Abstract
Neuroimaging studies have demonstrated reduced hippocampal volume in trauma-exposed individuals without posttraumatic stress disorder (PTSD). However, the implications of such a deficit in this non-clinical population are still unclear. Animal and human models of PTSD suggest that hippocampal deficit may result in impaired learning and use of associations between contextual information and aversive events. Previous study has shown that individuals with PTSD have a selective impairment in reversing the negative outcome of context-related information. The aim of this study was to test whether non-PTSD individuals who are repeatedly exposed to traumatic events display similar impairment. To that end, we compared the performance of active-duty firefighters who are frequently exposed to traumatic events as part of their occupational routine and civilian matched-controls with no history of trauma-exposure. We used a novel cue–context reversal paradigm, which separately evaluates reversal of negative and positive outcomes of cue and context-related information. As predicted, we found that while both trauma-exposed firefighters and unexposed matched-controls were able to acquire and retain stimulus-outcome associations, firefighters struggled to learn that a previously negative context is later associated with a positive outcome. This impairment did not correlate with levels of PTSD, anxiety or depressive symptoms. The results suggest that similar to individuals with PTSD, highly exposed individuals fail to associate traumatic outcomes with their appropriate context. This impairment may reflect a possible hidden price of repeated traumatic exposure, which is not necessarily associated with PTSD diagnosis, and may affect the way highly exposed individuals interpret and react to their environment.
Introduction
Numerous neuroimaging studies have shown that not only individuals with posttraumatic stress disorder (PTSD) but also trauma-exposed individuals without PTSD have a reduced hippocampal volume compared to trauma-unexposed controls (for meta analysis, see Karl et al., Citation2006; Woon et al., Citation2010). These findings suggest that independent of PTSD, trauma exposure itself may be associated with hippocampal volume reduction. However the effect of hippocampal deficit on cognitive functions and its relations to PTSD symptoms in trauma-exposed individuals is still unclear.
The item-in-context model argues that the hippocampus integrates object and context-related information (Davachi, Citation2006; Diana et al., Citation2012; Dickerson & Eichenbaum, Citation2010). Animal and human models of PTSD suggest that a hippocampal deficit may result in impaired associations between contextual information and aversive events (Acheson et al., Citation2012; Goosens, Citation2011; Moustafa et al., Citation2013; Rudy, Citation2009, for review, see Maren et al., Citation2013). Such impairment may explain, for example, why a person, who was exposed to a terror attack in a coffee shop, may associate all coffee shops with a negative outcome.
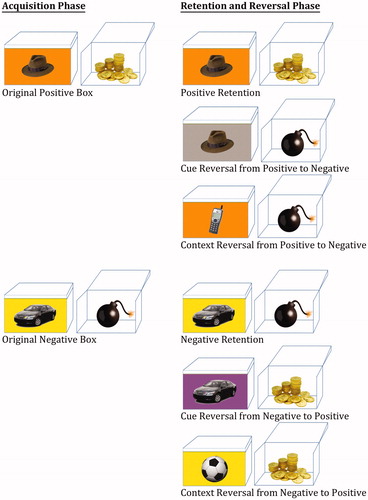
In order to test whether non-PTSD individuals, with repeated exposure to trauma, experience similar deficits, we used an innovative cue–context reversal paradigm (Levy-Gigi et al., Citation2011, Citation2014). In a common reversal paradigm, participants acquire a stimulus-outcome association (S → Positive) and later need to reverse the outcome of the same stimulus (S → Negative). Such a paradigm does not take into account that a stimulus usually contains a cue that occurs in a specific context (Mayes et al., Citation1992; Murnane et al., Citation1999). In our paradigm, participants learn stimulus-outcome associations (A hat on an orange background → Positive) and later view new associations, which require reversing the outcome of either the cue (A phone on an orange background → Negative) or the context (A hat on a grey background → Negative) of the acquired stimuli. This unique manipulation enables us to detect selective impairments in reversing positive and negative outcomes of cue and context-related information.
Performance on our paradigm significantly correlated with hippocampal functions (Levy-Gigi et al., Citation2011) and volume reduction (Levy-Gigi et al., Citation2014). Specifically, we found that individuals with PTSD showed a selective deficit in reversing the outcome of negative context; after they learned that a specific context is associated with a negative outcome, they struggled to learn that the same context predicts a positive outcome when presented later with a new cue.
