaptive state of physiological overload. A prolonged state of overload is certainly detrimental to health (McEwen, Citation2007), which reveals an important limitation of exercise – too much is as bad as too little. Such a phenomenon may also explain differences between the current data and aforementioned studies, although this suggestion is purely speculative.
Differences may also be due to variations in study design (prospective versus cross-sectional) and/or the population studied. The current study was delimited to a group of individuals (ages 18–50 years) with no current diagnosis or treatment for physical or mental health disorders. This likely constrained variability in measures of adverse events and health complaints, and results may not replicate in these populations. Additionally, over 40% of the sample reported 150 min or more of exercise at a moderate or greater intensity. Therefore, this group could be characterized as a relatively high functioning and resilient group of individuals. For older and less healthy populations, which generally have low levels of leisure-time PA, the relationship between stress and health may be stronger, but differing interactions between stress and exercise are additionally plausible (Rueggeberg et al., 2012).
Exercise also moderated the association between adverse life events and psychological health problems. Such findings support prospective work, which demonstrates that engaging in exercise helps to protect an individual from the deleterious effects of stress on depression (Toker & Biron, Citation2012). In contrast, regressions examining musculoskeletal health outcomes did not detect interactive effects of stress and exercise behavior. This is unexpected as cumulative stress and MLE are related to disrupted cortisol production, functional impairments, and lower back pain among other maladies (Seery et al., Citation2010).Footnote1
In the investigation of physical health, an interactive effect was detected between sex, exercise, and either MLE and CALE indices of stress. For women, the interaction between exercise and stress emerged as significant, such that higher exercise volume was related to better health at low levels of stress but the benefits of exercise became weaker at higher levels of stress (e.g. poor health outcomes regardless of exercise volume). In contrast, for men, higher exercise volume was related to fewer health problems at any level of stress. Several factors may explain this difference, such as sex differences in coping style and personality (Kjelsas & Augestad, Citation2004). Recent evidence suggests that women are more stress sensitive (Oldehinkel & Bouma, 2011). Indeed, women in our sample reported more adverse life events, more MLE and more RLE than men did. Thus, the interplay of sex differences in stress sensitivity and subjective report of stress may influence the present pattern of results. It is unlikely that dysregulated eating and excessive exercise, maladaptive coping mechanisms, explained this pattern of results. Men reported higher levels of exercise than women, there was no significant gender difference in BMI, and individuals with current psychiatric problems, such as disordered eating, were eliminated from this analysis. Future research may further clarify these results by incorporating assessments of individual differences in subjective or physiological sensitivity to stress.
Research in this area could be expanded in several respects. First, future longitudinal research may build on the present findings and provide insight into the sequence of stress and health effects. Milani and Lavie (Citation2009) demonstrated that among a large group in cardiac rehabilitation, those with high stress with large increases in exercise behavior had lower mortality than those who did not change this healthy behavior or changed it very little (0 versus 19%). The current study design and analytic approach explored associations between exercise, stress, and health from a cross-sectional perspective (Gerber & Pühse, Citation2009; Norris et al., 1992), which identified associations but not causal effects. For example, it could be proposed that an individual’s health status actually has causal effects on cumulative adversity and leisure-time PA: the combination of high stress and a great number of cardiovascular problems could impede effort to be physically active. Such a model seems feasible given longitudinal evidence of an effect of stress on PA along with findings that numerous factors moderate this relationship (Lutz et al., Citation2010). A study with a longitudinal design would better explain the direction and sequence of effects of stress, exercise and health.
In addition, the measurement of exercise behavior is an important limitation in this area of research, and improving such measurement is an important target for future research. In the present study, we utilized an exercise measure that contained a limited number of items used to quantify average minutes per week of exercise at a moderate (or greater) intensity. This measure is consistent with previous research (Shephard, Citation2003), enabling us to compare our results with other recent studies. Similar instruments are also utilized in medical practice to determine whether patients meet a threshold of health-enhancing PA (Sallis, Citation2009). However, the assessment of participant engagement in neuromotor exercises and resistance training, modes of activity recommended for optimal musculoskeletal health, was lacking (Garber et al., 2011). In addition, relying exclusively on subjective reports of exercise provides limited information (Shephard, Citation2003), so future research would be enhanced by the use of objective measures of exercise and leisure-time PA, such as accelerometry and the use of energy expenditure instead of exercise duration. Melding this information with fitness data would also be appropriate, as this factor is more strongly related to health than exercise and has stress-buffering properties in its own respect (Carmack et al., Citation1999). In a similar vein, measuring objective markers of health (e.g. blood pressure, C-reactive protein, uric acid, cholesterol levels) would greatly enhance the understanding of these relationships. For instance, Emeny et al. (Citation2012) recently found that leisure-time PA was a mediator of the relationship between stress and C-reactive protein, an indicator of chronic inflammation that has been linked to heart disease.
