Abstract
Adversity during early life can lead to diverging endocrine and behavioral responses to stress in adulthood. In our laboratory, we evaluated the long-term effects of early life adversity and its interaction with chronic stress during adulthood. We propose this as a model of vulnerability to dysregulation of the stress response. We hypothesized that rats subjected to both protocols would show differential expression of corticosteroid receptors measured as number of neurons immunoreactive for glucocorticoid receptors (GR) or mineralocorticoid receptors (MR), in limbic areas related to the control of anxiety-like behavior. We also evaluated the effect of amitriptyline expecting to prevent the outcomes of the model. Male Wistar rats were separated from the mother (MS) for 4.5 h every day for the first 3 weeks of life. From postnatal day 50, rats were subjected to chronic variable stress (CVS) during 24 d (five types of stressor at different times of day). During the stress protocol, the rats were administered amitriptyline (10 mg/kg i.p.) daily. MS evoked lower MR expression in the central amygdaloid nucleus and this was reversed by amitriptyline. Furthermore, CVS increased MR immunoreactivity in the hippocampal area CA2 and increased anxious behavior; both effects were prevented by the antidepressant. When MS was combined with CVS during adulthood, there was a reduction of locomotor activity, with no corrective effect of amitriptyline. The differential effects among groups could mean that MS would promote an alternative phenotype that is expressed when facing CVS (a double hit) later in life.
Introduction
Aversive situations during early life may lead to dysfunction of the stress response during adulthood (Oitzl et al., Citation2010). Throughout life, alterations in the ability to respond to stressors can constitute a risk factor for disease, especially psychiatric disorders such as depression (De Kloet, Citation2008; Miller et al., Citation2009). An imbalance of central glucocorticoid signaling has been proposed to underlie such dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (De Kloet et al., Citation1998). Glucocorticoid functions are exerted through the glucocorticoid receptors type I (mineralocorticoid receptor; MR), and type II (glucocorticoid receptor; GR). MR has a ten-fold greater affinity for the endogenous ligand compared to GR (Reul & De Kloet, Citation1985), hence they perform as a biphasic system of control such that MR interacts with glucocorticoids at baseline levels of the hormone while GR activates at stress or diurnal peak concentrations (De Kloet et al., Citation1998).
The control of glucocorticoid levels depends mainly on the regulation of the HPA axis but also on the interaction of the HPA axis with the limbic system. Signaling through GR and MR has an important role in the communication between these two systems (Herman et al., Citation2005). Both receptors are abundant in hippocampus, amygdala and septum (Ahima et al., Citation1991; Ahima & Harlan, Citation1990), which are brain areas involved in emotion, cognition and HPA axis control (Calfa et al., Citation2006, Citation2007; Degroot & Treit, Citation2004; Gray & McNaughton, Citation2000; Herman et al., Citation2005; McEwen, Citation2001; Sah et al., Citation2003; Witter & Amaral, Citation2004).
Early life adversity as well as chronic stress can alter glucocorticoid dependent feedback since the HPA system can become less sensitive to such control (De Kloet et al., Citation2005; Herman et al., Citation2005; Mizoguchi et al., Citation2001). This has been proposed to be due to the imbalance between the actions exerted through MR and GR that occurs under these circumstances. This is known as the MR/GR imbalance hypothesis (De Kloet et al., Citation1998) and it has been associated with the development and progress of mental disorders such as depression, anxiety-related conditions and post-traumatic stress disorder (De Kloet et al., Citation1998, Citation2005; Ladd et al., Citation2004). This relationship would occur due to the disruption of the balance between the function and expression of GR in the brain, which may comprise the resilience of the individual, i.e. the ability to recover after adverse stimuli (De Kloet et al., Citation2005). Other authors have also proposed that the dysregulation of HPA axis function would be evoked by the gradual loss of inhibitory control exerted by the hippocampus which would leave the excitatory actions of the amygdala to take over the regulation of glucocorticoid secretion, causing its continual activation (Vyas et al., Citation2002). In this case there would be an imbalance between the inhibitory and excitatory actions on the HPA axis.
Numerous antidepressants are known to affect the activity of the HPA axis in different situations. They can correct glucocorticoid hypersecretion in humans during depression (Gillespie & Nemeroff, Citation2005) and in rodents in chronic variable stress models (Cotella et al., Citation2013; Katz & Hersh, Citation1981; Roth & Katz, Citation1981; Soblosky & Thurmond, Citation1986). They can also affect the expression of glucocorticoid receptors in the brain. This has been widely studied, particularly in the hippocampus (Makino et al., Citation2002; Reul et al., Citation1993; Yau et al., Citation1995). Amitriptyline actions, as for other tricyclic antidepressants, have been proposed to be exerted through the unspecific inhibition of serotonin and noradrenaline reuptake in the brain (Gould et al., 2006). Nevertheless, it also has anticholinergic effects and it is an agonist at N-methyl-D-aspartate (NMDA) glutamate receptors (Ashina et al., 2004). Yet, there are other actions of antidepressants that are poorly understood, for instance, these involve changes in chemical balances in the brain, expression of different proteins and even neurogenesis, which are often only observed after chronic treatment with these drugs and may account for the effects of the antidepressant and correction of the HPA axis dysregulation (Pariante, Citation2004; Pittenger & Duman, Citation2008).
Adverse experiences in early life may sensitize specific neurocircuits to subsequent acute stressors thereby increasing individual risk for the onset of physiopathology and psychopathology (Ladd et al., Citation2005). Hence, in our laboratory we evaluated the long-term effects of early-life adversity and its interaction with chronic stress during adulthood. We proposed this as a model of vulnerability for dysregulation of the stress response, in accordance with the two-hit hypothesis for the development of psychopathology. This hypothesis postulates that early life factors may act as a “first hit” that would increase relative vulnerability to developing a given psychiatric disease when additional stressful events (“second hit”) are superimposed later in life (Llorente et al., Citation2011; Maynard et al., Citation2001). Our previous results showed that maternal separation can evoke several changes related to HPA axis control, catecholamine secretion and behavior (Cotella et al., Citation2013). These effects are context-dependent since they are expressed differently whether the rats are subjected to stress during adulthood or not.
The aim of the present study was to analyze the long-term effects of maternal separation in early life, under chronic stress conditions during adulthood, expecting to observe a differential phenotype concerning anxious behavior and immunoreactivity of corticosteroid receptors in limbic areas such as the central and medial nuclei of amygdala, the septohippocampal nucleus, the lateral septum and the dorsal hippocampus. We also evaluated the effect of treatment with the tricyclic antidepressant amitriptyline in such situations. We found that these receptors are variably affected by our experimental double hit model and that some of the effects observed can be differentially expressed depending on the life history of the individual.
Materials and methods
Animals
Wistar derived rats were bred and reared in our animal facility under controlled temperature conditions (22 ± 2 °C) and artificial illumination (12:12 h light/dark; lights on at 07:00 h), with water and food available ad libitum. Rats were housed in 45 cm × 30 cm × 18 cm plastic cages with pine-shavings as bedding.
