Abstract
The stress of dental treatment often elicits negative emotions in children, expressed as dental fear or anxiety. Highly anxious children obstruct treatment and avoid therapy, further amplifying oral health problems. The aim of this study was to examine the neuroendocrine and autonomic nervous system responses to dental treatment and their possible interactions and associations with psychometric indices of anxiety, caries, previous dental experience, anesthesia, age and gender in school children. Upon informed consent, saliva was obtained from 97 children (59% males, mean age ± SD: 89.73 ± 15 months) in the Clinic of pediatric dentistry before treatment, immediately post-treatment and at the recall visit to determine cortisol and salivary alpha-amylase (sAA) levels. Dental and general anxiety was assessed through specific questionnaires completed by the children. Compared to pre-treatment, cortisol levels were increased following treatment, while sAA levels were higher at the recall. Pre- and post-treatment cortisol and sAA responses were positively correlated. Dental and general anxiety questionnaire scores were also significantly correlated with each other. The integrated autonomic and neuroendocrine responses prior to treatment were correlated with state anxiety and those following treatment with dental anxiety. However, univariable and multivariable linear regression analysis associated post-treatment cortisol, but not sAA, levels with dental anxiety. No associations of cortisol or sAA responses with caries, age, gender, previous dental experience or anesthesia were detected. These data provide some evidence that both sAA and cortisol levels are altered in children in anticipation or during dental treatment, but only cortisol levels are associated to dental anxiety.
Introduction
Dental treatment elicits negative emotions in most children, which are often articulated as dental fear and/or anxiety. The mean prevalence of dental anxiety in children is approximately 10% (Klingberg & Broberg, Citation2007), but it has been shown to vary considerably in different populations (Ten Berge et al., Citation2002). Dental anxiety is more prevalent among younger children (Ten Berge et al., Citation1998) and factors linked to it include personality traits, previous painful dental experiences, parental fear and severity of dental treatment (Klingberg et al., Citation1995). Dentally fearful children, not only impede treatment in the clinic settings but can also subsequently develop avoidance to therapy that amplifies oral health problems.
Most of the perceived knowledge on children’s anxiety to dental environment is based on the analysis of specific psychometric tests completed by the child or the parent (Aartman et al., Citation1998). Recent studies have utilized salivary cortisol levels to assess the hypothalamic–pituitary–adrenal (HPA) axis’ response to dental visit or treatment, reporting either no effects (Blomqvist et al., Citation2007; dos Santos et al., Citation2012) or increased responses (Furlan et al., Citation2012; Kandemir et al., Citation1997; Rodrigues Gomes et al., Citation2013). Salivary cortisol is an indicator of unbound cortisol levels in the circulation and is considered a reliable non-invasive marker for stress-induced HPA axis activation (Hellhammer et al., Citation2009). Associations of salivary cortisol levels to psychometric indices have been reported in studies applying other psychological stressors in children (Allwood et al., Citation2011; Strahler et al., Citation2010). Similarly, salivary cortisol levels prior to a recall examination have been correlated with dental anxiety in 13-year-old children (Blomqvist et al., Citation2007).
Measurement of urinary catecholamines and their metabolites has previously been applied to assess children’s sympathetic nervous system response during dental treatment (Sakuma & Nagasaka, Citation1996), the risk for dentofacial injuries (Vanderas & Papagiannoulis, Citation1997) or to relate emotional state with dental caries (Vanderas et al., Citation1995). Recently, salivary alpha-amylase (sAA) has been introduced as an alternative biomarker of autonomic nervous system (ANS) activation since its secretion in the oral cavity is regulated by both sympathetic and parasympathetic stimulation (Bosch et al., Citation2011; Nater & Rohleder, Citation2009). Measurement of sAA is an easy and non-stressful method often used to estimate children’s anxiety in response to psychosocial stressful stimuli (Allwood et al., Citation2011; Strahler et al., Citation2010); however, limited data are available for sAA association with dental anxiety. Anticipatory responses to dental treatment (dos Santos et al., Citation2012; Furlan et al., Citation2012) or no significant immediate responses in children receiving dental treatment under local anesthesia (Arhakis et al., Citation2012) have been reported.
