Abstract
Intimate partner violence (IPV) perpetrators have been categorized into two groups based on their heart rate (HR) reactivity to stress following Gottman’s studies. Overall, type I perpetrators tend to show autonomic underarousal, whereas type II or reactive perpetrators present a hyper-reactivity in anticipation of stress. In this study, changes in HR, pre-ejection period (PEP), vagal ratio as well as psychological state variables (anxiety and anger) in response to stress were assessed, comparing a group of type II IPV perpetrators (based on violence reports and psychological assessment; n = 17; mean age = 37) with non-violent controls (n = 17; mean age = 35) using modified version of the Trier Social Stress Test. IPV perpetrators had higher HRs and lower vagal ratios than controls, particularly during the recovery period. Moreover, the former presented shorter PEPs than controls. There were no differences between groups in the magnitude of response of the HR, PEP or vagal ratio. High baseline anxiety and anger were associated with an HR increase during the preparation time in IPV perpetrators but not in controls. These findings indicate a different cardiovascular pattern of response to psychosocial stress in IPV perpetrators, especially during recovery. Thus, they contribute to understanding the biological functioning of violence sub-types, supporting the validity of cardiovascular measures as diagnostic indicators for IPV classification.
Introduction
Approximately 30% of women worldwide are victims of intimate partner violence (IPV) at some point in their lives (WHO, Citation2013). Many researchers have been studied aggressors and most have taken a psychological approach, paying little interest to the potential influence of biological variables (Pinto et al., Citation2010). Due to the poor results obtained with psychotherapy programs developed for these individuals, it is worthwhile exploring different approaches based on biopsychosocial models (Babcock et al., Citation2004a) that consider psychobiological variables such as cardiovascular parameters. Such data could increase our understanding of the complex phenomena of IPV.
One of the most analyzed cardiovascular measures in IPV perpetrators is heart rate (HR), and this variable has been employed to build IPV typologies. HR regulation is a complex feedback system, the result of a balance between the sympathetic and parasympathetic components (Umhau et al., Citation2002). In line with this, hyper-reactivity in a sympathetic indicator such as the cardiac pre-ejection period (PEP), a systolic time interval that reflects cardiac contractility (Reyes del Paso et al., Citation2013), was found to be associated with antisocial personality disorders in men (Sylvers et al., Citation2010). Moreover, a low cardiac vagal or parasympathetic tone has been shown to reflect antisocial traits in IPV perpetrators (Gottman et al., Citation1995). Consistent with this, an inability of the vagal tone to control the HR in IPV perpetrators was related to difficulties in autonomic regulation during conflict, with negative expression for emotion and aggression modulation (Umhau et al., Citation2002).
IPV perpetrators have been categorized into two groups based on their autonomic function, specifically on their HR reactivity to a marital conflict discussion (Gottman et al., Citation1995). Type I IPV perpetrators tend to show under arousal, reflected in a significant decrease in HR between baseline and a marital conflict task. They perpetrate a type of violence that is often called “proactive” (Tweed & Dutton, Citation1998). Type II IPV perpetrators tend to present a more reactive physiological profile (Gottman et al., Citation1995), with increased HRs during the beginning of a conflict interaction or high autonomic nervous system (ANS) reactivity to stress. This kind of violence is known as “reactive”. However, two studies have failed to replicate Gottman’s IPV classification based on perpetrators HR reactivity to stress (Babcock et al., Citation2004b; Meehan et al., Citation2001).
The majority of studies of men who are IPV perpetrators have employed HR measures in response to a conflict discussion with the subject’s romantic partners and/or an anger induction procedure. Nevertheless, in the conflict discussion, IPV perpetrators must repress their own anger feelings due to social pressures such as being present with their girlfriends/wives in a laboratory situation. Furthermore, in anger induction tasks, participants are not required to cope with real stress situations, and instead they are only faced with imagined scenarios. These procedures have been criticized for not being strictly standardized experimental tasks (Ornduff et al., Citation1995). To analyze the autonomic reactivity of IPV perpetrators, we thought it could be interesting to employ a standardized laboratory task such as the Trier Social Stressor Test (TSST; Carrillo et al., Citation2001; González-Bono et al., Citation2002; von Dawans et al., 2010), which combines high levels of social-evaluative threat and uncontrollability (Dickerson & Kemeny, Citation2002). Moreover, in tasks with social-evaluative threat, men tend to show higher autonomic reactivity than women (Denson et al., Citation2012).