The aim of this study was to test whether non-PTSD individuals with repeated traumatic exposure would show a deficit in reversing the outcome of negative context similar to what we recently found in individuals with PTSD. To that end, we concentrated on a unique population of active-duty firefighters and compared them to trauma-unexposed matched controls.
We postulated that both groups would equally learn and retain positive and negative stimulus–outcome associations. However, we expected that similar to previous findings in individuals with PTSD, non-PTSD highly exposed individuals would show a selective impairment in reversing the outcome of negative context compared to trauma-unexposed individuals.
Methods and materials
Participants
Thirty-two active-duty firefighters who are repeatedly exposed to trauma as part of their daily routine and thirty-one unexposed controls matched for age, gender and years of education volunteered to participate in the study (see for a detailed description of the sample). Firefighters were randomly recruited from five different fire stations in southern Israel, which are all located in a similar setting within a radius of 40 miles. All firefighters reported multiple exposures to Diagnostic and Statistical Manual for Mental Disorders-Fifth Edition (DSM-V) Criterion A events. In order to further validate the firefighter’s exposure to traumatic events, we used the fire and rescue department archive to collect data on potential traumatic events that were encountered by firefighters from the five studied fire stations during the past 10 years (see ). Participants in the unexposed control group were civilians who work in an industrial factory. They were recruited by a clinical psychologist who interviewed them to ensure no past exposure to DSM-V criteria A events. Three participants were excluded from the study due to past exposure to potential traumatic event. Individuals in both groups showed high rates of consent; hence, approximately 95% of the people we sampled agreed to participate in the study. All participants were interviewed using the Structured Clinical Interview for Diagnostic and Statistical Manual for Mental Disorders-Forth Edition (DSM–IV) Axis I Disorders (SCID-CV) (First et al., Citation1996). Exclusion criteria included any current DSM-IV psychopathology including PTSD, and any history of psychiatric or neurological disorders, alcohol abuse or dependence. Two firefighters were excluded from the sample due to a clear diagnosis of PTSD. The rest thirty-two Non-PTSD firefighters were also interviewed using the SCID Non-Patient PTSD module interview (Spitzer et al., Citation1990) to assess the levels of subclinical PTSD symptoms. All interviews were conducted by a well-trained and regularly supervised clinical psychologist. The experiment was done in accordance with the Declaration of Helsinki for the protection of human participants. All participants provided a written informed consent at the beginning of the experiment.
Table 1. Demographic characteristics of trauma exposed firefighters and trauma-unexposed matched controls.
Table 2. Mean number of exposures to different potential traumatic events per year in the past 10 years in Israel southern fire and rescue stations.
Tools
Cue and context reversal paradigm
In this paradigm, participants view a series of boxes on a computer screen (). On each box, there is a picture of a cue (one of various objects, e.g. a hat) presented against a specific context (different background colors, e.g. orange) (see Hockley, Citation2008; Isarida & Isarin, Citation2007; Lang et al., Citation2009; Macken, Citation2002; Rutherford, Citation2004 for studies that manipulated context in a similar way). When opened, each box is associated with a specific outcome (positive or negative). Participants receive the following instructions: “In this experiment you will be shown various boxes. For each box you have the option to open it or to leave it closed. If you open a box you will either win or lose 25 points (see for example of the different trials). If you do not open the box you will not win or lose any points. Your job is to earn as many points as possible. Through trial and error you will learn to open the boxes that earn you points and not open the boxes that cost you points. Note that in order to learn whether a box earns or costs you points, you should open each box in the first time you see it”. The experimenter verifies then that the participants understand the instructions. Afterward, participants take part in a practice phase under close supervision of the experimenter. This phase demonstrates the task of using two boxes; one associated with a positive outcome and the other associated with a negative outcome. They see a closed box, with a picture of an object presented against a background color, and receive the following instructions: “Suppose you see a box for the first time. You should open it”. After opening the box, participants see gold inside of it (positive box) accompanied with a matching voice, a smiley face and a numeric indication that they earned 25 points. These points are added to the participants’ total amount of points indicated at the side of the screen (). “Great job! There is gold inside”. In the following screen, they see the same reward box, with