The existing literature provides some evidence that dental anxiety in children can be deciphered by anxiety-recording questionnaires and salivary cortisol or sAA levels, but to our knowledge, there is no study to examine associations of the self-reported emotional state during dental treatment with the biohormonal responses of the HPA and ANS systems, taking into consideration possible confounding factors. Based on previous findings, we hypothesized heightened HPA and ANS responses of children to dental treatment. Given the difference in responsiveness of the two systems following stress and their possible interaction, we aimed to examine these responses prior and following dental treatment and search for possible associations with self-reported dental and general anxiety status of the children. In addition, since some studies report anticipatory responses of both systems to treatment, we compared the initial and recall visit responses to examine for possible adaptations. The potential confounding effects of gender, age, prior dental experience, anesthesia, caries and day-time of saliva collection on the above measures were also considered.
Methods
Participants
Ninety-seven mentally and physically healthy children (58 boys and 39 girls), 6–10 years old (range: 62–124 months, mean = 89.73, SD = 15) who presented to the Postgraduate Clinic of Pediatric Dentistry, School of Dentistry, National and Kapodistrian University of Athens (NKUA) for a planned dental appointment were included in the study. All subjects had previously visited the Clinic for dental examination, and 78 of them (80.4%) also had a previous dental treatment experience in the same place. The exclusion criteria were based on factors known to influence cortisol and sAA levels, including children with active respiratory problems and children under medication for any reason, especially corticosteroids (Kirschbaum & Hellhammer, Citation1994; Woodside et al., Citation1991). The experimental protocol was approved by the ethical committee of the School of Dentistry, NKUA.
Study design
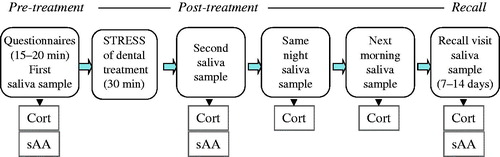
This was a prospective cohort study, and the stages and data collections points are outlined in . Upon arrival to the Clinic, parents and children were informed for the project, and after a written parent’s consent, the children were first asked to fill out the three questionnaires and then to provide the first saliva sample (within 15–20 min from arrival). They were then brought in the treatment room where they underwent clinical examination and recording of caries lesions, followed by either a cleaning with rotary instrument or a small restorative procedure with the use of local anesthesia (articaine HCl 4% with epinephrine 1:200,000). Forty-two of the 97 children (17 girls and 25 boys) received local anesthesia during treatment. Immediately after the end of the procedure, which lasted approximately 30 min, the second saliva sample was collected. The parents were given pre-labeled collection tubes and instructions in order to collect the next two saliva samples at home, which were scheduled for the same evening (at bed time) and the next morning (within 30 min from awakening), without eating or drinking anything prior to morning sampling. These samples were collected as an index of the diurnal variation (morning/evening ratio) in saliva cortisol levels. The parents were instructed to store the samples at 4 °C immediately after collection and to mail them back to the School of Dentistry in prepaid, self-addressed envelopes. Patients were asked to return for a recall appointment after 7–14 d and another sample was collected at that time, within 15–20 min after arrival and prior to dental treatment. Patients’ appointments were limited between 9 a.m. and 2 p.m. To control for the effect of diurnal rhythm on cortisol and sAA levels, the exact sampling time was included in the statistical analysis. The two “home samples” were used solely to determine salivary cortisol due to the sensitivity of sAA enzyme activity at room temperature storage.
Saliva collection
The saliva samples were collected with the help of cotton swabs (Salivettes, Sarstedt, Nuembrecht, Germany), which the participants were instructed to keep in their mouth for 1–3 min until they were soaked with saliva. All saliva samples were centrifuged at room temperature for 5 min at 1500 × g to collect saliva, and this was stored at −20 °C until assayed.
Caries activity
Caries activity was assessed by recording the caries index DMFT or dmft (number of decayed, missing and filled teeth), DMFS or dmfs (number of decayed, missing and filled tooth surfaces), for permanent and primary teeth, respectively, based on the Koch criteria (Koch, Citation1970). In our study, dmft was used for the statistical analysis since some of the children did not have permanent teeth. The caries index for primary teeth (dmft) is one of the simplest and most commonly used indices in epidemiologic surveys of dental caries. It quantifies dental health status based on the number of carious, missing and filled primary teeth. Children with dmft ≥ 3 were defined as having a high degree of dental caries, whereas those with dmft ≤ 2 as having a low degree of caries (Oulis et al., Citation2012).