In conclusion, the present data indicate that CALE stress, as measured in a structured interview format, is associated with greater physical and psychological health problems. These data also suggest that aerobic exercise volume (average minutes per week) moderates these relationships. In a population of healthy adults with a low incidence of lifetime psychiatric disorders, a combination of low stress and high exercise was associated with fewer health problems. At higher levels of cumulative stress, however, exercise may fail to yield health benefits. When examining the relationships of stress and physical health problems, sex also interacted with the effects of stress and exercise: at all levels of stress, greater exercise was associated with less health problems for men, but for women exercise was only related to better health at low levels of stress. Future research should determine the mechanisms responsible for these protective or absence of protective effects.
Declaration of interest
The authors report no conflicts of interest.
This research was supported by the National Institute of Health Grants (AA013892, UL1-DE019586 and PL1-DA024859).
Supplementary Material
Download PDF (50.2 KB)Acknowledgements
The authors wish to thank Roselinde Kaiser Henderson for her assistance in the production of this article.
Notes
1An earlier exploratory analysis with this same dataset, in which exercise was coded as a dichotomous variable (attaining or not attaining at least 150 min of moderate or greater intensity exercise per week) revealed a stress by exercise interaction for major life events (p = 0.029) but not CALE.
References
- Aiken LS, West SG. (1991). Multiple regression: testing and interpreting interactions. Newbury Park, CA: Sage
- Ansell EB, Gu PH, Tuit K, Sinha R. (2012a). Effects of cumulative stress and impulsivity on smoking status. Hum Psychopharmacol 27(2):200–8
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. (2012b). Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry 72(1):57–64
- Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Waugh R, Georgiades A, Bacon SL, et al. (2005). Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease – a randomized controlled trial. JAMA 293(13):1626–34
- Brodman K, Erdmann AJ, Lorge I, Wolff HG. (1949). The Cornell Medical Index – an adjunct to medical interview. JAMA 140(6):530–4
- Carmack CL, Boudreaux E, Amaral-Melendez M, Brantley PJ, de Moor C. (1999). Aerobic fitness and leisure physical activity as moderators of the stress-illness relation. Ann Behav Med 21(3):251–7
- Chrousos G, Gold PW. (1992). The concepts of stress and stress system disorder: overview of physical and behavioral homeostasis. JAMA 267:1244–52
- Dohrenwend BP. (2006). Inventorying stressful life events as risk factors for psychopathology: toward resolution of the problem of intracategory variability. Psychol Bull 132(3):477–95
- Emeny RT, Lacruz ME, Baumert J, Zierer A, von Eisenhart RA, Autenrieth CS, Herder C, et al. (2012). Job strain associated CRP is mediated by leisure time physical activity: results from the MONICA/KORA study. Brain Behav Immun 26:1077–84
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. (2007). Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Dev Psychol 43(2):341–51
- First MB, Spitzer RL, Miriam G, Williams JBW. (1996). Structured clinical interview for DSM-IV Axis I Disorders, Clinical Version (SCID-CV). Washington, DC: American Psychiatric Press, Inc
- Fondell E, Lagerros YT, Sundberg CJ, Lekander M, Balter O, Rothman KJ, Balter K. (2011). Physical activity, stress, and self-reported upper respiratory tract infection. Med Sci Sports Exerc 43(2):272–9
- Forcier K, Stroud LR, Papandonatos GD. (2006). Links between physical fitness and cardiovascular reactivity and recovery to psychological stressors: a meta-analysis. Health Psychol 25(6):723–39
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, et al. (2011). Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43(7):1334–59
- Gerber M, Brand S, Elliot C, Holsboer-Trachsler E, Pühse U, Beck J. (2013a). Aerobic exercise training and burnout: a pilot study with male participants suffering from burnout. BMC Res Notes 6:78
- Gerber M, Kellmann M, Hartmann T, Pühse U. (2010). Do exercise and fitness buffer against stress among Swiss police and emergency response service officers? Psychol Sport Exerc 11(4):286–94
