Abstract
We investigated whether children’s performance on working memory (WM) and delayed retrieval (DR) tasks decreased after stress exposure, and how physiological stress responses related to performance under stress. About 158 children (83 girls; Mage = 10.61 years, SD = 0.52) performed two WM tasks (WM forward and WM backward) and a DR memory task first during a control condition, and 1 week later during a stress challenge. Salivary alpha-amylase (sAA) and cortisol were assessed during the challenge. Only WM backward performance declined over conditions. Correlations between physiological stress responses and performance within the stress challenge were present only for WM forward and DR. For WM forward, higher cortisol responses were related to better performance. For DR, there was an inverted U-shape relation between cortisol responses and performance, as well as a cortisol × sAA interaction, with concurrent high or low responses related to optimal performance. This emphasizes the importance of including curvilinear and interaction effects when relating physiology to memory.
Introduction
At school, children are confronted with diverse stressors, which raises the question how stress may affect school performance. Two memory processes crucial to school performance are working memory (WM) and the retrieval of previously learned information. Although stress effects on these processes have been investigated in adults, research in children is limited, despite the high societal relevance of such knowledge. The current study sought to increase our understanding of the effects of stress on children’s WM and retrieval.
Declarative long term memory refers to “the explicit storage of facts and events, which can later be intentionally retrieved” (Wolf, Citation2007). This retrieval is also called delayed retrieval (DR). In adults, high glucocorticoid levels during DR impaired memory for stressor-unrelated information (Tollenaar et al., Citation2008). Rodent studies indicate that this results from noradrenergic activity in the basolateral amygdala interacting with the hippocampus and prefrontal cortex (Roozendaal, Citation2002), suggesting that concurrent glucocorticoid (HPA axis) and noradrenergic (SNS) activation are required for memory impairment. Findings in human adults support this hypothesis, as glucocorticoid effects on memory are most pronounced for emotional stimuli (Smeets et al., Citation2008), or under emotionally arousing conditions (Kuhlmann & Wolf, Citation2006). Taken together, this indicates that concurrent HPA axis and SNS activation reduces declarative memory retrieval capacities.
WM refers to the temporary storage and manipulation of task-related information (Baddeley, Citation1992). WM can be assessed with digit span tasks, where forward tasks (WMfw) measure passive short term storage (Quesada et al., Citation2012) involving differential activation of the inferior frontal gyrus (BA 44/45; Sun et al., Citation2005), and backward tasks (WMbw) measure executive functioning (Quesada et al., Citation2012) involving differential activation of the left PFC (BA 9) and left occipital visual cortex (Sun et al., Citation2005).
Findings regarding stress effects on WM in human adults are inconsistent. Some studies found decreased performance for WMfw only (Elzinga & Roelofs, Citation2005) or WMbw only (Schoofs et al., Citation2009), whereas others reported no effects at all (Smeets et al., Citation2006), or even enhanced performance (Lewis et al., Citation2008). A rodent study suggests that noradrenergic activity in the amygdala enables glucocorticoid effects in the prefrontal cortex on WM (Roozendaal et al., Citation2004). In line with this, Elzinga and Roelofs (Citation2005) found in adult humans that impairments in WM were only present when both cortisol levels and adrenergic activity were elevated. This suggests that stress effects on WM also depend on concurrent HPA axis and SNS activation.
Hippocampus, amygdala and prefrontal cortex activation are involved in DR and WM changes over age (Casey et al., Citation2000), as does the susceptibility of the prefrontal cortex to glucocorticoids (Perlman et al., Citation2007). Therefore, stress effects on memory might differ in children versus adults. To date, research in children has focused on stress effects during memory encoding (Quas et al., Citation2012) and did not assess the interaction effect of HPA axis and SNS activation (Quesada et al., Citation2012).
This study investigated the interactive effects of the HPA axis and the SNS on both DR and WM. We hypothesized WM and DR to be worse under stress than in a control condition, and HPA axis activation to lead to worse memory performance only if SNS activation is also high. As there are indications that glucocorticoid effects on memory follow an inverted U-shape [Lupien & McEwen, Citation1997; known as the Yerkes–Dodson law (Yerkes & Dodson, Citation1908)], we also hypothesized memory performance to be best in children with intermediate HPA axis reactivity.