the following text: “Now suppose you see the same box again. You just learned there is gold inside. You should open it”. After opening the box again, they see an open box with gold inside of it a smiley face and a numeric indication that they earned 25 points, and receive the following feedback. “Very good. You won gold”. Later, they see a screen with a new box that has a different object presented against a different background color on it. “Next suppose you see another new box. You should open it”. After opening the box, participants see an open box with a bomb inside of it (negative box) accompanied with a matching voice, a frown face and a numeric indication that they lost 25 points. “Oops, there is a bomb inside”. In the following screen, they see the same negative box, with the following text: “Now, suppose you see the same box again. You just learned that there is a bomb inside. You should decide not to open it”. After choosing the “Do not open” option, participants receive the following feedback: “You were right not to open it. There is a bomb inside”. The experiment starts at the end of the practice phase. We created new boxes for the experiment, different from those presented in the practice phase, using eight cue objects and eight distinctive context colors (for a schematic description see ). Boxes were 4′′ × 3′′ size, presented on a 13′′ screen. The outcome of each box was counterbalanced across participants. The paradigm has two phases. In the acquisition phase, participants learn by trial and error to predict the outcome of four different boxes (i.e. open the two positive boxes and skip the two negative boxes). Each box has a unique cue and context (i.e. a box with a hat on an orange background has gold inside while a box with a car on a yellow background has bomb inside). The acquisition phase contains a minimum of 40 trials. However, in order to ensure learning of the stimulus–outcome associations in this phase, participants have to reach a criterion of six consecutive correct responses before they move on to the next phase. Participants who do not reach this criterion within 64 trials are automatically opt-out from the experiment. Correct responses refer to conditions in which participants open positive boxes or leave negative boxes closed. Similarly, incorrect responses refer to conditions in which participants open negative boxes or leave positive boxes closed. A subsequent retention and reversal phase starts immediately after the acquisition phase without any signaled switch or delay. In this phase, participants receive retention trials with the original boxes that keep the same learned outcome (e.g. a hat on an orange background has gold inside) in addition to two new types of boxes that share either the cue (e.g. a hat on a gray background) or the context (e.g. a phone on an orange background) with an original box (). The new boxes are associated with the opposite outcome relative to the original boxes (i.e. if the box with the hat on the orange background has gold inside, then the boxes with the hat on a grey background and a phone on the orange background will have bomb inside and vice versa). Therefore, in order to successfully learn these new associations, participants need to reverse the association rule of either the original cue or the original context. Boxes in this phase are presented in 10 blocks of 12 boxes each (two boxes from each of the following conditions: positive/negative retention, positive/negative cue reversal and positive/negative context reversal). These sums up to a total of 120 trials; 20 trials per condition. At the end of the task, participants see their total earned points; however, the experiment includes no actual payment.
Figure 2. Example of experimental trials in which participants chose to (a) open a positive-outcome box and (b) open a negative-outcome box.
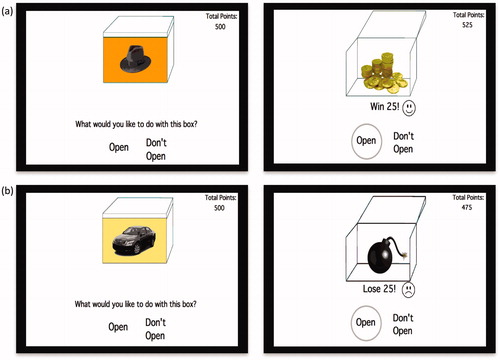
Table 3. Schematic description of the Cue–Context Reversal Task.
Self-report questionnaires and cognitive assessment
All participants completed self-report questionnaires in order to control for possible effects of depression and anxiety symptoms. Depressive symptoms over the previous two weeks were assessed using the revised version of the Beck Depression Inventory-II (BDI-II; Beck et al., Citation1996). General anxiety was measured using the State–Trait Anxiety Inventory (STAI; Spielberger et al., Citation1983) questionnaire. Finally, we used the scaled scores of the Wechsler Adult Intelligence Scale III (WAIS-III) vocabulary subtest to estimate IQ levels (Wechsler, 1997). Previous studies showed that scores from this subtest are the best predictor of full IQ scale scores (Spreen, Citation1998).
Data analysis
We used SPSS (version 19) software (SPSS Inc., Chicago, IL) to analyze the data. All data were checked for normality of distribution using Kolmogorov–Smirnov tests. Since participants are instructed to open boxes when they first see it, in our analyses, we did not include the first response to each new box in the acquisition and reversal trials (note that retention trials include only old boxes, and therefore all trials are analyzed). This was done in order to avoid artificial errors (i.e. when participants open a negative box for the first time) and possible effects of task compliancy.