- Gerber M, Lindwall M, Lindgård A, Börjesson M, Jonsdottir IH. (2013b). Cardiovascular fitness protects from stress-related symptoms of burnout and depression. Patient Educ Couns 93(1):146–52
- Gerber M, Pühse U. (2009). Do exercise and fitness protect against stress-induced health complaints? A review of the literature. Scand J Public Health 37(8):801–19
- Godin G, Jobin J, Bouillon J. (1986). Assessment of leisure-time exercise behavior by self-report: a concurrent validity study. Can J Public Health 77(5):359–62
- Holtermann A, Mortensen OS, Burr H, Sogaard K, Gyntelberg F, Suadicani P. (2010). Physical demands at work, physical fitness, and 30-year ischaemic heart disease and all-cause mortality in the Copenhagen Male Study. Scand J Work Environ Health 36(5):357–65
- Kivimaki M, Leino-Arjas P, Luukkonen R, Riihimaki H, Vahtera J, Kirjonen J. (2002). Work stress and risk of cardiovascular mortality: prospective cohort study of industrial employees. Br Med J 325(7369):857–60
- Kjellberg A, Wadman C. (2007). The role of the affective stress response as a mediator of the effect of psychosocial risk factors on musculoskeletal complaints – part 1: assembly workers. Int J Ind Ergonomics 37(4):367–74
- Kjelsas E, Augestad LB. (2004). Gender, eating behavior, and personality characteristics in physically active students. Scand J Med Sci Sports 14(4):258–68
- Klaperski S, von Dawans B, Heinrichs M, Fuchs R. (2013). Does the level of physical exercise affect physiological and psychological responses to psychosocial stress in women? Psychol Sport Exerc 14(2):266–74
- Kobasa SC, Maddi SR, Puccetti MC. (1982). Personality and exercise as buffers in the stress-illness relationship. J Behav Med 5(4):391–404
- Korkeila J, Vahtera J, Korkeila K, Kivimaki M, Sumanen M, Koskenvuo K, Koskenvuo M. (2010). Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart 96(4):298–303
- Krueger PM, Chang VW. (2008). Being poor and coping with stress: health behaviors and the risk of death. Am J Public Health 98(5):889–96
- Kruk J. (2012). Self-reported psychological stress and the risk of breast cancer: a case-control study. Stress 15(2):162–71
- Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. (2003). Life course epidemiology. J Epidemiol Community Health 57(10):778–83
- Lee S, Colditz GA, Berkman LF, Kawachi I. (2003). Caregiving and risk of coronary heart disease in US women – a prospective study. Am J Prev Med 24(2):113–19
- Lutz RS, Stults-Kolehmainen MA, Bartholomew JB. (2010). Exercise caution when stressed: stages of change and the stress-exercise participation relationship. Psychol Sport Exerc 11(6):560–7
- McEwen BS. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87(3):873–904
- Milani RV, Lavie CJ. (2009). Reducing psychosocial stress: a novel mechanism of improving survival from exercise training. Am J Med 122(10):931–8
- Mirowsky J, Ross CE. (1995). Sex differences in distress – real or artifact? Am Sociol Rev 60(3):449–68
- Norris R, Carroll D, Cochrane R. (1992). The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. J Psychosom Res 36(1):55–65
- Oldehinkel AJ, Bouma EMC. (2011). Sensitivity to the depressogenic effect of stress and HPA-axis reactivity in adolescence: a review of gender differences. Neurosci Biobehav Rev 35(8):1757–70
- Pendleton N, Clague JE, Horan MA, Rabbitt PMA, Jones M, Coward R, Lowe C, McInnes L. (2004). Concordance of Cornell Medical Index self-reports to structured clinical assessment for the identification of physical health status. Arch Gerontol Geriatr 38(3):261–9
- Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E. (2010). The power of exercise: buffering the effect of chronic stress on telomere length. PLoS ONE 5(5):e10837
- Rimmele U, Seiler R, Marti B, Wirtz PH, Ehlert U, Heinrichs M. (2009). The level of physical activity affects adrenal and cardiovascular reactivity to psychosocial stress. Psychoneuroendocrinology 34(2):190–8
- Rimmele U, Zellweger BC, Marti B, Seiler R, Mohiyeddini C, Ehlert U, Heinrichs M. (2007). Trained men show lower cortisol, heart rate and psychological responses to psychosocial stress compared with untrained men. Psychoneuroendocrinology 32(6):627–35