Pregnancy and maternal separation
Two non-related females were mated with a male and they remained together until there was physical evidence of pregnancy. After that the male was removed. The females were housed together until 2 days before the expected day of birth when they were put in separate cages. Mothers were randomly divided into two groups of twelve rats each: maternal separation (MS) or no maternal separation (NMS) to obtain a minimum of 80 male pups. The day of birth was considered as postnatal day 0. On postnatal day 1, litters were culled to eight pups (four males, four females) and submitted to the MS protocol. For MS, pups were separated daily from their mother for 4.5 h during the first 3 weeks of life (Ogawa et al., 1994) by removing the dam from the home cage and placing it alone into another cage in the same room. The litters were kept at room temperature during the separation with water and food ad libitum, and allowed to thermoregulate by gathering them together in the nest. Separations were carried out between 08:00 h and 12:30 h until postnatal day 21. The NMS group remained undisturbed in the maternal cage except for a bedding change twice a week, until the weaning age at postnatal day 25.
After weaning, male rats were selected and housed in standard cages in groups of four. They were handled daily by the same researcher to minimize stress reactions to manipulation at the moment of terminal perfusion-fixation.
Adult male offspring: chronic variable stress
Both NMS and MS groups (approximately 40 rats each) were randomly divided into another two groups of 20 rats each: one that was submitted to chronic variable stress (CVS), and a second that remained unstressed (NCVS). Unrelated rats were used to form the groups to avoid confounding litter effects. The CVS protocol as well as the antidepressant and vehicle treatments started at 50 d of age (200–250 g body weight).
The CVS model consists of five stressors with varying intensities that were presented randomly in time and order to the animals over the course of 24 d. The stressors were (a) noise produced for 4 h by an alarm bell (85 dB; 2.5 Hz); (b) loss of consciousness by ether anesthesia and subsequent exposure for 2 min; (c) two intraperitoneal (i.p.) injections of 0.5 ml isotonic (0.9%) saline at 4 h intervals; (d) food deprivation for 24 h; (e) restraint for 1 h by placement inside a 6 cm diameter metal grid cylinder which could be adapted to the length of the rat. The stressors used in this paradigm did not affect body weight. With the exception of the last day, stressors were assigned randomly in each replication of the experiments. Noise was always used on the last day of the chronic stress protocol.
Amitriptyline treatment
Concomitant with the application of the 24 day CVS, half of the animals of each of the above groups were given i.p. amitriptyline (10 mg/kg) or vehicle (10–11 rats each). Amitriptyline was prepared diluted in 0.5 ml of 0.9% saline solution (vehicle). amitriptyline or vehicle was administered every day, between 12:00 h and 14:00 h in order to keep the administration separated in time from the application of the stressors.
Experimental design
In summary, we used a factorial (3 × 2) experimental design with: rearing condition [non-maternally separated (NMS) or maternally separated (MS)], adult stress [stressed (CVS) or unstressed (NCVS)] and antidepressant [vehicle (V) or amitriptyline (AMI)] as factors. The resulting experimental groups were: NMS-NCVS (Vehicle; AMI); MS-NCVS (Vehicle; AMI); NMS-CVS (Vehicle; AMI); MS-CVS (Vehicle; AMI). Each group had 10–11 rats. At the end of the experiment some rats were not included in the analysis as the behavioral test was not done under the appropriate conditions of silence or the rats escaped from the maze.
Elevated plus maze
At day 75, rats were tested for anxiety-like behavior using the plus maze test. The elevated plus maze test is based on creating a conflict between the rat’s exploratory drive and its innate fear of open and exposed areas. Thus, decreased open arms exploration was taken to indicate enhanced anxiety-related behavior. The apparatus consisted of a plus-shaped platform elevated 50 cm from the floor. Two of the opposing arms (50 × 10 cm) were enclosed by 40-cm high side and end walls (closed arms), whereas the other two had no walls (open arms). At the beginning of the test, each rat was placed onto the central area (10 × 10 cm) of the maze facing a closed arm and was allowed to explore the plus maze freely. During the 5-min exposure, the following parameters were recorded: number of entries into open arms, number of entries into closed arms, and time spent on the open arms. Three indices of anxiety were obtained: the number of (1) entries into open arms expressed as a percentage of the total number of entries, (2) the amount of time spent in the open arms expressed as a percentage of total time and (3) the number of total entries (open arms + closed arms). Between each session, the maze was wiped clean with 20% ethanol. Behavioral testing was conducted in a quiet room. Rats were transported to the experimental room 2 h before the behavioral test to eliminate the stressor effects of the new environment.
Brain collection and processing
At day 76, rats (350–400 g body weight) were perfuse-fixed between 09:00 h and 12:00 h The rats were deeply anesthetized with chloral hydrate (6%); 0.54 g/kg i.p) and transcardially perfused with heparinized 0.9% saline followed by a 4% paraformaldehyde, 0.2 % picric acid alcoholic saturated solution in 0.1 M phosphate buffer (PB); brains were removed and stored at 4 °C in 20% sucrose-PB solution overnight. Forty-µm coronal sections were cut using a freezing microtome. Brains were processed for localization of GR and MR immunoreactivity. Sections were placed in a mixture of normal horse serum (NHS) 10% in PB for 1 h to block sites of non-specific binding to serum constituents. Free-floating sections were carefully matched for anatomical location to ensure comparability of regions of interest across conditions. Sections were washed 3 times for 5 min in PB, and blocked with 10% NHS in 0. M PB for 1 h at room temperature. GR primary antibody was diluted 1:500 in PB containing 2% NHS and 0.01% Triton X-100 and incubated for 72 h at 4 °C [rabbit anti-GR antibody (P- 20, sc-1002); Santa Cruz Biotechnology, Inc., Dallas, TX]. MR primary antibody was diluted 1:500 in PB containing 2% NHS and 0.01% Triton X-100 and incubated for 36 h at 4 °C [mouse anti-MR antibody MR (1-18 1D5), kindly donated by Dr. Gomez-Sanchez, Endocrinology, G.V. (Sonny) Montgomery VA Medical Center (C.E.G.-S., E.P.G.-S.) and University of Mississippi Medical Center (C.E.G.-S., A.F.d.R., D.G.R., J.E., M.P.W., E.P.G.-S.) (Gomez-Sanchez et al., Citation2006)]. After incubation, sections were rinsed with PB and incubated in the appropriate biotinylated secondary antiserum and avidin-biotin-peroxidase complex (ABC kit; Vectastain, Vector Laboratories, Burlingame, CA) for 2 h at room temperature. Immunoreactivity was detected by diaminobenzidine hydrochloride (0.025% in PB) which produces a brown reaction product. Finally, free-floating sections were mounted on gelatinized slides, air-dried overnight, dehydrated, cleared in xylene, and placed under a coverslip with DPX mounting (Fluka, Buchs, Switzerland). Samples in which primary antibodies were excluded were processed in parallel and used as negative controls (not shown). The same antibody has been used by others who showed specificity in western blot and ChIP analysis (Diefenbacher et al., Citation2010; Hong et al., Citation2009).