Biochemical analyses
Salivary alpha-amylase was measured by enzymatic chromatometry in an automatic analyzer (Cobas Integra 800, Roche, Basel, Switzerland). Saliva samples (diluted 1:400 with double distilled water) were incubated with the substrate reagent (alpha-amylase EPS ver.2, Roche) according to manufacturer’s instructions. A standard curve was created from known concentrations of sAA, provided in the detecting kit. Intra- and inter-assay precision expressed as a percent coefficient of variation was below 5% and 10%, respectively.
Salivary cortisol was detected by electrochemiluminescence in an automatic analyzer (Cobas e411, Roche) using a commercially available kit (Roche). Saliva samples were treated according to manufacturer’s instructions. The results were calculated from a standard curve created from known cortisol concentrations provided in the kit. Positive control samples of high and low cortisol concentrations were included. Initial cortisol units (μg/dl) were converted in nmol/L (S.I.), according to the equation μg/dl × 27.586 = nmol/L. The sensitivity was 0.500 nmol/L and the intra- and inter- assay coefficients of variation were less than 5% and 10%, respectively.
Psychometrical analyses
Three self-reporting questionnaires were used in this study. The Greek version of the Children’s Fear Survey Schedule-Dental Subscale (CFSS-DS) (Arapostathis et al., Citation2008; Cuthbert & Melamed, Citation1982) was used for the subjective assessment of dental fear/anxiety. CFSS-DS is the most commonly used questionnaire for assessing children’s dental anxiety and it is considered to be of higher validity compared to other scales (Aartman et al., Citation1998). It consists of 15 questions related to situations in the dental office, on a scale of 1–5, with 1 meaning “not afraid at all” and 5 meaning “very afraid”. Sum scores may range from 15 to 75, with higher scores indicating higher dental fear.
Anxiety was measured using the Greek version of the State-Trait Anxiety Inventory for Children scale (Psychountaki et al., Citation2003). Both state (STAIC-S) and trait (STAIC-T) versions were used. They consist of two forms of 20 items each, asking children how they feel (Spielberger et al., Citation1973). STAIC-S was used to assess the anxiety due to a specific situation (in our case dental visit) and children were asked to rate their momentary affective experience. STAIC-T is a psychometric instrument assessing general anxiety that is stable across time and situations (trait anxiety). The questionnaires were completed by the children before their entrance to the operatory room.
Statistical analyses
Due to deviations from normality, log10 transformation was used for cortisol and sAA values. Paired t-tests were used to compare the means of sAA or cortisol measurements between pre-treatment and post-treatment and between pre-treatment and recall. The Spearman’s correlation coefficient was calculated between cortisol and amylase levels at pre-treatment, post-treatment and recall visit, and between the ratios of amylase over cortisol and the three questionnaires.
Univariable and multivariable linear regression analyses were implemented in order to evaluate potential associations between cortisol or sAA levels (at pre-treatment, post-treatment and recall, separately) and dental anxiety, state and trait general anxiety, dental caries, gender, age, previous dental treatment experience, exact sampling time and anesthesia (where applicable). In the multivariable analysis, only the variables with alpha <0.20 from the respective univariable analysis were included. All analyses were conducted using Stata 13.1 (College Station, TX). Statistical significance was accepted for p ≤ 0.05.
Results
Sample characteristics
The descriptive statistics of non-transformed values for the psychometric tests, the salivary cortisol and alpha-amylase measures, dental caries (dmft score) and age are listed in . Morning cortisol levels were significantly higher than the evening levels (paired t-test, t(91) = −18.9; p < 0.001). The morning/evening cortisol ratio in is provided as an index of baseline HPA functioning.
Table 1. Sample characteristics.