The primary objective of this study was to determine whether reactive type II IPV perpetrators have different cardiovascular responses to psychosocial stress to non-violent men in HR, PEP and the balance between the low-frequency (LF) and high-frequency (HF; vagal ratio) parameters. IPV perpetrators in this study were classified as type II based on violence reports and psychological assessment (see “Methods” section). As previously demonstrated with baseline cardiovascular measures (Gottman et al., Citation1995), they would be expected to present lower resting HR and higher PEP and vagal tone than controls. Moreover, IPV perpetrators would present higher HR and PEP reactivity and lower vagal reactivity to stress than controls, as an indicator of a potential emotional control deregulation and sympathetic predominance (Beauchaine, Citation2001; Beauchaine et al., Citation2001). Second, in the general population the TSST usually induces increases in anxiety and anger feelings (Dickerson et al., Citation2004; Heinz et al., Citation2003). Hence, we expected to find greater increases in anxiety and anger feelings to social stress tasks in IPV perpetrators than controls. Third, we explored the relationships of cardiovascular markers with anxiety and anger. Higher negative affect (high anxiety and anger) has been related to greater cardiovascular responses to social stressors (Carrillo et al., Citation2001). Finally, it was hypothesized that stronger anger and anxiety responses would be associated with high sympathetic predominance in IPV perpetrators.
Methods
Participants
The final sample was composed of 34 healthy men volunteers (17 IPV perpetrators and 17 controls). IPV perpetrators were recruited from the participants in the CONTEXTO psycho-educational and community-based treatment program (mandatory for male abusers) at the Department of Social Psychology, University of Valencia. Having been sentenced to less than 2 years in prison and having no previous criminal record, participants had been given a suspended sentence on the condition that they attend an intervention program (Catalá-Miñana et al., Citation2013; Lila et al., Citation2013). Participating IPV perpetrators were initially classified as type II following the classification of Gottman et al. (Citation1995) due to the fact that their criminal record indicated that they had committed violence against their romantic partner and it was not premeditated violence, as it was described as impulsive violence in reaction to marital conflicts. Moreover, they obtained low dependent and antisocial personality trait scores (assessed using the Millon Clinical Multiaxial Inventory-III) as in the studies of Gottman et al. (Citation1995). Candidates who did not present these characteristics were excluded from the study.
We advertised in the University of Valencia for male volunteers for the control group, establishing contact by email and then screening applicants in interviews. The inclusion criteria for controls included: having no physical or mental problems, and similar anthropometrical and demographic characteristics to the IPV perpetrators, as well as not having perpetrated severe violence, defined as assaulting a partner or other individual outside the home or engaging in any severely violent act. Control individuals were required to provide criminal record certificates, to confirm that they had no history of violence.
All candidate participants were interviewed by trained researchers (with extensive experience treating IPV perpetrators) to assess their mental health. Cohen’s kappa, used to assess the inter-rater agreement between qualitative interviewers in the nine psychopathological dimensions evaluated (the same dimensions as the Symptom Checklist 90-R or SCL-90-R), ranged from 0.67 to 0.84. Regardless of the objective SCL-90-R results, participants were considered not to have any psychopathological signs and symptoms if they scored less than the mean for their age for each dimension.
All participants were right handed, lived in Valencia (Spain), and gave written informed consent. The experiment was performed in accordance with the Helsinki Declaration and approved by the University of Valencia Ethics Committee.
Procedure
Each subject participated in three sessions in the psychobiology laboratories of the University of Valencia. In the first sessions, participants were interviewed (as described above) to exclude any individuals with physical or mental illnesses. The second sessions all took place between 4:00 and 7:00 p.m. After arriving at the laboratory, participants were taken to a room where they signed informed consent forms and anthropometric data (height and weight) were obtained. Participants were then conducted to another noise-insulated room with a constant temperature 22 + 1 °C where they underwent an adapted version of the TSST (Kudielka et al., Citation2004). During the entire experimental session, which lasted approximately 75 min, participants remained seated and ECG parameters were continuously monitored and recorded out of sight of the participant. The procedure included the following periods: resting, preparatory, stressor (speech task and arithmetical mental task) and recovery (post-task), each period lasting 15 min, except that for the speech and arithmetic tasks which lasted 5 min each. All participants had to talk about their views of IPV and related Spanish legislation, in the case of perpetrators including their own experiences and problems due to their officially documented experience with IPV, and their opinions regarding Spanish legislation dealing with their own cases as well as IPV laws in general. Finally, after the TSST, participants completed a brief task appraisal questionnaire, comprising three items based on previous studies (de Andrés-García et al., Citation2012; Romero-Martínez et al., Citation2013a,Citationb). Perceived stress and satisfaction were assessed using two items, and ranked on a 10-point Likert scale from 0 (low stress and dissatisfied) to 10 (high stress and satisfied). The third item evaluated internal (e.g. personal effort and physical and technical abilities) and external (e.g. luck) attribution of the outcome and ranged from 0 (low external locus of control) to 10 (high external locus of control). Further, their psychological state (specifically, anxiety and anger) was assessed before and after the psychosocial stressor.