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, et al. (2004). Association of psychosocial risk factors with risk of acute myocardial infarction in 11,119 cases and 13,648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 364(9438):953–62
- Rozanski A, Blumenthal JA, Kaplan J. (1999). Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 99(16):2192–217
- Rueggeberg R, Wrosch C, Miller GE. (2012). The different roles of perceived stress in the association between older adults' physical activity and physical health. Health Psychol 31(2):164–71
- Sallis RE. (2009). Exercise is medicine and physicians need to prescribe it! Br J Sports Med 43(1):3–4
- Seery MD, Leo RJ, Holman EA, Silver RC. (2010). Lifetime exposure to adversity predicts functional impairment and healthcare utilization among individuals with chronic back pain. Pain 150(3):507–15
- Segerstrom SC, Miller GE. (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 130(4):601–30
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R. (2013). Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology. [Epub ahead of print]. doi: 10.1038/npp.2013.250
- Shephard RJ. (2003). Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med 37(3):197–206
- Sigfusdottir ID, Asgeirsdottir BB, Sigurdsson JF, Gudjonsson GH. (2011). Physical activity buffers the effects of family conflict on depressed mood: a study on adolescent girls and boys. J Adolesc 34(5):895–902
- Silcott NA, Bassett DR, Thompson DL, Fitzhugh EC, Steeves JA. (2011). Evaluation of the Omron HJ-720ITC pedometer under free-living conditions. Med Sci Sports Exerc 43(9):1791–7
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. (2000). Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med 343(1):16–22
- Stein DJ, Scott K, Abad JMH, Aguilar-Gaxiola S, Alonso J, Angermeyer M, Demytteneare K, et al. (2010). Early childhood adversity and later hypertension: data from the World Mental Health Survey. Ann Clin Psychiatry 22(1):19–28
- Stults-Kolehmainen MA, Bartholomew JB. (2012). Psychological stress impairs short-term muscular recovery from resistance exercise. Med Sci Sports Exerc 44(11):2220–7
- Thoits PA. (2010). Stress and health: major findings and policy implications. J Health Soc Behav 51:S41–53
- Toker S, Biron M. (2012). Job burnout and depression: unraveling their temporal relationship and considering the role of physical activity. J Appl Psychol 97(3):699–710
- Turner RJ, Lloyd DA. (2003). Cumulative adversity and drug dependence in young adults: racial/ethnic contrasts. Addiction 98(3):305–15
- Turner RJ, Lloyd DA. (2004). Stress burden and the lifetime incidence of psychiatric disorder in young adults – racial and ethnic contrasts. Arch Gen Psychiatry 61(5):481–8
- Turner RJ, Wheaton B. (1995). Checklist measurements of stressful life events. In: Cohen S, Kessler RC, Gordon LU, editors. Measuring stress. New York: Oxford University Press. p 29–53
- Turrell G, Lynch JW, Leite C, Raghunathan T, Kaplan GA. (2007). Socioeconomic disadvantage in childhood and across the life course and all-cause mortality and physical function in adulthood: evidence from the Alameda County Study. J Epidemiol Community Health 61(8):723–30
- Unger JB, Johnson CA, Marks G. (1997). Functional decline in the elderly: evidence for direct and stress-buffering protective effects of social interactions and physical activity. Ann Behav Med 19(2):152–60
- Vitaliano PP, Zhang JP, Scanlan JM. (2003). Is caregiving hazardous to one's physical health? A meta-analysis. Psychol Bull 129(6):946–72
- Wadden TA, West DS, Delahanty LM, Jakicic JM, Rejeski WJ, Berkowitz RI, Williamson DA, et al. (2006). The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. Obesity 14(5):737–52
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. (2000). Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 132(8):605–11
- Whelton SP, Chin A, Xin X, He J. (2002). Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med 136(7):493–503
- Williams JBW, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, et al. (1992). The Structured Clinical Interview for DSM-III-R (SCID): multisite test–retest reliability. Arch Gen Psychiatry 49(8):630–6
- Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, et al. (1994). Reproducibility and validity of a self-administered physical-activity questionnaire. Int J Epidemiol 23(5):991–9