Immunoreactivity quantitative analysis
The brain areas of interest were identified and delimited according to the rat brain atlas of Paxinos & Watson (Citation2007). The numbers of GR and MR immunoreactive neurons were counted in the dorsal hippocampus (CA1–CA3 and dentate gyrus, bregma: −2.80 to −3.60 mm), central and medial nuclei of amygdala (bregma: −2.16 to −2.76 mm), septohippocampal nucleus (bregma: 2.28 to 0.96 mm), and lateral septum (bregma: 1.68 to 0.72 mm). Between 5 and 7 sections per rat were analyzed depending on the size of the brain area. The images were analyzed using a computerized system that included an Olympus BX41 microscope equipped with a high resolution camera (Olympus Corporation, Tokyo, Japan). Magnification was 400X (40× objective; 10× ocular).
The number of GR-immunoreactive (GR-IR) or MR-immunoreactive (MR-IR) cells was counted using a semi automatized method with ImageJ software (National Institutes of Health, Bethesda, MD). Investigators were blinded to the grouping while taking the photomicrographs and performing the image analysis, within grids of defined size that were placed over each area. All images used in the analysis were taken on the same microscope and with the same optical settings. Counts of GR- and MR-labeled cells were obtained from each area of interest, maintaining constant background intensity across different sections and rats such that a GR-, MR- labeled cell was counted only if it reached a defined darkness threshold above background. The counting procedure was done in 4 to 7 rats from each condition. Once the number of positive GR and MR nuclei was determined in each section, the relative density of the population of cells immunopositive for each receptor was calculated by dividing this number by the area measured in each case. Then the results were represented as number of GR or MR positive cell per arbitrary area of 0.1 mm2 to set the scale of data between 0–100 cells per area unit.
Statistical analysis
Data are shown as group mean ± SEM and were evaluated considering three factors: rearing condition × adult stress ×antidepressant. Plus maze results were analyzed by a three-way ANOVA followed by the LSD post hoc test using Infostat software (www.infostat.com.ar). When necessary, homogeneity of variance was obtained by performing analysis on the values after square-root transformation. Immunoreactivity data were evaluated through a Mixed Model with three fixed effects (rearing condition × adult stress × antidepressant) and a random effect (animal) using an R interphase of the Infostat software to estimate Generalized Linear and Mixed Models (GLMM) followed by the LSD post hoc test. The most parsimonious model was determined in case of probing non-homogenous variance. Significance was accepted for p < 0.05. For immunoreactivity and MR:GR ratio results, the figures only display the limbic areas where significant results were observed.
The experiments were performed in full accordance with protocols approved by the animal care committee of the National University of Córdoba, Argentina.
Results
Hippocampus GR and MR immunoreactivity
Glucocorticoid receptor
There was a main effect of adult stress in the CA3 region, F(1,37) = 6.03, p < 0.05. The post hoc analysis revealed that MS-CVS vehicle rats had significantly greater GR-IR than the corresponding NCVS group (p < 0.05) (). There were no significant changes for GR-IR in CA1, CA2 or dentate gyrus.
Figure 1. Early life and adult stress with/without amitriptyline on GR and MR in hippocampal sub-fields. LEFT. Mean number per unit area (0.1 mm2) of (A) glucocorticoid receptor-immunoreactive (GR-IR) neurons in the CA3 subfield and (B) mineralocorticoid receptor-immunoreactive (MR-IR) neurons in CA2 of non-maternally separated (NMS) and maternally separated (MS) rats submitted to chronic variable stress (CVS) or not (NCVS) under amitriptyline (10 mg/kg; AMI) or vehicle (Vehic) treatment. Data were analyzed by a linear mixed effect model followed by LSD post hoc test. Data are mean + SEM. Number of rats per group is included inside each bar. Significant differences: a, indicates p < 0.05 versus respective Vehic group; b, indicates p < 0.05 versus respective NCVS; c, indicates p < 0.05 versus respective NMS. RIGHT. Representative photomicrographs. (C) GR-IR neurons in the CA3 subfield of the hippocampus of the MS (left two columns) and CVS rats under AMI or Vehic treatment. Note that MS rats exposed to CVS showed increased GR expression. Statistically significant difference shown in graph A is indicated (b). (D) MR-IR neurons in the CA2 subfield of the hippocampus of NMS (columns 1 and 3) and MS rats, NCVS (columns 1 and 2) and CVS under AMI treatment. Statistically significant differences shown in graph B are indicated (a,b,c).
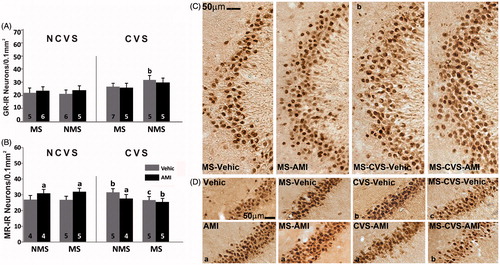
Mineralocorticoid receptor
Regarding MR-IR, in CA2 there was a positive interaction for adult stress × antidepressant F(1,29) = 4.50, p < 0.05. In the NCVS groups, AMI increased MR-IR compared to the vehicle groups (p < 0.05). In the NMS rats under CVS, those treated with vehicle presented greater MR-IR than its corresponding CVS group (p < 0.05). This rise was prevented by AMI (p < 0.05). The effect of CVS was not found in vehicle treated rats subjected to both MS and CVS. This group displayed significantly lower levels of MR-IR than observed in the NMS-CVS vehicle group (p < 0.05). The MS-CVS AMI group had lower MR-IR than its corresponding NCVS group (p < 0.05) in which AMI increased MR immunoreactivity by itself (). There were no changes in MR-IR for CA1, CA3 and dentate gyrus.
Amygdala GR and MR immunoreactivity
Glucocorticoid receptor
Regarding GR-IR, there was a positive interaction for adult stress × treatment in the medial amygdaloid nucleus F(1,28) = 6.69; p < 0.05. The LSD test showed that CVS-AMI rats had lower GR-IR than its corresponding vehicle group and NCVS-AMI group (p < 0.05 respectively) ().
Figure 2. Early life and adult stress with/without amitriptyline on GR and MR in amygdala LEFT. Mean number per unit area (0.1 mm2) of (A) glucocorticoid receptor-immunoreactive (GR-IR) neurons in the medial amygdaloid nucleus; (B,C) mineralocorticoid receptor-immunoreactive (MR-IR) neurons in (B) the central, and (C) medial amygdaloid nuclei of non-maternally separated (NMS) and maternally separated (MS) rats submitted to chronic variable stress (CVS) or not (NCVS) under amitriptyline (10 mg/kg; AMI) or vehicle (Vehic) treatment. Data were analyzed by a linear mixed effect model followed by LSD post hoc test. Data are mean + SEM. Number of rats per group is included inside each bar. Significant differences: a, p < 0.05 versus respective Vehic; b, p < 0.05 versus respective unstressed; c, p < 0.05 versus respective NMS. RIGHT. Representative photomicrographs. (D) GR-IR neurons in the medial amygdaloid nucleus of the NMS and MS NCVS (Rows 1 and 2) and CVS rats under AMI or Vehic treatment. Significant differences shown in graph A are indicated (a,b). (E) MR-IR neurons in the central amygdaloid nucleus of NMS (left 2 columns), MS, NCVS (upper 2 rows) and CVS rats under AMI or Vehic treatment. Significant differences shown in graph B are indicated (a,b,c).