Psychosocial stressor
The TSST is a standardized psychosocial laboratory stress protocol that consists of preparing a speech on a specific topic (usually as part of a mock job interview) during a brief preparation period, followed by a test period in which participants deliver this speech and then perform mental arithmetic tasks (Kirschbaum et al., Citation1993). In our study, as described above, the topic of the speech was their views of IPV for all participants, and they had to put forward their opinion for a period of 2.5 min, with interviewers asking a set of related questions for 5 min at the end of the participant’s presentation. Participants then performed an arithmetic task consisting of subtracting a constant from a series, and calculating simple arithmetical operations, such as additions, subtractions, multiplications and divisions. To increase the perceived social-evaluative threat throughout the TSST, there was an audience of four evaluators (two men and two women) and a video camera was turned on (though it was not recording). The variation in the psychosocial stress protocol has been demonstrated to elicit psychophysiological responses in previous studies (de Andrés-García et al., Citation2013; Romero-Martínez et al., Citation2013a,Citationb).
Psychological trait profiles
State anxiety was assessed using the “State-Trait Anxiety Inventory” (STAI-S) (Spielberger et al., Citation1983) suitably adapted, which contains 20 items, ranked on a four-point Likert scale. The reliability coefficient was 0.62.
State-trait anger and its expression were measured by an adapted version (Miguel-Tobal et al., Citation2001) of the State-Trait Anger Expression Inventory-2 (STAXI-2) (Spielberger, Citation1999). This test is composed of three subscales for state anger (feelings, verbal and physical expression), all being rated on a four-point Likert-type scale (1 = “not at all” to 4 = “very much so”). To reduce the number of tests, increase the effect size and simplify the interpretation of the results, state anger subscales were combined into single variable (State Anger, S-Ang). Cronbach’s alpha ranged from 0.67 to 0.89.
Electrophysiological recording
A physiological recording system (BIOPAC Systems, Inc, Santa Barbara, CA) was used to capture, process and analyze the ECG and impedance signals. In this system, signals were routed through a signal pre-amplifier UIM-150 (universal interface module), which was connected to a computer with data acquisition hardware (MP150) and data storage software (AcqKnowledge 4.1. for Windows, Biopac Systems, Montreal, Canada).
HR (in beats per minute, BPM) was calculated in accordance with published guidelines (Task Force, Citation1996). Electrocardiogram files were visually screened and R-waves of problematic files were manually marked. The second-by-second heart rate (in BPM) was then calculated from the resultant file.
We used impedance cardiography to assess PEP: Ag/AgCl electrodes were arranged on the participant’s chest in a standard three-lead configuration with four additional Mylar band electrodes in a tetrapolar configuration (two bands over cervical vertebra C4 and another two between vertebrae T8 and T9) in accordance with the manufacturer instructions and previous research (Sherwood et al., Citation1990). The electrodes were then connected to the aforementioned BIOPAC MP150 data acquisition system. PEP was calculated as the time in milliseconds between the Q wave of the ECG (onset of ventricular depolarization) and the first derivative of pulsatile changes in transthoracic impedance.
Analysis of heart rate variability in the frequency domain is a widely used tool in the investigation of autonomic cardiovascular control. The quotient between LF and HF bands has been used to quantify the balance between sympathethic and parasympathethic nerves (Task Force, Citation1996). In our study, we analyzed the vagal predominance. Specifically, the vagal activity, in terms of power spectral density, was calculated using the expression: vagal ratio hf = shf/(slf + shf) where shf and slf are the sums of the powers within the high and low frequencies bands, respectively (Billman, Citation2013). Increases in this ratio are assumed to reflect a shift to “parasympathetic dominance” and decreases in this index correspond to a “sympathetic dominance”. Nonetheless, the interpretation of the index is controversial because recent studies have demonstrated that the LF/HF ratio does not accurately measure cardiac sympatho-vagal balance (Billman, Citation2013; Reyes del Paso et al., Citation2013).