Method
Participants
Children (aged 9–11) were recruited through 31 general education primary schools in Nijmegen and surrounding areas (The Netherlands) for participation in a study on different aspects of responses to stress and their consequences for cognitive functioning. Exclusion criteria were stuttering, a diagnosis of a developmental disorder, and the use of psychotropic or centrally acting corticosteroid medication. Recruitment (for details, refer de Veld et al., Citation2012) resulted in 165 participants. Five children were excluded because they did not complete the entire data collection protocol, and two children were excluded when subsequently discovered to meet one of the exclusion criteria (during testing). Thus, the final sample for this study consisted of 158 children (83 girls; Mage = 10.61 years, SD = 0.52). The majority of the participants was Caucasian (94%), and had at least one parent with a college or university degree (79%). Two participants were excluded from the analyses relating physiological stress responses and memory performance within the stress condition due to missing sAA data for baseline (S1 and S2; n = 1) or S3 (n = 1).
This study was approved by the ethics committee of the Faculty of Social Sciences of the Radboud University Nijmegen, The Netherlands. The children participated in this study voluntarily, and all parents provided written informed consent prior to their child’s participation.
Procedure
The study used a within subjects design, with all children performing a WM task and a DR memory task first in a control condition in a mobile lab at home, and approximately 1 week later in a stress condition in the laboratory of the Behavioural Science Institute of the Radboud University Nijmegen. For an overview of the procedures in the control and stress condition, refer . All testing took place after school (Md = 15:45 h, IQR = 14:11–16:03 h).
Figure 1. Overview of the procedures for the control condition (top), and the stress condition (bottom). Intro = introduction; Sq = short questionnaire; Qs = questionnaires; C = control condition saliva sample; S = stress condition saliva sample. The shaded areas in the stress condition indicate the presence of the TSST-C jury.
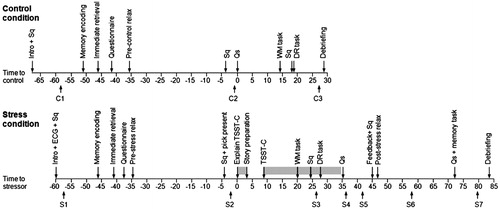
Control condition
During the control condition at home, all testing took place in a mobile lab (van parked in front of the home). After a short introduction in which children were told that they would be asked to do some tasks and fill out several questionnaires, children practiced providing a saliva sample (C1), and filled out a short questionnaire. This was followed by the encoding phase of the DR memory task (see “Instruments and measures” section), immediate retrieval for this task and a questionnaire. Then, a 30-min relaxation period commenced, during which children could read magazines or work on puzzles while listening to relaxing music. After relaxation, they filled out a short questionnaire, provided a saliva sample (C2), and filled out two other questionnaires. Then they performed the WM task (see “Instruments and measures” section), filled out a short questionnaire and performed delayed retrieval for the DR memory task (see “Instruments and measures” section). The procedure ended with a last saliva sample (C3). The entire procedure took approximately 1.5 h.
Stress condition
During the lab visit, children first received a short introduction. Thereafter, they provided a saliva sample (S1) and completed a short questionnaire. This was followed by the encoding phase of the DR memory task (see “Instruments and measures” section), immediate retrieval for this task and a questionnaire. This was followed by a 30-min relaxation period during which children could read a magazine or work on puzzles, while listening to relaxing music. Right after relaxation, they filled out a short questionnaire, and provided a second saliva sample (S2). After this, children were led to an adjacent room where a stress task was administered (adapted and extended TSST-C; Buske-Kirschbaum et al., Citation1997). The TSST-C consists of a public speaking task in which children provide the ending to a story, and perform a mental arithmetic task in which they count backwards from 758 to zero by repeatedly subtracting seven from the most recently acquired number. During both tasks, a jury of two confederates in white lab coats watches the child perform. In this study, before starting the TSST-C children had been asked to pick a favorite and least preferred present out of six small items (e.g. an inflatable ball or toilet brush) right before entering the TSST-C room (Jones et al., Citation2006), and had been told that a favorable judgment by the jury would earn them their favorite present, whereas in case of an unfavorable judement they would get the least preferred present. After the TSST-C, children were seated in front of the TSST-C jury, and were joined by the experimenter. The experimenter then conducted the WM task (see “Instruments and measures” section), asked children to supply a saliva sample (S3) and fill out a short questionnaire, and conducted the DR memory task (see “Instruments and measures” section). The stress task lasted approximately 34 min. Afterwards, the children were escorted back to the first room, where they provided another saliva sample (S4), and completed two questionnaires. This was followed by a fifth saliva sample (S5), the completion of another questionnaire, positive feedback on their performance during the stress task and a short questionnaire. Then, a 25-min post-stress relaxation period was initiated. Ten minutes into this relaxation period, a saliva sample was obtained (S6). After relaxation, children completed several questionnaires, performed a memory task, provided a last saliva sample (S7), and completed a last questionnaire. The entire procedure took approximately 2.5 h.