Results
Acquisition and retention of stimulus–outcome associations
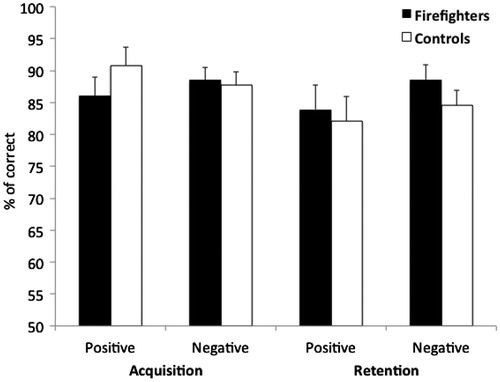
The vast majority of the participants (60 of 63) acquired the stimulus–outcome associations within the minimum of 40 trials. One trauma-exposed participant and two unexposed matched controls needed 1–2 additional blocks in order to reach a criterion of six consecutive correct responses. We conducted a Group (trauma-exposed firefighters vs. trauma-unexposed controls) by Acquisition (positive vs. negative stimuli) by Retention (positive vs. negative stimuli) mixed model ANOVA on the percentage of correct responses. In this model, Group was the between-subjects factor, while Acquisition and Retention were the within-subjects factors. The results are depicted in . As predicted, the ANOVA revealed no significant main effects of Group (F(1,61) = 0.06, p = 0.81) and no significant interactions of Acquisition by Group (F(1,61) = 1.26, p = 0.27) Retention by Group (F(1,61) = 1.18, p = 0.28) nor Acquisition by Retention by Group (F(1,61) = 0.18, p = 0.68). These results indicate that there were no significant differences in performance between acquisition and retention trials. In addition, it shows that both firefighters and unexposed matched controls are equally able to learn and retain positive and negative stimulus–outcome associations.
Cue and context reversal
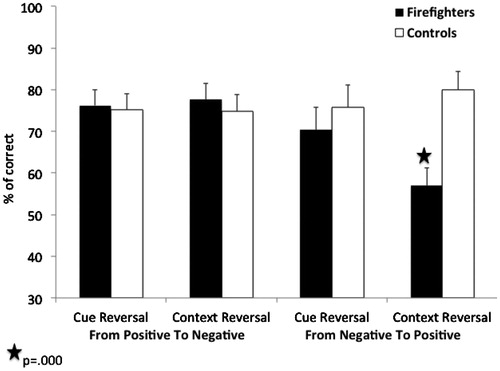
We conducted a Group (trauma exposed firefighters vs. trauma-unexposed controls) by Reversal Type (cue vs. context) by Outcome (reversal from positive to negative vs. reversal from negative to positive) mixed model ANOVA on the percentage of correct responses. In this model, Group was the between-subjects factor, while Reversal Type and Outcome were the within-subjects factor. The results are depicted in . There were no significant main-effects of Group, Reversal Type or Outcome (ps > 0.1). However, we found a significant triple interaction between Group, Reversal Type and Outcome (F(1,61) = 4.44, p < 0.05, = 0.07). Follow-up analysis revealed a significant interaction of Group by Reversal Type in negative-to-positive reversals (F(1,61) = 4.69, p < 0.05,
= 0.07) but not in positive-to-negative reversals (F(1,61) = 0.11, p = 0.74). Follow-up pairwise comparisons with Bonferroni correction (α = 0.01) showed that, as predicted, relative to controls firefighters were significantly impaired in reversing negative outcomes of context-related information (t(57) = −3.7, p = 0.000). There were no significant differences between the groups in reversing negative outcomes of cue-related information (t(57) = −0.73, p = 0.47). These results indicate that after firefighters learn that a specific context is associated with a negative outcome, they struggle to learn that the same context is associated with a positive outcome when it is presented later with a different cue. As can be seen in , in the three other reversal conditions, both groups preformed equally well.
Figure 4. Percentage of correct responses for the new associations as a function of Reversal Type (Cue vs. Context), Outcome (Reversal from Positive to Negative vs. Reversal from Negative to Positive) and Experimental Group (Trauma Exposed Firefighters vs. Trauma-Unexposed Controls). Cue reversal refers to conditions of old cue, which is presented against a new context; Context reversal refers to conditions of new cue, which is presented against an old context.