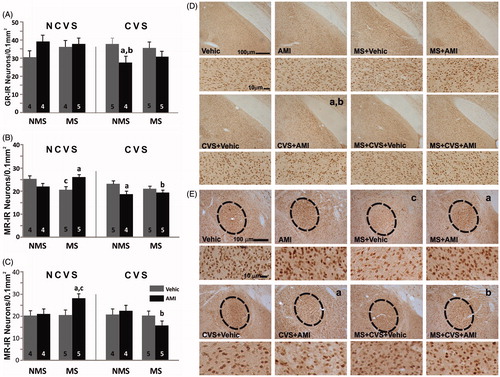
Mineralocorticoid receptor
Concerning MR, adult stress had a main effect on the central amygdaloid nucleus F(1,33) = 7.80, p < 0.01. This determined that, in general, rats under CVS had lower MR immunostaining than the NCVS groups. There was also a positive interaction for adult stress × antidepressant F(1,33) = 4.27; p < 0.05, and rearing condition × antidepressant F(1,33) = 8,29; p < 0.01. The post hoc test indicated that the MS-vehicle group had fewer MR stained nuclei than the corresponding NMS group (p < 0.05). AMI significantly prevented this reduction (p < 0.05). CVS by itself did not evoke a change in the immunostaining of NMS rats. Nevertheless, the adult stress × antidepressant interaction determined that the group subjected to both showed less MR-IR than the corresponding NCVS-AMI group (p < 0.05). Lastly, while MS-CVS vehicle rats did not differ from the corresponding NCVS group, AMI did not show the preventive effect that it had in the MS-NCVS group and indeed, MS-CVS rats treated with AMI had less MR staining than the AMI MS-NCVS group (p < 0.05) ().
In the medial amygdaloid nucleus there was an interaction, for rearing condition × adult stress F(1,32) = 4.29; p < 0.05. This determined that in general, MS rats subjected to CVS had less MR-IR than the MS rats without CVS. The post hoc analysis showed that MS-AMI rats had greater MR immunostaining compared with vehicle and the NMS control groups (p < 0.05 respectively). In the MS-CVS group, those treated with AMI presented the opposite effect from that in the corresponding NCVS rats and had significantly less MR staining (p < 0.05) ().
Septum GR and MR immunoreactivity
Glucocorticoid receptor
In lateral septum there was a main effect of rearing condition, F(1,26) = 7.27; p < 0.05, which determined that in general separated rats had more GR stained nuclei than the NMS groups. The analysis of the individual group differences showed that NMS-CVS rats under AMI treatment had less GR immunostaining than its corresponding NCVS group (p < 0.05). The MS-CVS groups both showed more GR immunostaining than their correspondent NMS control groups (p < 0.05) ().
Figure 3. Early life and adult stress with/without amitriptyline on GR and MR in septum. UPPER ROW. Mean number per area unit (0.1 mm2) of (A) glucocorticoid receptor-immunoreactive (GR-IR) neurons in the lateral septum, and (B) mineralocorticoid receptor-immunoreactive (MR-IR) neurons in the septohippocampal nucleus of non-maternally separated (NMS) and maternally separated (MS) rats submitted to chronic variable stress (CVS) or not (NCVS) under amitriptyline (10 mg/kg; AMI) or vehicle (Vehic) treatment. Data were analyzed by a linear mixed effect model followed by LSD post hoc test. Data are mean + SEM. Number of rats per group is included inside each bar. Significant differences: b, p < 0.05 versus respective NCVS group; c, p < 0.05 versus respective NMS group. LOWER ROWS. Representative photomicrographs. (C) GR-IR neurons in the lateral septum of NMS (left 2 columns), MS (right two columns), NCVS (upper 2 rows) and CVS (lower two rows) rats under AMI or Vehic treatment. The statistical differences shown in graph A are indicated (a,b,c). (D) MR-IR neurons in the septohippocampal nucleus of NMS (left 2 columns), MS (right two columns), NCVS (upper row) and CVS (lower row) rats under AMI or Vehic treatment. Significant differences shown in graph B are indicated (b,c).

Mineralocorticoid receptor
Concerning MR staining, in the septohippocampal nucleus there was a main effect of the rearing condition F(1,28) = 7.20; p < 0.05. In general, MS rats had more stained nuclei than the rest of the groups. The post hoc test indicated that the increase was significant in the MS vehicle and AMI groups compared to their NMS controls (p < 0.05 respectively). In the MS-CVS groups, those treated with saline solution presented the same increase compared to its corresponding NMS group (p < 0.05), while the AMI group remained at the level of the controls, NMS-NCVS ().
In the lateral septum there was a main effect of the stress F(1,30) = 4.57; p < 0.05. In general MR-IR was lower in the CVS rats. There were no differences when comparing the individual groups ().
Analysis of the MR/GR ratio
The MR/GR ratio in hippocampus was significantly affected in dentate gyrus by the interaction rearing condition × stress F(1,27) = 6.08; p < 0.05. The MS-CVS vehicle group showed the lowest ratio which was different from the corresponding NMS-CVS group (p < 0.05) ().
Figure 4. Ratio of density of mineralocorticoid: glucocorticoid receptor-immunoreactive neurons (MR:GR). Data are for dentate gyrus of non-maternally separated (NMS) and maternally separated (MS) rats submitted to chronic variable stress (CVS) or not (NCVS) under amitriptyline (10 mg/kg; AMI) or vehicle (Vehic) treatment. Data are mean + SEM. Number of rats per group is included inside each bar. Significant difference (ANOVA followed by the LSD post hoc test): c, p < 0.05 versus respective NMS group.
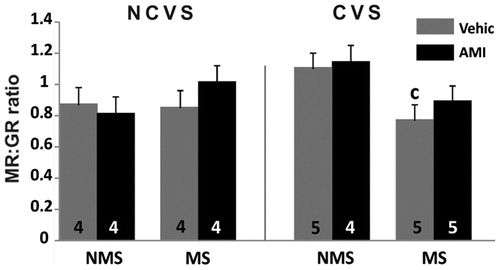
There were no differences for this variable in amygdala or in septum.
Elevated plus maze: anxiety-like behavior
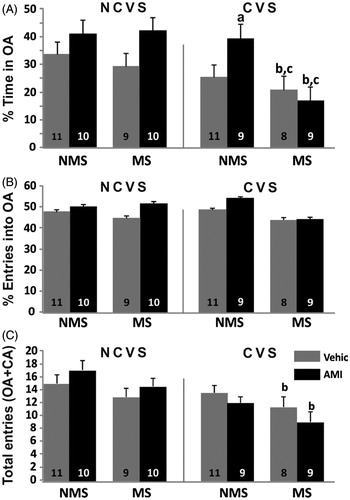
When analyzing the percentage of time in open arms, the ANOVA showed a main effect of every factor: rearing condition F(1,69) = 4.88; p < 0.05, adult stress F(1,69) = 10.64; p < 0.01 and antidepressant F(1,69) = 4.88; p < 0.05. In general, NMS, NCVS and AMI rats spent more time in the open arms than the rest. The CVS vehicle treated group did not differ significantly from its controls. Nevertheless, AMI treated rats under CVS were significantly less anxious than their corresponding vehicle controls (p < 0.05). Rats subjected to both MS and CVS spent significantly less time in the open arms than the rest of the groups with no effect of AMI in reversing that effect (p < 0.05 respectively compared to corresponding NCVS and NMS).