Instruments and measures
Delayed retrieval memory task
To fit the purpose of the current study, a new DR memory task was devised based on materials from De Deyne et al. (Citation2008b). For the encoding phase of the DR memory task, children were seated in front of the black screen of a laptop and listened to a pre-recorded short story played on the laptop (see Appendix). Parts of this story contained five word categories, with eight exemplars each. Upon hearing a category in the story (e.g. professions), this category’s name appeared in yellow capital letters on the black laptop screen. Upon hearing an exemplar (e.g. pilot, dentist), this exemplar appeared in white lowercase letters underneath the category name. Exemplars were presented on screen for 4 s each; the category name stayed on screen until all exemplars of that category had been presented. The order in which categories were presented within the task was fixed; the order in which exemplars were presented within each category was randomized across participants.
Right before the encoding phase of the DR memory task, the experimenter had outlined the stimulus presentation to the children, and had instructed them to do their best to remember as many of the presented words as possible. Children had also been told that the experimenter would ask them to name as many of the words of one of the categories as possible later during the procedure. When a child indicated that he/she had understood the nature of the task, the experimenter started the encoding phase.
To allow for comparison of memory performance over the two conditions, we constructed two versions of the memory task (version A and B). Task order over conditions was counterbalanced across participants. Categories and exemplars were derived from De Deyne et al. (Citation2008a,Citationb). Words in version A and B were matched on typicality, goodness of example of category, exemplar generation frequency, estimated age of acquisition, familiarity and imageability according to the norms presented in De Deyne et al. (Citation2008a).
DR memory performance was assessed by asking children to name as many exemplars of a randomly selected category as possible in 2 min. If a child indicated not to remember any more words within those in 2 min, he or she was told that there was still time to think. Memory performance was defined as the number of correctly retrieved exemplars of the tested category. This could result in a score between 0 and 8.
Working memory task
Working memory was assessed with a digit span test based on that from the Wechsler Intelligence Scale for Children (Wechsler, Citation1991). Again a version A and B were constructed, the order of which was counterbalanced across participants. In the digit span test, digit sequences of increasing length are presented, with two trials for each sequence length. In the forward condition, indicating passive storage, digits are to be repeated in the order presented. In the backward condition, indicating executive functioning, digits are to be repeated in reversed order. If responses to both trials of a particular sequence length are incorrect, the current condition is terminated. One point is given for each correct answer. Participants’ performance in each condition was determined by summing all points received in that condition. This could result in a score between 0 and 16 in the forward condition, and between 0 and 14 in the backward condition. Because WM forward (WMfw) and backward (WMbw) have been argued to assess different memory processes (Reynolds, Citation1997), and data in adults suggests different underlying neural mechanisms (Sun et al., Citation2005), the two subtests were analyzed separately.
Cortisol and sAA
To obtain reliable saliva samples, participants were asked to only drink water in the 2 h before participation, to limit physical exercise in the hour prior to participation and to abstain from meals at least 45 min before participation. These instructions were identical for the control and the stress conditions.
The sampling procedure was as follows. Participants swallowed all saliva to empty their mouths, and collected all subsequently secreted saliva in their mouths for 2 min, after which they used a short straw to spit the saliva into a small tube. This procedure was repeated until at least 0.25 ml of saliva was collected, with a maximum total collection time of 5 min. The procedure was identical for the control and the stress conditions.
During the control condition, three saliva samples were obtained, namely, at −55 (C1), −1 (C2) and 27 (C3) min from the onset of the control task. During the stress condition, seven saliva samples were obtained, namely, at −57 (S1), −2 (S2), 26 (S3), 36 (S4), 42 (S5), 58 (S6) and 80 (S7) min from the onset of the stressor. Timing of samples C2 and C3 in the control condition corresponded to the timing of samples S2 and S3 in the stress condition. Due to practical constraints, we analyzed saliva samples during the control condition in a subsample of n = 53 participants.
All samples were kept frozen at −20 °C until their shipment to the analysis lab.
Cortisol concentrations were determined at the Endocrinology Laboratory of the University Medical Center Utrecht, with an in-house competitive radio-immunoassay uses a polyclonal anticortisol-antibody (K7348). [1,2-3H(N)]-Hydrocortisone (Amersham Biosciences Limited, Amersham, UK; TRK407) was used as a tracer. The lower limit of detection was 1 nmol/l, and inter-assay and intra-assay variations were below 10%.
sAA concentrations were determined from the same saliva samples that were used to determine cortisol concentrations. Analysis was performed at the Endocrinology Laboratory of the University Medical Center Utrecht. Alpha amylase was measured on the D × I analyzer (Beckman Coulter Inc., Fullerton, CA). Saliva samples were diluted 500× with 0.2% BSA in 0.01 M Phosphate buffer pH 7.0. Inter-assay variation was <2.2%.