HPA axis and ANS responses during dental treatment and recall
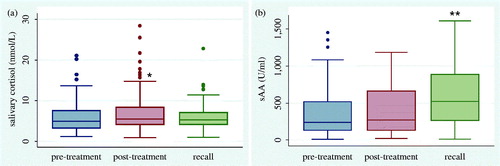
The HPA axis and ANS responses in the environment of the dental Clinic, as depicted from salivary cortisol and sAA levels, respectively, are illustrated in . Paired t-tests between pre- and post-treatment measures showed that dental treatment significantly increased cortisol levels (t(96) = −3.07; p = 0.003), whereas it did not significantly modify sAA levels (t(95) = −1.66; p = 0.10). Comparisons between the pre-treatment levels of the initial visit and those of the recall revealed a significant increase in sAA levels (t(87) = −4.51; p < 0.001) at the recall, but no differences in respective cortisol levels (t(86) = −0.14; p = 0.88).
Correlations of biohormonal responses and self-reported anxiety
Salivary cortisol responses of the same child at the pre-treatment, post-treatment and recall samples were significantly correlated to each other (). The same was true for sAA responses. Relevant positive correlations were also detected between pre-treatment cortisol and pre- and post-treatment sAA, as well as between post-treatment cortisol and sAA ().
Table 2. Correlations between salivary cortisol and alpha-amylase levels.
Children’s responses to the three psychometric tests used in the study were positively correlated with each other. Specifically, Spearman’s two-tailed revealed correlations of dental anxiety questionnaire with that of state anxiety (r = 0.33; p = 0.002) and trait anxiety (r = 0.37; p = 0.0004). State and trait anxiety questionnaires were also positively correlated (r = 0.42; p = 0.0001) ().
Table 3. Correlations between biological responses and self-reported anxiety.
To assess the integrated response of the two stress systems at each time point, the ratios of sAA over cortisol (AOC) were tested for possible correlations with questionnaire scores of dental, state and trait anxiety (). Spearman’s analysis showed that an increased sAA over cortisol ratio was associated to lower anxiety levels. More specifically, before treatment, the AOC ratio was negatively correlated with state anxiety (r = −0.26; p = 0.015). Following dental treatment, the AOC was negatively correlated with dental anxiety (r = −0.24; p = 0.029), whereas correlation with state anxiety did not reach significance (r = −0.19; p = 0.077).
Associations of biohormonal responses to anxiety and other parameters
Univariable linear regression analysis for cortisol samples at pre-treatment, post-treatment and recall () showed that pre-treatment cortisol levels were significantly associated with dental anxiety (β = 0.01, 95% CI: 0.002–0.03, p = 0.03). Pre-treatment cortisol was also associated with the time of sampling (β = −0.16, 95% CI: −0.24, −0.08, p < 0.001). Similarly, post-treatment cortisol levels were associated with dental anxiety (β = 0.02, 95% CI: 0.004–0.03, p = 0.01) and sampling time (β = −0.11, 95% CI: −0.20, −0.01, p = 0.03). Recall cortisol was significantly associated with state anxiety (β = 0.01, 95% CI: 0.0004, 0.02, p = 0.04) and sampling time (β = −0.10, 95% CI: −0.16, −0.05, p < 0.001).
Table 4. Univariable and multivariable linear regression analysis for the association of cortisol levels to study variables.
The subsequent multivariable linear regression analysis for cortisol included only the variables with alpha < 0.20 from the previous univariable analysis. A significant association between cortisol levels and sampling time was noted at pre-treatment (β = −0.14, 95% CI: −0.22, −0.06, p = 0.001) and recall (β = −0.09, 95% CI: −0.15, −0.04, p = 0.001), whereas the association of pre-treatment and recall cortisol with dental anxiety (β = 0.00, 95% CI: −0.003, 0.02, p = 0. 13) and state anxiety (β = 0.00, 95% CI: −0.001, 0.01, p = 0. 10) did not reach significance (). In the adjusted model, the significant association of post-treatment cortisol activity with dental anxiety was retained (β = 0.01, 95% CI: 0.001, 0.03, p = 0.03), whereas the effect of sampling time did not reach statistical significance (β = −0.09, 95% CI: −0.18, 0.002, p = 0.06).
No significant associations were detected between cortisol levels and the other variables (i.e. trait anxiety, caries, gender, age, previous dental experience or anesthesia). Univariable linear regression analysis for sAA samples at pre-treatment, post-treatment and recall showed no significant associations to any of the variables tested, including exact time of sampling ().
Table 5. Univariable linear regression analysis for the association of salivary alpha-amylase levels to study variables.