Data analysis
t-Tests with Levene’s test for equality of variances and/or Chi-square analyses were performed where appropriate to check for significant differences in age, BMI and socio-demographic variables (between IPV and control men). Effect sizes for the between-group differences were calculated using Cohen’s d (Cohen, Citation1988).
For exploring psychological state responses, after confirming the normality of the data using the Kolmogorov–Smirnov test, repeated measures ANOVAs were performed with “time” (pre and post) as the within-subject factor and “group” as the between-subject factor. Considering the cardiovascular responses, the effectiveness of the stressor in the total sample was confirmed by general linear model repeated-measures ANOVA with “time” (at four levels: for resting, preparation, stressor and recovery) as a within-participants factor. To examine group effects, repeated-measures ANOVA was conducted with “time” as the within-subject and “group” as the between-subject factors.
Greenhouse–Geisser corrections for degrees of freedom and Bonferroni corrections for multiple comparisons were applied where appropriate. For significant results, partial eta squared () is reported as a measure of effect size.
The magnitude of stress responses was estimated by the area under the curve with respect to the increase (AUCi). The AUCi enabled HR, PEP and vagal ratio responses to the stressor to be quantified, using the trapezoid formula originally employed for estimating the magnitude of hormonal responses (Pruessner et al., Citation2003). Partial AUC values were calculated by only considering the time points before the current measurement. To achieve this, differences between each of the three times (preparation, stressor and recovery) and the resting period were summed to obtain a single AUC.
Spearman’s and Pearson’s correlations were used for assessing relationships between psychophysiological resting and reactivity with psychological state variables where appropriate. Bonferroni corrections for multiple comparisons with a p value of 0.05 were applied following the recommendations of Curtin and Schulz (Citation1998).
Data analyses were performed using SPSS, version 17.0 (SPSS IBM, Armonk, NY). All reported p values are two-tailed, and p ≤ 0.05 was considered significant. Average values are expressed as mean ± SEM.
Results
Participant characteristics and appraisal scores
IPV perpetrators did not differ from controls in age (36.88 ± 2.59 and 34.82 ± 1.47, respectively), BMI (26.84 ± 3.34 and 27.20 ± 3.22, respectively) or socio-demographic variables (see details in Romero-Martínez et al., Citation2013b). Moreover, no differences were found in baseline values of cardiovascular variables.
IPV perpetrators obtained similar appraisal scores to controls in satisfaction (5.13 ± 0.46 and 5.38 ± 0.45, respectively), as well as the internal (5.53 ± 0.41 and 5.00 ± 0.48, respectively) and external (4.47 ± 0.41 and 5.00 ± 0.48, respectively) loci of control; but IPV perpetrators perceived the TSST to be less stressful than controls (t[30.10] = −2.83, p = 0.008, d = 1.03).
Stress responses
Effectiveness of the TSST to elicit cardiovascular responses
The psychosocial stressor employed in this study was found to be effective, as indicated by the significant “time” effect on HR in the total sample [ε = 0.66, F(1.97, 65.03) = 17.68, p = 0.000, = 0.35]. Dividing the sample into groups, intra-group comparisons revealed that a significant “time” effect in both IPV perpetrators [F(3, 48) = 7.73, p = 0.000,
= 0.33] and controls [F(3, 48) = 12.41, p = 0.000,
= 0.44]. In IPV men, HR increased from resting to the preparatory time and from then to the tasks. Afterwards, HR decreased from then to recovery (for all p < 0.05). In controls, HR followed a similar pattern to the IPV participants (for all p < 0.05).
Regarding PEP, no significant “time” effect was found. In the case of vagal ratio, a significant “time” effect was found in the total sample [F(1.92, 63.45) = 5.42, p = 0.007, = 0.14]. Dividing the sample into groups, intra-group comparisons revealed that a significant “time” effect in both IPV perpetrators [F(3, 48) = 4.79, p = 0.005,
= 0.23] and controls [F(3, 48) = 3.70, p = 0.018,
= 0.19]. In IPV men, the vagal ratio decreased from resting to the preparation time and from then to the stressor. Afterwards, the LF component decreased from then to recovery (for all p < 0.05). The vagal ratio followed a similar pattern in controls, though their vagal ratio increased from the stressor to the recovery time.