All physiological data were screened for outliers, which were defined within each assessment point as values greater than 3 SD above the mean. On the assessment points relevant to the current study, there were 11 outliers out of a total of 580 data points for cortisol (S1: 3; S2: 3; S3: 5), and 14 outliers out of a total of 576 data points for sAA (C1: 1; C2: 1; S1: 4; S2: 4; S3: 4). All outliers were winsorizedFootnote1 by replacing their values with the value of 3 SD above the mean (Tukey, Citation1977). For the manipulation check (see “Results” section) C2 and S2 served as pre-task measurement, and C3 and S3 as post-task measurement.
To determine children’s physiological responses to the stress task at the time of the WM and DR task in the stress condition, we first determined a baseline value for cortisol and sAA by selecting the lowest pre-stress value for each participant from S1 (n = 17 for cortisol, 27 for sAA) and S2 (n = 139 for cortisol, 129 for sAA). Then, a delta increase was computed by subtracting this baseline value from the value at S3.Footnote2
Potential confounders
Participant’s stage of pubertal development was assessed through a self-report on a five-point scale using Tanner criteria (breast development and pubic hair for girls, genital development and pubic hair for boys; Marshall & Tanner, Citation1969, Citation1970), with higher scores indicating more advanced physical development.
Parental education level was assessed for both parents on an eight-point scale (1 = primary education, 8 = university degree). Values for both parents were averaged to obtain a single score for analysis.
Statistical analyses
Square root (sqrt; for WMfw, sAA values, sAA reactivity, puberty) and logarithm (log10; for cortisol values, cortisol reactivity, parental education level, time between encoding and retrieval) transformations were applied to normalize variables for which the Kolmogorov–Smirnov test indicated deviation from a normal distribution.
To check whether our control condition was indeed non-stressful, and our stress condition induced a cortisol and sAA response, we conducted a repeated measures MANOVA (n = 53) with Condition (control versus stress) and Time (pre- versus post-control/stressor) as within subject factors and cortisol and sAA values as outcomes.
To examine the effect of stress on memory performance, we performed a repeated measures MANOVA (n = 158) with Condition (control versus stress) as a within subject factor and WMfw, WMbw and DR as outcome variables.
To examine whether the strength of children’s physiological responses in the stress condition was related to their memory performance in the stress condition, we performed two hierarchical regression analyses (n = 156) for each dependent variable (WMfw, WMbw and DR). Variables used in interaction terms were centered prior to their inclusion. In the first model, all possible confounders (sex, age, parental education and pubertal stage) and predictors were entered in separate steps. These first models are presented in a footnote to the tables with the final models (see “Results” section). For the second (final) model, only variables that individually explained at least 1% of variance in the first model [calculated as (part correlation)2 × 100] were retained to eliminate irrelevant confounders and increase power. Significant interaction and quadratic effects were plotted based on Aiken & West (Citation1991).
Results
Preliminary analyses
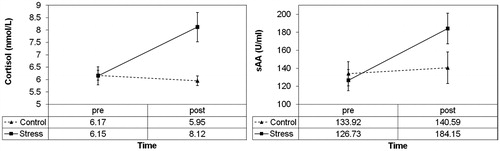
Raw cortisol and sAA values for the stress condition are presented in . Descriptives of study variables and correlations between stress condition memory variables, physiological variables and confounders are presented in .
Table 1. Raw cortisol and sAA levels for the stress condition.
Table 2. Descriptives and correlations for the study variables.
A Mann–Whitney U test reveals that the children from whom control condition saliva was analyzed (n = 53) were slightly younger (Md = 10.3) than the others (Md = 10.8), U = 1349.5, p < 0.01. No significant differences were found for the distribution of boys/girls, parental education level, puberty, stress condition cortisol reactivity and stress condition sAA reactivity.
A Wilcoxon Signed Rank Test reveals that the time between encoding and DR was shorter in the control condition (Md = 64 min) than in the stress condition (Md = 69 min), z = −7.62, p < 0.001. To test whether this timing difference was related to the DR performance difference between conditions, we calculated the difference in timing between conditions and the difference in DR performance between conditions for each participant separately, and then correlated these difference scores. This correlation was not significant (r = 0.04, p = 0.64), indicating that any difference between DR performance between conditions was unrelated to differences in time between encoding and retrieval.