Discussion
This study aimed to examine the children’s HPA and ANS responses to dental treatment by combining psychometric and biohormonal measures. In agreement with our hypothesis, both cortisol and sAA responses were modified during dental visit and treatment though at different time points. The pre-treatment sAA and cortisol levels in our study reflect anticipatory responses of both systems to impending therapy and cannot be considered as baseline, compared to the previously reported values in same-age children at resting conditions (Strahler et al., Citation2010). Based on the kinetics of HPA axis activation following stress, the post-treatment salivary cortisol levels indicate the axis arousal due to dental treatment. However, due to the rapid excitation and recovery of the ANS at stress, this design cannot conclude if the absence of significant differences between the pre- and post-treatment sAA samples reflects ANS adaptation to the initial stimulus and/or its ongoing stimulation due to oral manipulations.
Increased salivary cortisol levels due to dental treatment have previously been reported following treatment (Kandemir et al., Citation1997), in compliance with our findings or in anticipation to it (Furlan et al., 2012; Rodrigues Gomes et al., Citation2013). On the other hand, Blomqvist et al. (Citation2007) reported no differences in salivary cortisol of young adolescents before and after dental visit. However, those patients were subjected only to clinical examination without treatment and they were of an older age, known to diminish dental anxiety (Klingberg et al., Citation1995; Ten Berge et al., Citation2002; Wogelius et al., Citation2003).
In line with our observations, previous studies in children reported either anticipatory to dental treatment increases in sAA levels (dos Santos et al., Citation2012; Furlan et al., Citation2012) or no immediate responses to dental treatment under anesthesia (Arhakis et al., Citation2012). Upon exposure to other stressors, sAA levels of children and adolescents have been reported to either increase (Gordis et al., Citation2006; Strahler, et al., Citation2010) or remain unchanged (Stroud et al., Citation2009), implying the dependence of this biomarker on co-existing factors and the type of stress stimulus.
The two major stress systems, HPA axis and ANS, interact in a complex manner to fine-tune stress reactivity. Deregulation of their concurrent actions has been related to behavioral problems in children (Bauer et al., Citation2002) and adults (Ali & Pruessner, Citation2012). Previous studies in different stress settings have reported interactions between the two systems in children (Nater et al., Citation2005, Citation2006; Strahler et al., Citation2010); however, the exact nature of these interactions is still a matter of debate (Allwood et al., Citation2011; Granger et al, Citation2007). This study detected significant correlations between cortisol and sAA at pre- and post-treatment time points. In an effort to detect possible concerted effects of the aforementioned systems, we used the ratio of alpha-amylase over cortisol, recently suggested as a reliable marker of ANS/HPA interactions (Ali & Pruessner, Citation2012) to test for possible correlations with children’ s emotional status. The detected significant correlations suggest a possible concurrent action of ANS and HPA on the self-reported dental anxiety. Our data show negative correlations indicating that the lower the alpha-amylase over cortisol ratio, the higher the children’s anxiety. This observation, along with the lack of any association of sAA to dental anxiety in subsequent regression analyses, implies that the “key regulator” of this ratio may be saliva cortisol. A heightened activity of the latter could reduce the AOC ratio toward increased dental anxiety. This assumption is supported by the results of regression analysis; however, further studies are required to delineate the ANS/HPA interaction.
Previous literature has revealed the implication of several parameters in the expression of dental anxiety in children, including previous dental experience, oral health, anesthesia, gender and age. In this study, we aimed to quantify the impact of the aforementioned parameters on cortisol and sAA responses during dental visit and treatment by using multivariable regression analysis. To our knowledge, this is the first attempt to collectively examine the possible contribution of an assortment of factors in children’s response to dental treatment. Cortisol levels and dental anxiety were significantly associated following dental treatment, upon correction for a possible confounding by sampling time, denoting that saliva cortisol is a relevant predictor of children’s anxiety following dental treatment. In a previous study (Blomqvist et al., Citation2007), a positive correlation was reported between dental anxiety (measured by a different scale) and salivary cortisol levels before dental examination. This is in line with our findings, though the nature of dental stress (clinical examination vs. treatment) and patients’ age may explain differences between the studies. The median dental anxiety score in the children of this study was 30 (range 15–63), which is within the range previously reported for Greek children (Arapostathis et al., Citation2008).