Psychological responses to the TSST
For anxiety, a significant effect of “time” and “time × group” interaction was found [F(1, 32) = 34.90, p = 0.000, = 0.52); F(1, 32) = 13.69, p = 0.001,
= 0.30], respectively). Although anxiety increased after the stressor in both groups, the rise was smaller in the case of IPV perpetrators (p < 0.05; ).
Table 1. Mean ± SD of psychological states before and after task.
Regarding anger, significant effects of “time” and “time × group” interaction were found [(F(1, 32) = 4.98, p = 0.033, = 0.149; F(1, 32) = 8.79, p = 0.006,
= 0.21)], with IPV perpetrators having higher baseline S-Ang than controls (p < 0.05; ).
Differences between IPV perpetrators and controls in cardiovascular responses to the TSST
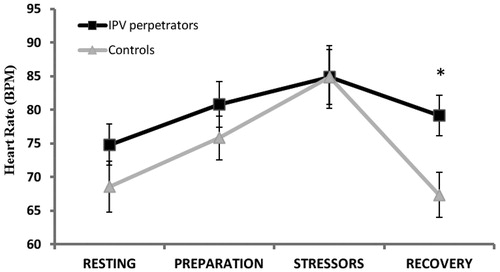
In the case of HR, a significant “time × group” interaction effect was found [ε = 0.68; F(2.05, 65.58) = 3.17, p = 0.047, = 0.09], with IPV perpetrators having higher HR than controls in the recovery time (p < 0.05; ). Considering PEP, a significant “group” effect was found [F(1, 32) = 3.93, p = 0.050,
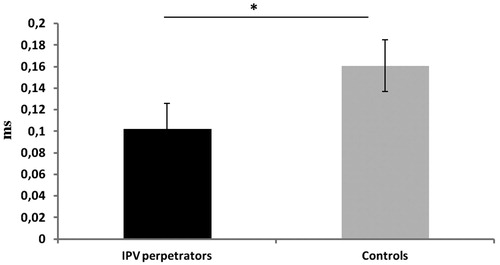
= 0.11], with IPV perpetrators having shorter PEP values than controls ().
Figure 1. Heart rate (BPM) during resting, preparation, stressor and recovery times for groups (IPV perpetrators and controls; *p < 0.05).
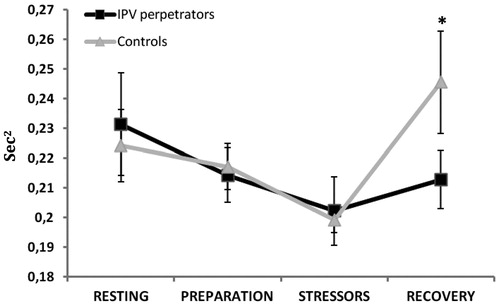
Regarding the vagal ratio, a significant effect of “time × group” interaction was found [ε = 0.64, F(1.92, 61.49) = 3.08, p = 0.050, = 0.09], with IPV perpetrators having lower vagal ratio values than controls in the recovery time (p < 0.05; ).
Figure 3. HF/LF ratio or vagal ratio values (s2) during resting, preparation, stressor and recovery times for groups (IPV perpetrators and controls; *p < 0.05).
No significant effects were detected in the magnitude of the response (AUCi) in any cardiovascular measure. Relationships between psychological and cardiovascular responses to the TSST in IPV perpetrators ().
Discussion
Regarding the cardiovascular profile of type II IPV perpetrators (), the participants in our study had higher HRs and lower vagal ratios than controls, particularly during the recovery time. Moreover, the IPV perpetrators had shorter PEPs than controls. On the other hand, there were no differences between the two groups in the magnitude of response in the HR, PEP or vagal ratio. With respect to psychological stress response, IPV perpetrators had smaller increases in anxiety than controls. Moreover, they present higher state anger (S-Ang) than controls. Finally, the relationship between cardiovascular measures and psychological variables revealed that high baseline anxiety and anger were associated with the increase in the HR during the preparatory time only among IPV perpetrators. The power of the analyses was moderate to high as revealed the values (which ranged from 0.09 to 0.44) and, hence, we conclude there is adequate power to conduct the analyses presented.
Table 2. Relationships of cardiovascular parameters (HR, PEP and vagal ratio) with psychological state profiles (STAI and S-Ang) in IPV perpetrators and controls.