Manipulation check
We first checked whether our control condition was indeed non-stressful, and our stress condition induced a cortisol and sAA response. A repeated measures MANOVA with Condition (control versus stress) and Time (pre- versus post- control/stressor) as within subject factors and cortisol and sAA values as outcomes showed a significant multivariate Condition × Time interaction, Wilks’ Lambda = 0.56, F (2, 51) = 20.21, p < 0.001, multivariate partial eta squared = 0.44. Univariate tests showed a significant Condition ×Time interaction for both cortisol, F (1, 52) = 27.70, p < 0.001, partial eta squared = 0.35, and sAA, F (1, 52) = 21.34, p < 0.001, partial eta squared = 0.29. Post-hoc Bonferroni-corrected paired-samples t-tests showed that this effect was the result of stable control condition levels for both cortisol, t (52) = 1.49, p = 0.14, and sAA, t (52) = −0.14, p = 0.89, while in the stress condition there was an increase in both cortisol, t (52) = −4.90, p < 0.001, and sAA t (52) = −5.91, p < 0.001 (). This indicates that each condition worked as intended.
Effects of stress on memory performance
Next we examined the effect of stress on memory performance, using repeated measures MANOVA with Condition (control versus stress) as a within subject factor and WMfw, WMbw and DR as outcome variables. There was a significant multivariate effect of condition, Wilks’ Lambda = 0.94, F (3, 155) = 3.12, p < 0.05, multivariate partial eta squared = 0.06. Univariate tests show a significant effect of condition for WMbw, F (1, 157) = 4.93, p < 0.05, partial eta squared = 0.03. These results were due to lower memory scores in the stress condition versus the control condition (). There were no effects of condition for DR, F (1, 157) = 3.51, p = 0.06, and WMfw, F (1, 157) = 0.67, p = 0.41.
Relation between physiological stress responses and memory performance within the stress condition
Next, we used hierarchical regression analyses to examine whether within the stress condition children’s physiological stress responses were related to their performance on the different memory tasks.Footnote3 The final regression model for WMfw was significant, and is summarized in . There was a significant linear effect of cortisol, such that a stronger cortisol response was related to better WMfw performance.
Table 3. Final regression models for the prediction of WM forward and DR in the stress condition.
The final regression model for WMbw was also significant, F (7,148) = 11.78, p < 0.001. In this case, however, all coefficients for cortisol and sAA variables were non-significant.
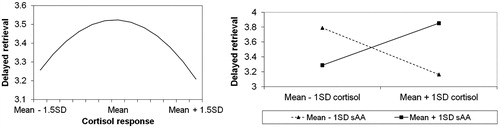
The final regression model for DR was also significant, and is summarized in . There was a significant quadratic effect of cortisol, which is depicted in (left), indicating that children with relatively small and large cortisol responses had poorer DR performance in the stress condition than children with intermediate cortisol responses. In addition, there was a significant sAA × cortisol interaction effect, which is shown in (right). When the sAA response to the stress task is small, larger cortisol responses are related to worse DR performance. When the sAA response to the stress task is large, larger cortisol responses are related to better DR performance.
Figure 3. The relation between stress condition cortisol responses and delayed retrieval memory performance. There was a significant quadratic relation (left panel), as well as a significant interaction between sAA and cortisol (right panel). Note: average DR memory performance in the control condition was 3.5.
To test whether the quadratic effect of cortisol was further moderated by sAA, we performed an additional regression analysis that included the cortisol × cortisol sAA interaction. Although the model as a whole was significant, F (7, 148) = 4.17, p < 0.001, the coefficient for the interaction was not. Thus, the quadratic effect of cortisol was not moderated by sAA.
Discussion
Effects of stress on memory performance
The expected memory performance decline under stress versus control was found only for WMbw, not for WMfw or DR. However, within the stress condition, cortisol and sAA responses were unrelated to WMbw performance, raising the question whether this performance decline resulted directly from increased HPA-axis and SNS activation in the stress condition. An alternative explanation could be that children’s attempts to regulate their emotional responses to the stress task decreased their task performance. Cognitive reappraisal, a strategy that involves reinterpreting a situation such that the emotional impact of the situation is changed (Gross, Citation1998), may be particularly interesting in this regard. Emotion regulation in general has been argued to be an aspect of executive functioning (Zelazo & Cunningham, Citation2007), and adult fMRI studies show that reappraisal activates brain regions that are also involved in working memory and executive functioning (Ochsner & Gross, Citation2008). As WMbw requires executive functioning to manipulate the stored material, it could be speculated that as children engaged in reappraisal during stress, their WMbw capacity decreased. As we measured reappraisal use during the stress task in the current sample to answer a different research question (de Veld et al., Citation2012), we were able to perform post-hoc analyses that showed that higher self-reported use of reappraisal during the stress task was related to lower WMbws scores.Footnote4 As WMfw merely consists of passive storage, this aspect of WM would remain unaffected, as evidenced by the absence of a correlation between reappraisal use and WMfw scores in the post-hoc analysis.