There were no effects on the parameter percentage of entries into open arms. Regarding the number of total entries, which is considered an index of total locomotor activity, there was a main effect of rearing condition, F(1,72) = 5.55; p < 0.05 and stress F(1,72) = 11.3; p < 0.01. The post hoc analysis showed that in the groups with the combination of both stress factors, the summation of their effects evoked significantly less locomotor activity (p < 0.05) compared to the NCVS groups and this effect was not reversed by the treatment with AMI (p < 0.05 compared to NCVS) ().
Figure 5. Plus Maze Test. Data are behaviors during a 5-min exposure of non-maternally separated (NMS) and maternally separated (MS) rats submitted to chronic variable stress (CVS) or not (NCVS) under amitriptyline (10 mg/kg; AMI) or vehicle (Vehic) treatment. Data are mean + SEM. Number of rats per group is included inside each bar. (A) %Time spent in the open arms (OA); (B) % Number of entries into the OA; (C) Number of total entries (open+closed arms). Significant differences (ANOVA followed by the LSD post hoc test): a, p < 0.05 versus respective Vehic; b, p < 0.05 versus respective NCVS; c, p < 0.05 versus respective NMS.
Discussion
To evaluate the long-term effects of early life adversity and its interaction with chronic stress during adulthood, in this study, we characterized the immunoreactive expression of GR and MR in different limbic brain areas and we evaluated anxious behavior in rats submitted to early maternal separation and chronic stress in adulthood. We also analyzed the effect of treatment with the tricyclic antidepressant amitriptyline in such situations. According to the two-hit hypothesis for the development of psychopathology, we expected to observe a differential phenotype in the different experimental conditions. Furthermore, considering that some antidepressants can modify neuroendocrine aspects in depressed patients and that previous results of our group using the same stress model showed effects of amitriptyline on HPA axis drive, we also expected amitriptyline to correct or prevent the changes evoked by the double model of stress. Our results showed that maternal separation reduced MR expression in the central amygdaloid nucleus which was reversed by amitriptyline. Chronic variable stress increased MR immunoreactivity in the hippocampal CA2 area. It also increased anxiety-like behavior. All those effects were prevented by the antidepressant. When maternal separation and chronic stress during adulthood were combined, there was also a reduction of the locomotor activity in the rats and amitriptyline had no corrective effect. This indicates that maternal separation promotes an alternative phenotype, which in turn evokes different responses later in life depending on the environment in which the animal lives.
Below we analyze the results group by group in order to understand some of the later implications of adversity during early-life and how this can modify the way individuals react to chronic stress in adulthood.
Normal controls or maternal separation, no adult variable stress: amitriptyline and MR in brain regions (amygdala, CA2, hippocampus)
Considering NMS and NCVS, we observed an effect of amitriptyline by itself. The increased MR immunoreactivity in CA2, which was also observed in the maternally separated group with no effect of the rearing condition, is in accordance with several authors who determined that amitriptyline can up-regulate the expression of MR (Makino et al., Citation2002; Yau et al., Citation1995) or its binding (Reul et al., Citation1993).
Regarding the long-term effects of maternal separation, the decrease in the number of MR positive neurons in the medial amygdaloid nucleus is in agreement with other reports of lower expression of MR in hippocampus after 24 h maternal deprivation during the stress hypo-responsive period (Sutanto et al., Citation1996). Notably, amitriptyline treatment during adulthood was able to correct the decrease of MR staining in the MS rats. A similar effect was observed in the medial amygdaloid nucleus. In this case, even though maternal separation did not evoke a change, MS-AMI rats showed an increase of MR-stained neurons. It is also remarkable that in the amygdala, the antidepressant did not evoke by itself an increase in the number of MR-positive neurons as was the case in hippocampus. In amygdala there was a state-dependent effect only observed in maternally separated individuals, which is supported by the statistical interaction between the rearing condition and the antidepressant. Furthermore, maternal separation increased MR-staining in the septohippocampal nucleus. This effect was not reversed by amitriptyline, which indicates that this antidepressant is capable of correcting the changes in MR immunoreactivity due to early life adversity only in some brain areas, i.e. these effects appear to be location-dependent. The septohippocampal nucleus has been implicated in the modulation of inhibitory inputs to the hippocampus. This affects the excitability of this structure, which for instance would have an impact on memory processes (Steffensen et al., Citation1993). Other authors have determined that this nucleus connects to the hypothalamus along with the lateral septum. This suggests some role in the regulation of the endocrine stress response (Risold & Swanson, Citation1997). Considering this evidence and the present results, we hypothesize that information regarding stress responses processed through MR in this nucleus is prone to be affected by early adversity. This may be maintained throughout life without being susceptible to amitriptyline action during adulthood.
Chronic variable stress and GR and MR in limbic brain regions (amygdala, hippocampus, septum): amitriptyline effects
GR in hippocampus
Analyzing the changes that occurred in the groups under chronic variable stress, the finding of lack of effect on GR in hippocampus opposes other authors’ results, which found that GR immunoreactivity was reduced in CA1 and dentate gyrus after 21 days of chronic restraint stress (Kitraki et al., Citation2004). This indicates an alternative way to respond to chronic stress depending on the variability of the stressors. This is supported by the finding that the HPA reacts differently to diverse type of chronic stress protocols (Aguilera, Citation1994).
MR in hippocampus
In accordance with the increase in MR immunoreactivity in CA2 with CVS, which was effectively prevented by the treatment with the antidepressant, other authors reported that between 8 and 24 h after submitting rats to certain acute psychological stressors there was increased MR immunoreactivity in all the hippocampal areas and in amygdala (Gesing et al., Citation2001). These authors suggested that such a delayed rise of MR immunoreactivity would imply an increase in the inhibitory control of the HPA through the MR receptors and therefore would denote a dynamic participation of the MRs in the regulation of the HPA drive. This might explain why our CVS model failed to show modifications in the basal concentration of corticosterone in our previous work (Cotella et al., Citation2013). Nonetheless, we cannot discard possible effects of our chronic stress model on the circadian cycle of glucocorticoid secretion or in the acute response to stress which might be related to the effect observed.
GR and amitriptyline: amygdala
Considering the effects of amitriptyline in the CVS group, the antidepressant reduced the number of GR positive cells in the medial amygdaloid nucleus and in the lateral septum, and reduced MR-staining in the central amygdaloid nucleus. This action was state-dependent, only observed in the CVS rats, even though there was no effect of CVS per se on these variables. Regarding the amygdala, other authors reported that amitriptyline can also significantly reduce the expression of CRH receptors in medial and basolateral amygdala and paraventricular nucleus (Aubry et al., Citation1999). These kinds of actions on stress related receptors could be involved in some of the endocrine and behavioral effects of amitriptyline (Katz & Hersh, Citation1981; Roth & Katz, Citation1981; Soblosky & Thurmond, Citation1986) observed in different models of stress and depression.