No significant associations were detected between general anxiety and biohormonal responses, upon corrections for sampling time. In the literature there is no study examining the association of general anxiety with saliva cortisol in children undergoing dental treatment. In children or adults exposed to other stressors, no correlation of neuroendocrine response with state anxiety has been found (Allwood et al., Citation2011; Maruyama et al. Citation2012; Strahler et al., Citation2010), in agreement with our findings. No effects of age and gender were detected in accordance with the literature for school children (Allwood et al., Citation2011; Knutsson et al., Citation1997; Netherton et al., Citation2004; Rohleder and Nater, Citation2009; Tzortzi et al., Citation2009). No association of caries with salivary cortisol response to dental treatment was found, in agreement with a previous study (Kambalimath et al., Citation2010). However, positive associations between dental caries and salivary cortisol levels have been reported in children with rampant caries (Rai et al., Citation2010) or socially disadvantaged (Boyce et al., Citation2010), implying that caries may not be the sole factor contributing to cortisol response following stress.
This study detected no associations of sAA levels with the self-reported dental anxiety. In agreement with our findings, no associations were found between sAA levels and psychographic indices of children or young adults exposed to other stressors (Allwood et al., Citation2011; Maruyama et al., Citation2012). In addition, no association has been found between sAA levels and the dental anxiety scale in adults (Sadi et al., Citation2013), while Nater et al. (Citation2007) reported no associations between self-reported anxiety and sAA levels. These results suggest that sAA may not represent a relevant biomarker of dental anxiety in children. However, more studies are required to define the meaning of the detected sAA increase in anticipation of dental treatment and its possible interplay with HPA axis arousal.
Our results extend previous findings on the relationship between salivary cortisol and anxiety for dental treatment and provide new evidence on the responsiveness of HPA and ANS to it. Inclusion of several variables with possible confounding effects in the analysis of results has helped detection of relevant associations between biological and emotional measures. Limitations of the study include the following: first, the lack of baseline measures for cortisol and sAA that did not allow the calculation of areas under the curve for these markers. Cortisol awakening response, which is informative for stress and mood state in adults, was not determined because this response is not always present in children at the age range of this study (Michels et al., Citation2012; Rosmalen et al., Citation2005). As an index of diurnal HPA axis variation, one morning sample, within the first 30 min from awakening, and an evening sample were collected. These samples are well in the range reported by previous studies in same-age children (Netherton et al., Citation2004; Tzortzi et al., Citation2009). Second, we have collected stimulated whole saliva, and we cannot confirm lack of chewing that could possibly modify sAA levels (DeCaro, Citation2008). Stressful stimuli also modify salivation and concomitantly saliva content. Stress-induced changes in the flow rate do not appear to affect salivary cortisol levels, whereas current literature on sAA levels is not conclusive, supporting either no effect (Rohleder et al., Citation2006; Sadi et al., Citation2013) or partial dependence on it (Bosch et al., Citation2003). Furthermore, it is not known whether saliva production due to oral manipulations can have a dilutional effect on its contents. Collection of unstimulated saliva along with measures of flow rate and inclusion of more sampling points during treatment would allow a more accurate determination of dental stress effect on sAA levels. Third, this was a cohort study and the potential for selection bias, information bias and confounding was present. The study sample included only children attending a university clinic, which may be different and less representative of the average population. Information bias is considered to be less likely, given that cortisol and sAA levels were measured in the laboratory. Residual confounding cannot be eliminated; however, efforts were made to decrease it by applying multivariable regression analyses.
Reliable bio-hormonal markers of children’s dental anxiety provide an objective approach to characterize children’s reactions. A better understanding of these responses and their relevant associations with subjective emotional inquiries could aid therapists to appropriately manage dental stress. These results could potentially help toward this direction.
Declaration of interest
All authors declare that have no conflicts of interest.