We did not find a decrease in WMfw and DR performance in the stress condition versus the control condition. For WMfw, this may have been the result of the limited range in scores on the WMfw task. Although the median and interquartile range in scores was the same for WMfw and WMbw, the overall range was smaller for WMfw (4–13 versus 2–14 for WMbw). This might explain a general effect of condition for WMfw. In general, the absence of a condition effect for both WMfw and DR might be the consequence of the relatively small average cortisol stress response in the current study: a median increase of 1.1 nmol/l, versus an average increase of approximately 10 nmol/l in Quesada et al. (Citation2012) and 4 nmol/l in Buske-Kirschbaum et al. (Citation1997). We speculate that larger increases are necessary for effects of stress versus control condition performance to emerge.
Relation between physiological stress esponses and memory performance within the stress condition
Although children did not show a significant decrease in WMfw performance across conditions, we did find a significant correlation between cortisol stress reactivity and WMfw performance. Contrary to our hypothesis, however, this correlation was positive: higher reactivity was related to better performance. One possible interpretation is that this finding results from a combination of two factors: (1) the Yerkes–Dodson phenomenon (Yerkes & Dodson, Citation1908). This is the idea that optimal performance occurs at some optimal stress level. Here, it would result in an inverted U-shape relation between glucocorticoids and memory in which both low and high levels are associated with worse performance, whereas intermediate levels are associated with optimal performance (Lupien & McEwen, Citation1997), and (2) relatively small cortisol increases in the current sample. This combination could have led to a pattern of results in which participants’ scores all fell on the left side of the inverted U, thus resulting in the appearance of a positive linear relation. This would also imply that in studies where cortisol responses are stronger, findings should shift to a curvilinear or negative linear relation between WMfw and cortisol reactivity.
For DR, the results were more complex. We found a quadratic effect of cortisol that indicated that intermediate cortisol responses were related to optimal DR performance. Such an inverted U-shape relation between cortisol and memory performance is consistent with the Yerkes–Dodson law (Yerkes & Dodson, Citation1908) and has been found both in the context of memory consolidation (in men only; Andreano & Cahill, Citation2006), and memory retrieval (Domes et al., Citation2005). In addition, we found that the linear correlation between cortisol responses and DR differed according to the child’s sAA response: for children with a high sAA response, higher cortisol responses were associated with better DR performance, whereas for children with a low sAA response, lower cortisol responses were associated with better DR performance. In other words, DR performance was optimal when there was concurrent activation or deactivation of the SNS and HPA-axis. At first glance, these two results may appear contradictory. However, as our regression analysis controlled for the linear main effect of sAA, the quadratic effect of cortisol was tested at mean levels of sAA reactivity. As such, it indicates that DR performance was optimal under conditions of concurrent average activation of the SNS and HPA-axis. Therefore, both effects found for DR indicate that children’s performance was optimal when the stress responses of the SNS and HPA-axis were of similar magnitude. Based on hypotheses that one of the functions of HPA axis reactivity to stress is suppressing stress-induced SNS activation (Sapolsky et al., Citation2000), it can be speculated such concurrent (de)activation is indicative of a well-coordinated stress system that prevents adverse outcomes on performance. This would be in line with a study by Quas et al. (Citation2012), where concurrent HPA-axis and SNS activation during encoding and consolidation were associated with better memory for a stressful event. These results signify the importance of incorporating interactions between HPA axis and SNS responses when investigating stress effects on memory.
Limitations and future directions
Some limitations to our study should be acknowledged. The manipulation check was performed on a subsample that was slightly younger than the remaining participants. It is therefore possible that the difference in physiological responses to the control versus stress condition differed for this latter group. This seems unlikely however, as subsample participants’ cortisol and sAA stress responses were similar to those for the remaining participants.