GR and amitriptyline: lateral septum
The lateral septum has been implicated in the integration of affective and environmental stimuli with regard to the control of the stress response (Gray & McNaughton, Citation2000; Mostalac-Preciado et al., Citation2011) and HPA drive (Herman et al., Citation2005; Singewald et al., Citation2011). It has also been considered an important target for antidepressant and anxiolytic treatments (Degroot & Treit, Citation2004; Calfa et al., Citation2007; Mostalac-Preciado et al., Citation2011). In our results, we observed a reduction of GR-IR in lateral septum of CVS rats administered with amitriptyline. Other authors reported that the inhibition of GR in lateral septum evoked less anxiety in the plus maze test in a social defeat paradigm, with no involvement of the MR (Calfa et al., Citation2006).
CVS and reduced anxiety: amitriptyline effects.
In our study the ANOVA revealed that in general CVS rats were more anxious since they spent less time in the open arms, and amitriptyline treated rats did not show this. The effect of amitriptyline could be related to the reduction of GR immunoreactivity in the lateral septum of the CVS rats. This supports the idea that the signaling through GR in lateral septum would be an important target for anxiolytic drugs. Thus, the decrease in the number of neurons expressing GR receptor could explain in part the anxiolytic effect observed in the present and in previous results of our group with this CVS model (Cotella et al., Citation2009). Furthermore, it is also possible that the changes in MR immunoreactivity detected in the other areas were related to the control of anxiety-like behavior. This is indicated by the finding that in the CVS group, amitriptyline also produced modifications in the number of MR stained nuclei in CA2 and the central amygdaloid nucleus. The action of glucocorticoids on hippocampal MR has been indicated previously to regulate anxiety-like behavior in the elevated plus maze. It has been reported that treatment with a specific MR antagonist in this area increased exploration of the open arms while there were no GR-dependent effects (Bitran et al., Citation1998). On the contrary, other authors observed that the over-expression of MR in the forebrain of transgenic mice determined less anxious behavior measured in the open field test and dark/light box test (Lai et al., Citation2007). Others also reported that the over-expression of MR in basolateral amygdala evoked less anxiety with more open arm activity in the elevated plus maze (Mitra et al., Citation2009). This clearly suggests a differential role of this receptor depending on the brain area involved.
Effects of maternal separation and chronic variable stress (“double hit”) on hippocampal GR and MR
As was expected, some of the outcomes observed in the measured variables were a product of the interaction of both stress protocols and not just the summation of the separate protocols. This even resulted in changes in the effect amitriptyline exerted compared to effects observed in the other groups.
Glucocorticoid receptor
The increased number of GR-stained neurons in CA3 in these rats according to the statistical analysis was only due to CVS, even though it was significant only in this group. This effect contrasts with other authors’ results. It has been reported that a different protocol of chronic stress evoked a reduction in GR mRNA in CA3 even when applying a monotypic (Gómez et al., Citation1996) or variable (Paskitti et al., Citation2000) model of chronic stress. Nevertheless, others reported the absence of effect of repeated immobilization stress on GR mRNA (Mamalaki et al., Citation1992). The latter supports our observation and Paskitti et al. (Citation2000) suggested that the down-regulation of GR is a variable response to stress. Also, we cannot directly compare these reports with our results since we used a semi-quantitative technique that determined the number of positive neurons for the receptor but not the overall amount of receptor in a region. Furthermore, recent human post mortem studies on hippocampi of depressed patients failed to show modifications in GR immunoreactivity and also reported a lack of effect of antidepressants on this variable (Wang et al., Citation2012). Nonetheless, the distribution pattern of expression of the receptor in the human hippocampus is comparable to the GR distribution observed in rodents (Wang et al., Citation2013). In light of those reports, perhaps the actual role of hippocampal GR in the etiology and course of human stress related disorders should be reconsidered. Hence, the changes in GR hippocampal expression in animal stress models need to be interpreted with caution as an explanation for symptomatology in humans. Nonetheless, our observation is that after 24 days of CVS there was a recruitment of GR-expressing cells in response to stress.
Mineralocorticoid receptor
Regarding MR in hippocampus, the finding that the effect observed in CVS rats, an increase in MR-IR in CA2, was not detected in the group that was previously subjected to maternal separation indicates a differential response to stress in adulthood depending on previous life history. This might reflect buffering by maternal separation of the effects of chronic variable stress in adulthood or, alternatively, an exhaustion of the capacity of the system to regulate MR expression in response to the chronic stress. However, we do not know if the difference in the number of MR positive neurons between MS and NMS rats under CVS is maintained throughout life, or whether it is triggered by the stressful situation in adulthood. Furthermore, we do not have information about levels of expression of MR mRNA or protein in these neurons.
Changes in relative expression of MR and GR in the “double hit” model.
Considering the MR/GR immunoreactivity ratio in hippocampus, the interaction between maternal separation and chronic variable stress yielded a lower ratio in the dentate gyrus. Other researchers found that under a 21-day restraint protocol, the balance of the relation between MR and GR was not altered in hippocampus (Kitraki et al., Citation2004). Nevertheless, as previously mentioned, their results probably differ from ours due to the difference in the stress model and also to interaction with early life environment. Even though in the dentate gyrus there were no individual effects of the double hit model on the immunoreactivity of GR or MR separately, this effect on the MR:GR ratio is of interest, considering that corticosteroid receptors participate in the regulation of antagonistic processes, for instance, long-term potentiation (LTP) or long-term depression (LTD) during stress and basal conditions (Joëls, Citation2001). Also, MRs have been implicated in processes of proliferation and neuronal survival while GR would be related to neuronal death (Hassan et al., Citation1999). Hence, in rats subjected to the double hit, the change in the MR:GR ratio in the dentate gyrus, a region where active neurogenesis and plastic processes are occurring (Mongiat & Schinder, Citation2011), indicates a different regulation of these processes under CVS depending on the previous early life experience of stress. It would be interesting to further investigate the implications of the decrease of the MR:GR ratio in the dentate gyrus in our model since such reduction would be in line with the MR/GR imbalance hypothesis for psychiatric disorders (De Kloet et al., Citation1998). Moreover, our previous results with the same double-hit model of stress showed hyper-secretion of corticosterone (Cotella et al., Citation2013), which dysregulation of the stress response may lead to the receptor imbalance. As the change in the MR:GR ratio and the augmented drive of the HPA axis were only observed in the group subjected to the double hit model, this would also be in agreement with the two-hit hypothesis for the development of psychopathology. Given that the MR:GR change in our model was prevented by amitriptyline, it seems that our maternal separation combined with adult chronic stress protocol could constitute a valid model for the study of at least some of the aspects of a phenotype vulnerable to stress. Furthermore, our MR:GR ratio result suggests a potential relevance for translational studies. Although absence of changes in hippocampal GR expression in depressed patients has been reported (Wang et al., Citation2012), changes in MR, and consequently in MR:GR balance, or effects of antidepressants have not been reported. Such human data would be interesting.