References
- Aartman IH, van Everdingen T, Hoogstraten J, Schuurs AH. (1998). Self-report measurements of dental anxiety and fear in children: a critical assessment. ASDC J Dent Child 65:252–8 and 229–30
- Ali N, Pruessner JC. (2012). The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiol Behav 106:65–72
- Allwood M, Handwerger K, Kivlighan K, Granger D, Stroud L. (2011). Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biol Psychol 88:57–64
- Arapostathis KN, Coolidge T, Emmanouil D, Kotsanos N. (2008). Reliability and validity of the Greek version of the Children’s Fear Survey Schedule-Dental Subscale. Int J Paediatr Dent 18:374–9
- Arhakis A, Menexes G, Coolidge T, Kalfas S. (2012). Heart rate, salivary α-amylase activity, and cooperative behavior in previously naïve children receiving dental local anesthesia. Pediatr Dent 34:225–30
- Bauer AM, Quas JA, Boyce WT. (2002). Associations between physiological reactivity and children’s behavior: advantages of a multisystem approach. J Dev Behav Pediatr 23:102–13
- Blomqvist M, Holmberg K, Lindblad F, Fernell E, Ek U, Dahllof G. (2007). Salivary cortisol levels and dental anxiety in children with attention deficit hyperactivity disorder. Eur J Oral Sci 115:1–6
- Bosch JA, de Geus EJ, Veerman EC, Hoogstraten J, Nieuw Amerongen AV. (2003). Innate secretory immunity in response to laboratory stressors that evoke distinct patterns of cardiac autonomic activity. Psychosom Med 65:245–58
- Bosch JA, Veerman EC, de Geus EJ, Proctor GB. (2011). α-Amylase as a reliable and convenient measure of sympathetic activity: don’t start salivating just yet! Psychoneuroendocrinology 36:449–53
- Boyce WT, Den Besten PK, Stamperdahl J, Zhan L, Jiang Y, Adler NE, Featherstone JD. (2010). Social inequalities in childhood dental caries: the convergent roles of stress, bacteria and disadvantage. Soc Sci Med 71:1644–52
- Cuthbert MI, Melamed BG. (1982). A screening device: children at risk for dental fears and management problems. ASDC J Dent Child 49:432–6
- DeCaro J. (2008). Methodological considerations in the use of salivary α-amylase as a stress marker in field research. Am J Hum Biol 20:617–9
- dos Santos MJ, Bernabé DG, Nakamune AC, Perri SH, de Aguiar SM, de Oliveira SH. (2012). Salivary alpha amylase and cortisol levels in children with global developmental delay and their relation with the expectation of dental care and behavior during the intervention. Res Dev Disabil 33:499–505
- Furlan NF, Gavião MB, Barbosa TS, Nicolau J, Castelo PM. (2012). Salivary cortisol, alpha-amylase and heart rate variation in response to dental treatment in children. J Clin Pediatr Dent 37:83–7
- Gordis EB, Granger DA, Susman EJ, Trickett PK. (2006). Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology 31:976–87
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis E, Stroud LR. (2007). Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann N Y Acad Sci 1098:122–44
- Hellhammer DH, Wüst S, Kudielka BM. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34:163–71
- Kambalimath HV, Dixit UB, Thyagi PS. (2010). Salivary cortisol response to psychological stress in children with early childhood caries. Indian J Dent Res 21:231–7
- Kandemir S, Okşan T, Alpöz AR, Ergezer G, Kabalak T. (1997). Salivary cortisol levels in children during dental treatment. J Marmara Univ Dent Fac 2:639–42
- Kirschbaum C, Hellhammer DH. (1994). Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 19:313–33
- Klingberg G, Berggren U, Carlsson SG, Noren JG. (1995). Child dental fear: cause-related factors and clinical effects. Eur J Oral Sc 103:405–12
- Klingberg G, Broberg A. (2007). Dental fear/anxiety and dental behaviour management problems in children and adolescents: a review of prevalence and concomitant psychological factors. Int J Paediatr Dent 17:391–406
- Knutsson U, Dahlgren J, Marcus C, Rosberg S, Bronnegard M, Stierna P, Albertsson-Wikland K. (1997). Circadian cortisol rhythms in healthy boys and girls: relationship with age, growth, body composition, and pubertal development. J Clin Endocrinol Metab 82:536–40
- Koch G. (1970). Selection and caries prophylaxis of children with high caries activity. Odontol Revy 21:71–81