The validity of this version of the TSST modified and adapted to marital violence was demonstrated by a significant increase in negative affect (high anxiety and anger) and HR, and a reduction in vagal activity in all participants. This reinforces the results as the differences observed cannot be attributed to the different emotionally charged topic used for the TSST. Specifically, a significant increase in HR and PEP from resting to stress periods was observed in both IPV perpetrators and non-violent controls. It has been previously pointed out that the TSST, as an acute psychosocial stress, produces an immediate increase in HR in healthy non-violent adults (Kudielka et al., Citation2004). This increase in HR is normally followed by a decrease at the recovery time (Kudielka et al., Citation2004), as we found in all participants. Nevertheless, IPV perpetrators recovered more slowly than controls, maintaining higher HRs after the stress had finished. This could be explained by the high predominance of the sympathetic component of the ANS response in IPV perpetrators (Umhau et al., Citation2002). This hypothesis was reinforced in our study, in that IPV perpetrators presented shorter average PEPs than controls (see below). Moreover, we previously demonstrated that type II IPV perpetrators had higher non-specific skin conductance responses (another measure of the ANS) during the recovery time than controls (Romero-Martínez et al., Citation2013c). That is, type II IPV perpetrators maintain vigilance for a long time (Dawson et al., Citation2000) increasing the risk of them becoming violent even after the stress disappears.
IPV perpetrators and controls did not differ in HR, PEP or vagal tone in the resting period. However, IPV perpetrators had shorter PEPs, which suggest that they probably have higher ANS activation than controls. PEP decreases correspond to HR increases (Newlin & Levenson, Citation1979). The short PEPs in IPV perpetrators could partially explain the slow recovery of their HR and vagal tone, and the fact that they maintain vigilance for a long time (Dawson et al., Citation2000). This sympathetic predominance, which could reflect more abnormal functioning, may result in difficulties in emotional regulation and a lowering of the threshold for impulsive aggressive behavior (Porges, Citation2001). Nevertheless, in some studies with reactive perpetrators, an increase in efferent vagal activity was not followed by a decrease in HR (Umhau et al., Citation2002).
IPV perpetrators showed higher baseline anger than controls, but smaller increases in anxiety. Our previous work found that the negative affect (high anxiety and anger) drives the hormonal (testosterone and cortisol) response to stress in IPV perpetrators but not in controls (Romero-Martínez et al., Citation2013a,Citationb). Our current results reinforce these previous findings in that negative affect was only associated with high HR preparatory response to stress in IPV perpetrators. This is in line with previous research and the most recent theoretical models for the prediction of anger and IPV (Anderson & Bushman, Citation2001). Higher feelings of anger could disrupt normal cognitive processes that would otherwise increase levels of sympathetic arousal (Houston, Citation1994) and in turn motivate aggressive responding.
This study is part of an ongoing research effort to improving our understanding of why IPV perpetrators use violence against their partners. The research design is strong, including a control group which was matched for the main demographic characteristics. The major limitation of this study is, however, the small sample size. For this reason, the findings should be considered preliminary. Further research is needed to explore these patterns in larger samples. Another limitation of this study is that only one indicator of parasympathetic arousal (namely, vagal tone from heart rate variability analysis) was used. Future studies might consider other vagal response-related measures such as respiratory sinus arrhythmia (RSA). Unfortunately, due to a lack of the necessary equipment, we were not able to collect this type of data. This is the first study to demonstrate sympathetic activity with IPV perpetrators, but we recognize that the experiment was not the ideal setting for an assessment of the PEP, and this should be considered in future studies. Further, differences between subtypes of IPV perpetrators were not explored, as all participants were classified as type II (Gottman’s classification) or reactive. In addition, the TSST has been argued to be a performance-based social stressor that induces social-evaluative threat in which men are generally much more reactive than women, but the topic of marital conflict we used in the TSST is not related to performance or social rejection. Furthermore, the vast variability in what individuals would include in the discussion of this topic is an important consideration in relation to future replication of this research. Moreover, as the control condition was based on Spanish social–cultural factors, future research could consider specific aspects of IPV law of each country in order to assess IPV’s response to this laboratory task. Finally, it would also be interesting to consider other biomarkers, such as serum cytokines and alpha amylase.