Interpretation of behavioral changes in double hit model
Finally, the rats subjected to the double hit model presented not only less time in the open arms of the plus maze, but also less locomotor activity, similar to our previous findings with this model (Renard et al., Citation2007). Because of the reduced locomotor activity, it is not possible to analyze the anxiety-like behavior in this group. Nevertheless, this reduction in locomotion indicates a particular effect of the double hit, which was unaffected by amitriptyline. Several authors have proposed that maternal separation can differentially sensitize neurocircuits involved in the stress response (Ladd et al., Citation2005) and that this would determine alternative behavioral and endocrine responses when confronting a stressful context later in life. For instance, freezing behavior is developed during the stress hypo-responsive period, and depends on corticosterone levels and GR signaling (Korte, Citation2001).
GR changes and locomotor behavior
In this regard, in the double hit group there was an increase in GR-IR in CA3 and lateral septum. Also, unlike in CVS rats, amitriptyline did not reduce GR-IR in the medial amygdaloid nucleus and in the lateral septum in double hit rats. Whether the changes in GR-IR observed in hippocampus, amygdala and septum are causally related to fear-related behavior in this model needs further study.
MR changes and locomotor behavior
Regarding the possible role of MR, in the group subjected only to CVS in which amitriptyline increased the time spent in the open arms, MR-IR was reduced in CA2 and in the central amygdaloid nucleus. These effects of amitriptyline on MR were not seen in the double hit group, which indicates possible involvement of MR signaling in the behavioral changes seen in these rats. Other authors reported that intrahippoccampal treatment with MR antagonists increased exploratory activity in several behavioral tests, although this may be through a non-genomic pathway (Korte, Citation2001).
Amitriptyline and locomotor behavior
The lack of effect of amitriptyline on locomotor activity in the double hit model contrasts with our previous finding that this antidepressant corrected the hyper-secretion of corticosterone in this model (Cotella et al., Citation2013). Hence, the reduced locomotor activity in the double hit model is evidently not a result of increased glucocorticoid level.
Mechanisms in double hit phenotype
There are several mechanisms that might be involved in the expression of the long-term effects of maternal separation when exposed to stress in adulthood. Global changes in homeostasis of the pup during the separation period, consequent for example, on changes in food intake or body temperature, may be involved (Macrí et al., Citation2004, Citation2008). During early life, the interaction with this adverse environment can determine epigenetic modifications and evoke differential expression of genes and proteins throughout life, especially in the brain (McEwen, Citation2003; Meaney, Citation2001, Citation2010).
Conclusions
The results presented here are in accordance with the idea that the disruption of the maternal-pup relationship promotes an altered phenotype, which increases vulnerability to stress later in life, as postulated by the double-hit hypothesis for the development of psychiatric disorders. In our study the occurrence of a different phenotype was evidenced by the effects observed in the rats submitted to a double model of stress in early and adult life compared to rats subjected just to one of the stress protocols. This is consistent with our previous finding that in our model only the combination of both stress protocols produced hypersecretion of corticosterone under basal conditions (Cotella et al., Citation2013). We also found similar differential responses to amitriptyline between the groups. Unexpectedly, the antidepressant did not correct the behavioral effects of this double hit stress model although it reduced anxiety in rats exposed only to CVS, and not exposed to the early life stress. The lack of effect of amitriptyline on outcomes of the double hit model contrasts with our previous study showing correction by amitriptyline of hypersecretion of corticosterone in this model. The effects of amitriptyline in modifying the expression of corticosteroid receptors varied according to the region of the limbic system studied. Future studies should seek differential effects of the double hit model and amitriptyline on expression of corticosteroid receptors in neurons and glial cells in limbic regions (Wang et al., Citation2013).
Declaration of interest
This research was supported by the National University of Córdoba (SECyT Grants 214/10 and 26/11) and the Science Ministry of the Province of Córdoba, Argentina (MINCyT PID 2008-Resol 82/09). Doctoral Fellowship CONICET PhD College of Biological Sciences, Faculty of Exact, Physical and Natural Sciences, National University of Córdoba, Argentina. The authors report no conflicts of interest and alone are responsible for the content and writing of this article and the funding sources had no influence in the content of this article.
Acknowledgements
We gratefully thank Dr. Gomez-Sanchez for kindly providing the MR1–18 1D5 antibody and to Dr. Ignacio Monedero for his guidance regarding image analyzing.
References
- Aguilera G. (1994). Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol 15:321–50
- Ahima RS, Harlan RE. (1990). Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 39:579–604
- Ahima RS, Krozowski Z, Harlan RE. (1991). Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by Corticosteroids. J Comp Neurol 313:522–38
- Ashina S, Bendtsen L, Jensen R. (2004). Analgesic effect of amitriptyline in chronic tension-type headache is not directly related to serotonin reuptake inhibition. Pain 108(1–2):108–14
- Aubry J-M, Pozzoli G, Vale W. (1999). Chronic treatment with the antidepressant amitriptyline decreases CRF-R1 receptor mRNA levels in the rat amygdala. Neurosci Lett 266:197–200
- Bitran D, Shiekh M, Dowd JA, Dugan MM, Renda P. (1998). Corticosterone is permissive to the anxiolytic effect that results from the blockade of hippocampal mineralocorticoid receptors. Pharmacol Biochem Behav 60:879–87
- Calfa G, Bussolino D, Molina VA. (2007). Involvement of the lateral septum and the ventral Hippocampus in the emotional sequelae induced by social defeat: role of glucocorticoid receptors. Behav Brain Res 181:23–34
- Calfa G, Volosin M, Molina VA. (2006). Glucocorticoid receptors in lateral septum are involved in the modulation of the emotional sequelae induced by social defeat. Behav Brain Res 172:324–32
- Cotella EM, Mestres Lascano I, Franchioni L, Levin GM, Suárez MM. (2013). Long-term effects of maternal separation on chronic stress response suppressed by amitriptyline treatment. Stress 16:477–81
- Cotella EM, Mestres Lascano I, Levin GM, Suárez M, Lascano I. (2009). Amitriptyline treatment under chronic stress conditions: effect on circulating catecholamines and anxiety in early maternally separated rats. Int J Neurosci 119:664–80
- De Kloet ER. (2008). About stress hormones and resilience to psychopathology. J Neuroendocrinol 20:885–92
- De Kloet ER, Joëls M, Holsboer F. (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6:463–75
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. (1998). Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301
- Degroot A, Treit D. (2004). Anxiety is functionally segregated within the septo-hippocampal system. Brain Res 1001:60–71
- Diefenbacher ME, Litfin M, Herrlich P, Kassel O. (2010). The nuclear isoform of the LIM domain protein Trip6 integrates activating and repressing signals at the promoter-bound glucocorticoid receptor. Mol Cell Endocrinol 320:58–66