- Maruyama Y, Kawano A, Okamoto S, Ando T, Ishitobi Y, Tanaka Y, Inoue A, et al. (2012). Differences in salivary alpha-amylase and cortisol responsiveness following exposure to electrical stimulation versus the Trier Social Stress Tests. PLoS One 7:e39375
- Michels N, Sioen I, De Vriendt T, Huybrechts I, Vanaelst B, De Henauw S. (2012). Children’s morning and evening salivary cortisol: pattern, instruction compliance and sampling confounders. Horm Res Paediatr 77:27–35
- Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, Ehlert U. (2005). Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int J Psychophysiol 55:333–42
- Nater UM, La Marca R, Florin L, Moses A, Langhans W, Koller MM, Ehlert U. (2006). Stress-induced changes in human salivary alpha-amylase activity – associations with adrenergic activity. Psychoneuroendocrinology 31:49–58
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. (2007). Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology 32:392–401
- Nater UM, Rohleder N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 34:486–96
- Netherton C, Goodyer I, Tamplin A, Herbert J. (2004). Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology 29:125–40
- Oulis CJ, Tsinidou K, Vadiakas G, Mamai-Homata E, Polychronopoulou A, Athanasouli T. (2012). Caries prevalence of 5, 12 and 15-year-old Greek children: a national pathfinder survey. Community Dent Health 29:29–32
- Psychountaki M, Zervas Y, Karteroliotis K, Spielberger C. (2003). Reliability and validity of the Greek version of the STAIC. Eur J Psychol Assess 19:124–30
- Rai K, Hegde AM, Shetty S, Shetty S. (2010). Estimation of salivary cortisol in children with rampant caries. J Clin Pediatr Dent 34:249–52
- Rodrigues Gomes SS, Barretobezerra AC, Maia Prado AC. (2013). Salivary biomarkers, vital signs and behaviour of pre-school children during their first dental visit. Eur J Paediatr Dent 14:279–83
- Rohleder N, Wolf JM, Maldonado EF, Kirschbaum C. (2006). The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology 43:645–52
- Rohleder N, Nater UM. (2009). Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology 34:369–485
- Rosmalen JG, Oldehinkel AJ, Ormel J, de Winter AF, Buitelaar JK, Verhulst FC. (2005). Determinants of salivary cortisol levels in 10–12 year old children: a population-based study of individual differences. Psychoneuroendocrinology 30:483–95
- Sadi H, Finkelman M, Rosenberg M. (2013). Salivary cortisol, salivary alpha amylase, and the dental anxiety scale. Anesth Prog 60:46–53
- Sakuma N, Nagasaka N. (1996). Changes in urinary excretion of catecholamines and their metabolites in pediatric dental patients. ASDC J Dent Child 63:118–22
- Spielberger CD, Auerbach SM, Wadsworth AP, Dunn TM, Taulbee ES. (1973). Emotional reactions to surgery. J Consult Clin Psychol 40:33–8
- Strahler J, Mueller A, Rosenloecher F, Kirschbaum C, Rohleder N. (2010). Salivary a-amylase stress reactivity across different age groups. Psychophysiology 47:587–95
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. (2009). Stress response and the adolescent transition: performance versus peer rejection stressors. Dev Psychopathol 21:47–68
- Ten Berge M, Veerkamp JSJ, Hoogstraten J, Prins PJM. (1998). The Dental Subscale of the Children’s Fear Survey Schedule: a factor analytic study in the Netherlands. Community Dent Oral Epidemiol 26:340–3
- Ten Berge M, Veerkamp JSJ, Hoogstraten J, Prins PJM. (2002). Childhood dental fear in the Netherlands: prevalence and normative data. Community Dent Oral Epidemiol 30:101–7
- Tzortzi Ch, Proff P, Redlich M, Aframian DJ, Palmon A, Golan I, Muessig D, et al. (2009). Cortisol daily rhythm in saliva of healthy school children. Int Dent J 59:12–18
- Vanderas AP, Manetas C, Papagiannoulis L. (1995). Urinary catecholamine levels in children with and without dental caries. J Dent Res 74:1671–8
- Vanderas AP, Papagiannoulis L. (1997). Urinary catecholamine levels and dentofacial injuries in children. Endod Dent Traumatol 13:238–44
- Wogelius P, Poulsen S, Sørensen HT. (2003). Prevalence of dental anxiety and behavior management problems among six to eight years old Danish children. Acta Odontol Scand 61:178–83
- Woodside DB, Winter K, Fisman S. (1991). Salivary cortisol in children: correlations with serum values and effect of psychotropic drug administration. Can J Psychiatry 36:746–8