Conclusions
In conclusion, our study reveals that IPV perpetrators present high activation after stress ends. Moreover, negative affect drives HR reactivity to stress in IPV perpetrators but not in controls. For this reason, we suggest that cardiovascular measures of both sympathetic and parasympathetic branches of the ANS could be valid diagnostic indicators for IPV classification. Our previous papers have also provided evidence of potential differences in neuropsychological and hormonal parameters between participants with and without history of IPV. This study builds on this evidence, with data that contributes to improving our understanding of the interactions between the ANS and psychological state in IPV perpetrators. Finally, the findings from this research shed light on the biological functioning of violence sub-types supporting the validity of cardiovascular measures as diagnostic indicators for IPV classification.
Declaration of interest
The authors report no conflicts of interest. This work was supported by the Spanish Ministry of Health, Social Services and Equality, National Drug Plan (2012/001), the Ministry of Economy and Competitiveness (PSI2011-25434), the Committee for Business, Research and Science of the Regional Government of Valencia beca VALi + d (ACIF/2011/075), research groups and networks of excellence (PROMETEO/2011/048; ISIC/2013/001) and the University of Valencia (UV-INV-AE11-40217).
Acknowledgements
The authors wish to thank to the Home Office Prison Administration (Instituciones Penitenciarias, Ministerio del Interior) for its cooperation in this research project.
References
- Anderson CA, Bushman BJ. (2001). Effects of violent video games on aggressive behavior, aggressive cognition, aggressive affect, physiological arousal, and prosocial behavior: a meta-analytic review of the scientific literature. Psychol Sci 12:353–9
- Babcock JC, Green CE, Robie C. (2004a). Does batterers’ treatment work? A meta-analytic review of domestic violence treatment. Clin Psychol Rev 23:1023–53
- Babcock JC, Green CE, Webb SA, Graham KH. (2004b). A second failure to replicate the Gottman et al. (1995) typology of men who abuse intimate partners…and possible reasons why. J Fam Psychol 18:396–400
- Beauchaine T. (2001). Vagal tone, development, and Gray's motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol 13:183–214
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. (2001). Disinhibitory psychopathology in male adolescents: discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. J Abnorm Psychol 110:610–24
- Billman GE. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 4:26
- Carrillo E, Moya-Albiol L, Gonzalez-Bono E, Salvador A, Ricarte J, Gomez-Amor J. (2001). Gender differences in cardiovascular and electrodermal responses to public speaking task: the role of anxiety and mood states. Int J Psychophysiol 42:253–64
- Catalá-Miñana A, Lila M, Conchell R, Romero-Martínez A, Moya-Albiol L. (2013). ¿Se benefician de los programas de intervención que no tratan específicamente el consumo de alcohol los maltratadores con problemas de consumo abusivo? Psychosoc Intervention 22:135–43
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc., Publishers
- Curtin F, Schulz P. (1998). Multiple correlations and Bonferroni’s correction. Biol Psychiatry 44:775–7
- Dawson ME, Schell AM, Filion DL. (2000). The electrodermal system. In Cacioppo JT, Tassinary LG, Bernston GG, editors. Handbook of psychophysiology. 2nd ed. Boston: Cambridge University Press. p 200–23
- de Andrés-García S, Moya-Albiol L, González-Bono E. (2012). Salivary cortisol and immunoglobulin A: responses to stress as predictors of health complaints reported by caregivers of offspring with autistic spectrum disorder. Horm Behav 62:464–74
- de Andrés-García S, Sariñana-González P, Romero-Martínez A, Moya-Albiol L, Gonzalez-Bono E. (2013). Cortisol response to stress in caregivers of offspring with autism spectrum disorder is associated with care recipient characteristics. Stress 16:510–19
- Denson TF, Creswell JD, Granville-Smith I. (2012). Self-focus and social evaluative threat increase salivary cortisol responses to acute stress in men. J Behav Med. doi:10.1007/s10865-011-9393-x. [Epub ahead of print]
- Dickerson SS, Gruenewald TL, Kemedy M. (2004). When the social self is threatened: shame, physiology, and health. J Pers 72:1191–216
- Dickerson SS, Kemeny ME. (2002). Acute stressors and cortisol reactivity: a meta analytic review. Psychosom Med 64:105