Findings regarding the memory performance differences over conditions are limited by the fact that the condition order was not counterbalanced. This leaves room for alternative interpretations like motivational changes, interference effects and practice effects. Practice effects potentially contributed to WMfw and DR performance stability between conditions, as task familiarity might have undone any stress-induced performance decline. In addition, slight differences between conditions with regard to time between encoding and retrieval, type and duration of the control/stress task and timing of C3 versus S3 could have decreased the strength of our stress manipulation. Future research would benefit from a more comparable control and stress condition. It should be stressed, however, that these limitations do not apply to our findings regarding the relations between physiological stress responses and memory performance within the stress condition.
The results of this study help uncover developmental processes by adding much needed information on stress responses and their relation to memory in middle childhood. However, lacking the inclusion of multiple age groups or a longitudinal design, we cannot draw definitive conclusions about the developmental changes in the effects of stress on memory. Therefore, future research would benefit from a cross-sectional or longitudinal design.
Conclusion
The current study showed that physiological responses to a stress task were related to children’s WMfw and DR performance under stress. The decline in WMbw over conditions, without a relation between physiological stress responses and WMbw under stress, inspires further research into factors such as emotion regulation strategies, that might contribute to adverse effects of stress on cognitive functioning. The relations found between physiological stress responses and WMfw and DR emphasize the importance of including curvilinear and interaction effects in models relating memory and physiology. In addition, our results indicate that during a mild stressor, children’s DR performance is optimal under conditions of concurrent (de)activation of the SNS and HPA-axis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We thank the children who kindly participated in this study, their parents and all research assistants and students who assisted with data collection.
Notes
1Analyzing the data without participants whose cortisol and/or sAA values had been winsorized yielded comparable results to those presented in the manuscript.
2It is important to note that although S3 was chosen to compute delta increase, cortisol and sAA levels were still elevated at S4, indicating that cortisol and sAA values were elevated throughout memory testing.
3Similar analyses performed for the control condition (n = 53) yielded no significant results.
4Reappraisal was measured with a Dutch version of the Emotion Regulation Questionnaire (Gross & John, Citation2003, Dutch version by Koole, Citation2004) that was adapted to a state measure to be used with children (for details, see de Veld et al., Citation2012). The Pearson correlation between sqrt WMfw and reappraisal was significant (r = −0.17, p < 0.05), and indicated that more use of reappraisal was related to worse WMbw performance.
References
- Aiken LS, West SG. (1991). Multiple regression: testing and interpreting interactions. Newberry Park, CA: Sage
- Andreano JM, Cahill L. (2006). Glucocorticoid release and memory consolidation in men and women. Psychol Sci 17:466–70
- Baddeley A. (1992). Working memory. Science 255:556–9
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med 59:419–26
- Casey BJ, Giedd JN, Thomas KM. (2000). Structural and functional brain development and its relation to cognitive development. Biol Psychol 54:241–57
- De Deyne S, Verheyen S, Ameel EEF, Vanpaemel W, Dry MJ, Voorspoels W, Storms G. (2008a). DeDeyne2-BRM-2008.zip, available at http://brm.psychonomic-journals.org/content/40/4/1030/suppl/DC1 (accessed 26 February 2009)
- De Deyne S, Verheyen S, Ameel EEF, Vanpaemel W, Dry MJ, Voorspoels W, Storms G. (2008b). Exemplar by feature applicability matrices and other Dutch normative data for semantic concepts. Behav Res Methods 40:1030–48
- de Veld DMJ, Riksen-Walraven JM, de Weerth C. (2012). The relation between emotion regulation strategies and physiological stress responses in middle childhood. Psychoneuroendocrinology 37:1309–19
- Domes G, Rothfischer J, Reichwald U, Hautzinger M. (2005). Inverted-U function between salivary cortisol and retrieval of verbal memory after hydrocortisone treatment. Behav Neurosci 119(2):512–17
- Elzinga BM, Roelofs K. (2005). Cortisol-induced impairments of working memory require acute sympathetic activation. Behav Neurosci 119:98–103
- Gross JJ. (1998). Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J Pers Soc Psychol 74:224–37
- Gross JJ, John OP. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 85:348–62