- Gesing A, Bilang-Bleuel A, Droste SK, Linthorst A C, Holsboer F, Reul JM. (2001). Psychological stress increases hippocampal mineralocorticoid receptor levels: involvement of corticotropin-releasing hormone. J Neurosci 21:4822–9
- Gillespie CF, Nemeroff CB. (2005). Hypercortisolemia and depression. Psychosom Med 67:S26–8
- Gómez F, Lahmame A, de Kloet R, Armario A. (1996). Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology 63:327–37
- Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. (2006). Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147:1343–8
- Gould GG, Altamirano AV, Javors MA, Frazer AA. (2006). Comparison of the chronic treatment effects of venlafaxine and other antidepressants on serotonin and norepinephrine transporters. Biol Psychiatry 59(5):408–14
- Gray J, McNaughton N. (2000). The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. Second. In: Mackintosh NJ, McGaugh JL, Shallice T, Schacter D, Treisman A, Weiskrantz L, editors. Oxford Psychology Series. Oxford: Oxford University Press. p 440
- Hassan A H, Patchev VK, von Rosenstiel P, Holsboer F, Almeida OF. (1999). Plasticity of hippocampal corticosteroid receptors during aging in the rat. FASEB J 13:115–22
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29:1201–13
- Hong W, Chen L, Liu Y, Gao W. (2009). ATP hydrolysis is essential for Bag-1M-mediated inhibition of the DNA binding by the glucocorticoid receptor. Biochem Biophys Res Commun 390:77–81
- Joëls M. (2001). Corticosteroid actions in the hippocampus. J Neuroendocrinol 13:657–69
- Katz RJ, Hersh S. (1981). Amitriptyline and scopolamine in an animal model of depression. Neurosci Biobehav Rev 5:265–71
- Kitraki E, Kremmyda O, Youlatos D, Alexis MN, Kittas C. (2004). Gender-dependent alterations in corticosteroid receptor status and spatial performance following 21 days of restraint stress. Neuroscience 125:47–55
- Korte SM. (2001). Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev 25:117–42
- Ladd CO, Huot RL, Thrivikraman K V, Nemeroff CB, Plotsky PM. (2004). Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol Psychiatry 55:367–75
- Ladd CO, Thrivikraman K V, Huot RL, Plotsky PM. (2005). Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology 30:520–33
- Lai M, Horsburgh K, Bae S-E, Carter RN, Stenvers DJ, Fowler JH, Yau JL, et al. (2007). Forebrain mineralocorticoid receptor overexpression enhances memory, reduces anxiety and attenuates neuronal loss in cerebral ischaemia. Eur J Neurosci 25:1832–42
- Llorente R, Miguel-Blanco C, Aisa B, Lachize S, Borcel E, Meijer OC, Ramirez MJ, et al. (2011). Long term sex-dependent psychoneuroendocrine effects of maternal deprivation and juvenile unpredictable stress in rats. J Neuroendocrinol 23:329–44
- Macrì S, Chiarotti F, Würbel H. (2008). Maternal separation and maternal care act independently on the development of HPA responses in male rats. Behav Brain Res 191(2):227–34
- Macrì S, Mason GJ, Würbel H. (2004). Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. Eur J Neurosci 20(4):1017–24
- Makino S, Hashimoto K, Gold PW. (2002). Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav 73:147–58
- Mamalaki E, Kvetnansky R, Brady LS, Gold PW, Herkenham M. (1992). Repeated immobilization stress alters tyrosine hydroxylase, corticotropin-releasing hormone and corticosteroid receptor messenger ribonucleic Acid levels in rat brain. J Neuroendocrinol 4:689–99
- Maynard TM, Sikich L, Lieberman JA, LaMantia AS. (2001). Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull 27:457–76
- McEwen B. (2001). Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 933:265–77
- McEwen B. (2003). Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev 9:149–54
- Meaney M. (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24:1161–92
- Meaney M. (2010). Epigenetics and the biological definition of gene × environment interactions. Child Dev 81:41–79
- Miller G, Chen E, Cole SW. (2009). Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol 60:501–24
- Mitra R, Ferguson D, Sapolsky RM. (2009). Mineralocorticoid receptor overexpression in basolateral amygdala reduces corticosterone secretion and anxiety. Biol Psychiatry 66:686–90
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. (2001). Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology 26:443–59
- Mongiat LA, Schinder AF. (2011). Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci 33:1055–61
- Mostalac-Preciado CR, de Gortari P, López-Rubalcava C. (2011). Antidepressant-like effects of mineralocorticoid but not glucocorticoid antagonists in the lateral septum: interactions with the serotonergic system. Behav Brain Res 223:88–98
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K. (1994). Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacol Biochem Behav 49(4):961–7
- Oitzl MS, Champagne DL, van der Veen R, De Kloet ER. (2010). Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev 34:853–66
- Pariante C. (2004). Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology 29(4):423–47
- Paskitti ME, McCreary BJ, Herman JP. (2000). Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res 80:142–52
- Paxinos G, Watson C. (2007). The rat brain in stereotaxic coordinates6. San Diego: Elsevier Academic Press
- Pittenger C, Duman RS. (2008). Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109
- Renard GM, Rivarola MA, Suárez M. (2007). Sexual dimorphism in rats: effects of early maternal separation and variable chronic stress on pituitary-adrenal axis and behavior. Int J Dev Neurosci 25:373–9
- Reul JM, De Kloet ER. (1985). Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117:2505–11
- Reul JM, Stec I, Söder M, Holsboer F. (1993). Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology 133:312–20
- Risold PY, Swanson LW. (1997). Connections of the rat lateral septal complex. Brain Res Brain Res Rev 24:115–95
- Roth KA, Katz RJ. (1981). Further studies on a novel animal model of depression: Therapeutic effects of a tricyclic antidepressant. Neurosci Biobehav Rev 5:253–8
- Sah P, Faber ESL, Lopez De Armentia M, Power J. (2003). The amygdaloid complex: anatomy and physiology. Physiol Rev 83:803–34
- Singewald GM, Rjabokon A, Ebner K. (2011). The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology 36:793–804
- Soblosky JS, Thurmond JB. (1986). Biochemical and behavioral correlates of chronic stress: effects of tricyclic antidepressants. Pharmacol Biochem Behav 24:1361–8
- Steffensen SC, Yeckel MF, Miller DR, Henriksen SJ. (1993). Ethanol-induced suppression of hippocampal long-term potentiation is blocked by lesions of the septohippocampal nucleus. Alcohol Clin Exp Res 17:655–9
- Sutanto W, Rosenfeld P, De Kloet ER, Levine S. (1996). Long-term effects of neonatal maternal deprivation and ACTH on hippocampal mineralocorticoid and glucocorticoid receptors. Brain Res Dev Brain Res 92:156–63
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–18
- Wang Q, Joels M, Swaab DF, Lucassen PJ. (2012). Hippocampal GR expression is increased in elderly depressed females. Neuropharmacology 62:527–33
- Wang Q, Van Heerikhuize J, Aronica E, Kawata M, Seress L, Joels M, Swaab DF, Lucassen PJ. (2013). Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol Aging 34:1662–73
- Witter MP, Amaral DG. (2004). Hippocampal formation. In: Paxinos G, editor. The rat nervous system. 3rd edition. Burlington: Academic Press. p 635–704
- Yau JL, Olsson T, Morris RGM, Meaney M, Seckl JR. (1995). Glucocorticoids, hippocampal corticosteroid receptor gene expression and antidepressant treatment: relationship with spatial learning in young and aged rats. Neuroscience 66:571–81