- González-Bono E, Moya-Albiol L, Salvador A, Carrillo E, Ricarte J, Gómez-Amor J. (2002). Anticipatory autonomic response to a public speaking task in women: the role of train anxiety. Biol Psychol 60:37–49
- Gottman JM, Jacobson NS, Rushe RH, Shortt JW. (1995). The relationship between heart rate reactivity, emotionally aggressive behavior, and general violence in batterers. J Fam Psychol 9(3):227–48
- Heinz A, Hermann D, Smolka MN, Rieks M, Gräf KJ, Pöhlau D, Kuhn W, Bauer M. (2003). Effects of acute psychological stress on adhesion molecules, interleukins and sex hormones: implications for coronary heart disease. Psychopharmacology 165:111–17
- Houston BK. (1994). Anger, hostility, and psychophysiological reactivity. In Siegman AW, Smith TW, editors. Anger, hostility, and the heart. Hillsdale, NJ: Erlbaum. p 97–116
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The “trier social stress test” – A tool for investigating psychobiology stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Kudielka B, Buske-Kirschbaum A, Hellhammer D, Kirschbaum C. (2004). HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology 29:83–98
- Lila M, Oliver A, Galiana L, Gracia E. (2013). Predicting success indicators of an intervention programme for convicted intimate-partner violence offenders: the Contexto Programme. Eur J Psychol Appl Legal Cont 5:73–95
- Meehan JC, Holtzworth-Munroe A, Herron K. (2001). Maritally violent men's heart rate reactivity to marital interactions: a failure to replicate the Gottman et al. (1995) typology. J Fam Psychol 15:394–408
- Miguel-Tobal J, Casado M, Cano-Vindel A, Spielberger C. (2001). Inventario de Expresión de Ira Estado-Rasgo (STAXI-2). Madrid: TEA Ediciones, SA
- Newlin DB, Levenson RW. (1979). Pre-ejection period: measuring beta-adrenergic influences upon the heart. Psychophysiology 16:546–53
- Ornduff SR, Kelsey RM, O'Leary KD. (1995). What do we know about typologies of batterers? Comment on Gottman et al. (1995). J Fam Psychol 9:249–52
- Pinto LA, Sullivan EL, Ronsebaum A, Wyngarden N, Umhau JC, Miller MW, Taft CT. (2010). Biological correlates of intimate partner violence perpetration. Aggress Violent Behav 15:387–98
- Porges SW. (2001). The polyvagal theory: phylogenetic substrates of a social nervous system. Int J Psychophysiol 42:123–46
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Reyes del Paso GA, Langewitz W, Mulder LJM, Van Roon A, Duschek S. (2013). The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 50:477–87
- Romero-Martínez A, González-Bono E, Lila M, Moya-Albiol L. (2013a). Testosterone/cortisol ratio in response to acute stress: a possible marker of risk for marital violence. Soc Neurosci 8:240–7
- Romero-Martínez A, Lila M, Sariñana-González P, González-Bono E, Moya-Albiol L. (2013b). High testosterone levels and sensitivity to acute stress in perpetrators of domestic violence with low cognitive flexibility and impairments in their emotional decoding process: a preliminary study. Aggress Behav 39:355–69
- Romero-Martínez A, Lila M, Williams RK, González-Bono E, Moya-Albiol L. (2013c). Skin conductance rises in preparation and recovery to psychosocial stress and its relationship with impulsivity and testosterone in intimate partner violence perpetrators. Int J Psychophysiol 90:329–33
- Sherwood A, Turner RJ, Light KC, Blumenthal JA. (1990). Temporal stability of the hemodynamics of cardiovascular reactivity. Int J Psychophysiol 10:95–8
- Spielberger CD. (1999). Manual for the state-trait anger expression inventory-2. Lutz, FL: Psychological Assessment Resources Odessa
- Spielberger CD, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. (1983). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press
- Sylvers P, Brennan PA, Lilienfeld SO, Alden SA. (2010). Gender differences in autonomic indicators of antisocial personality disorder features. J Pers Disord 1:87–96
- Task Force. (1996). Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93:1043–65
- Tweed RG, Dutton DG. (1998). A comparison of impulsive and instrumental subgroups of batterers. Violence Vict 13:217–30
- Umhau JC, George DT, Reed S, Petrulis SG, Rawlings R, Porges SW. (2002). Atypical autonomic regulation in perpetrators of violent domestic abuse. Psychophysiology 39:117–23
- von Dawans B, Kirschbaum C, Heinrichs M. (2010). The Trier social stress test for groups (TSST-G): a new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology 36:514–22
- WHO. (2013). World Health Statistics 2013: a wealth of information on global public health. Geneva: WHO/WHD