- Jones A, Godfrey KM, Wood P, Osmond C, Goulden P, Phillips DIW. (2006). Fetal growth and the adrenocortical response to psychological stress. J Clin Endocrinol Metab 91:1868–71
- Koole SL. (2004). Volitional shielding of the self: effects of action orientation and external demands on implicit self-evaluation. Soc Cogn 22:100–25
- Kuhlmann S, Wolf OT. (2006). A non-arousing test situation abolishes the impairing effects of cortisol on delayed memory retrieval in healthy women. Neurosci Lett 399:268–72
- Lewis RS, Nikolova A, Chang DJ, Weekes NY. (2008). Examination stress and components of working memory. Stress 11:108–14
- Lupien SJ, McEwen BS. (1997). The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev 24:1–27
- Marshall WA, Tanner JM. (1969). Variations in pattern of pubertal changes in girls. Arch Dis Childh 44:291–303
- Marshall WA, Tanner JM. (1970). Variations in the pattern of pubertal changes in boys. Arch Dis Childh 45:13–23
- Ochsner KN, Gross JJ. (2008). Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Curr Direct Psychol Sci 17:153–8
- Perlman WR, Webster MJ, Herman MM, Kleinman JE, Weickert CS. (2007). Age-related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiol Aging 28:447–58
- Quas JA, Yim IS, Rush E, Sumaroka M. (2012). Hypothalamic pituitary adrenal axis and sympathetic activation: joint predictors of memory in children, adolescents, and adults. Biol Psychol 89:335–41
- Quesada AA, Wiemers US, Schoofs D, Wolf OT. (2012). Psychosocial stress exposure impairs memory retrieval in children. Psychoneuroendocrinology 37:125–36
- Reynolds CR. (1997). Forward and backward memory span should not be combined for clinical analysis. Arch Clin Neuropsychol 12:29–40
- Roozendaal B. (2002). Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 78:578–95
- Roozendaal B, McReynolds JR, McGaugh JL. (2004). The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci 24:1385–92
- Sapolsky RM, Romero LM, Munck AU. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89
- Schoofs D, Wolf OT, Smeets T. (2009). Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav Neurosci 123:1066–75
- Smeets T, Jelicic M, Merckelbach H. (2006). The effect of acute stress on memory depends on word valence. Int J Psychophysiol 62:30–7
- Smeets T, Otgaar H, Candel I, Wolf OT. (2008). True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology 33:1378–86
- Sun X, Zhang X, Chen X, Zhang P, Bao M, Zhang D, Chen J, et al. (2005). Age-dependent brain activation during forward and backward digit recall revealed by fMRI. Neuroimage 26:36–47
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd WAM. (2008). The effects of cortisol increase on long-term memory retrieval during and after acute psychosocial stress. Acta Psychol 127:542–52
- Tukey JW. (1977). Exploratory data analysis. Reading, MA: Addison Wesley
- Wechsler D. (1991). The Wechsler intelligence scale for children. 3rd ed. San Antonio, TX: Psychological Corporation
- Wolf OT. (2007). Glucocorticoid effects on memory: the positive and the negative. In: Fink G, editor. Encyclopedia of stress. 2nd ed. New York: Academic Press. p 166–71
- Yerkes RM, Dodson JD. (1908). The relation of strength of stimulus to rapidity of habit formation. J Comp Neurol Psychol 18:31–9
- Zelazo PD, Cunningham WA. (2007). Executive function: mechanisms underlying emotion regulation. In: Gross JJ, editor. Handbook on emotion regulation. 1st ed. New York, NY: Guilford
Appendix
English translations of the Dutch stories used during the memory encoding phase.
Version A
Today was a busy day at school. First, we spoke about what we would like to be when we grow up. There were a lot of different PROFESSIONS
actor; lawyer; fire fighter; vet; pilot; butcher; dentist; dustman
After that, we went to the school’s garden. On our way, there we saw a lot of BIRDS
eagle; magpie; vulture; tit; falcon; pelican; heron; woodpecker
In the school’s garden, we checked up on our VEGETABLES
eggplant; beet; zucchini; watercress; leek; tomato; chicory; sprouts
Afterwards, we went back to school for gym class. We could choose from different SPORTS
ballet; boxing; rugby; running; horseback riding; table tennis; gymnastics; volleyball
On our way back from school, we saw a lot of different VEHICLES
jeep; tram; truck; scooter; helicopter; caravan; tractor; moped
We had a fun day. THE END
Version B
Today there was a neighborhood party. It started with a treasure hunt in which people had dressed up. Some were dressed as INSECTS
cricket; dragonfly; fruit fly; beetle; cockroach; caterpillar; butterfly; woodlouse
There were also other animals. Some were dressed as FISH
swordfish; trout; herring; carp; eel; piranha; pike; stickleback
After the treasure hunt we went to eat something, namely FRUIT
apricot; fig; melon; strawberry; pumpkin; lemon; kiwi; peach
There were also musicians at the party. They all played different MUSICAL INSTRUMENTS
accordion; banjo; bass guitar; harp; organ; flute; violin; trumpet
And, people used different fabrics to make their own CLOTHING
blouse; shirt; suit; cap; scarf; socks; top; swimsuit
We had a fun day. THE END