In order to test whether there are group-related differences in the tendency to open new reversal boxes when they are first presented, we conducted independent sample t-test in each of the four reversal conditions, with the number of opened boxes as the dependent variable. There are two new boxes in each reversal condition; therefore, participants could receive a score between zero (i.e. they did not open any of the new boxes when they first saw them) to two (i.e. they opened the two new boxes when they first saw them). The results revealed no significant differences between the groups in the tendency to open new reversal boxes (ts < 0.82; ps > 0.41). Hence, even when reversal boxes shared the same context with original negative boxes, the tendency of trauma-exposed participants to open these boxes when they first saw them did not differ from the tendency of unexposed matched controls (t(61) = 0.81, p = 0.42; M = 1.88, SD = 0.34; M = 1.94, SD = 0.25, for trauma exposed and unexposed participants, respectively).
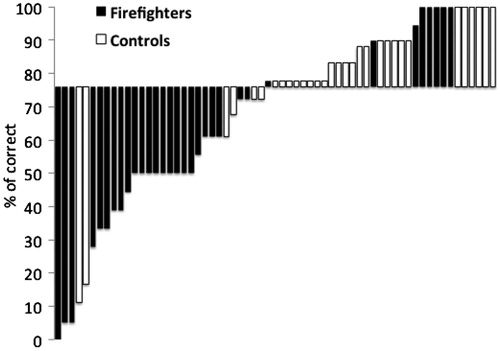
We used the median number of correct responses in reversal of negative context to divide the participants into two groups according to their performance. Chi-square test revealed that the number of trauma-exposed firefighters in the first group (number of correct responses above median) was significantly lower than the number of unexposed matched controls. In contrast, the number of trauma-exposed firefighters in the second group (number of correct responses below median) was significantly higher compared with the number of unexposed matched controls (X2(1) = 17.31, p < 0.0001). Finally, the distribution of correct scores for unexposed controls was significantly lower compared to the distribution of correct scores among trauma exposed firefighters (Levene’s test F = 4.82, p < 0.05) ().
Self-report questionnaires and cognitive assessment
depicts the comparison of trauma-exposed firefighters and unexposed controls on the BDI-II (Beck et al., Citation1996), the STAI (Spielberger et al., Citation1983) and on IQ assessment (WAIS-III, Wechsler, 1997). There were no significant differences in levels of depression, anxiety and IQ scores between the trauma-exposed firefighters and the unexposed controls. In addition, there were no significant correlations between reversal learning and symptoms of PTSD, depression or anxiety. Finally, in accordance with past findings (e.g. Levy-Gigi et al., Citation2012), there were significant correlations between PTSD symptoms and levels of state, trait and total symptoms of anxiety (r(32) = 0.37, p < 0.05; r(32) = 0.36, p < 0.05; r(32) = 0.37, p < 0.05, respectively).
Table 4. Questionnaires and cognitive assessment (means and standard deviation) of trauma exposed firefighters and trauma unexposed matched controls.
Discussion
The aim of this study was to test the effect of repeated traumatic exposure on the ability to reverse positive and negative outcomes of cue- and context-related information. To that end, we compared the performance of highly trauma-exposed firefighters without PTSD and trauma-unexposed matched controls on a novel cue–context reversal paradigm. As predicted, we found that both groups were equally able to learn and retain positive and negative stimulus–outcome associations. In addition, in accordance with previous findings (Levy-Gigi et al., Citation2011, Citation2014), both groups displayed spared cue reversal learning; they were able to learn that an object, which was first associated with positive or negative outcome is associated with the opposite outcome when presented later in a different context (e.g. a hat on an orange background is positive while a hat on a gray background is negative and vice versa). However, similar to previous findings in individuals with PTSD, firefighters who experience repeated traumatic exposure showed a selective deficit in reversing negative context; after they learned that a specific context is associated with a negative outcome (e.g. a car on a yellow background is negative) they could not learn that it predicts a positive outcome when presented later with a new object (e.g. a football on a yellow background is positive). Moreover, the magnitude of the effect in this group was similar to the one we previously observed in fully PTSD-diagnosed people (Levy-Gigi et al., Citation2014).
This study is the first to show associations between repeated traumatic exposure and impairment in reversing the negative outcome of context-related information in non-PTSD individuals. There are several possible ways to interpret the current results. First, the results may suggest that individuals with repeated traumatic exposure fail to associate traumatic outcomes with their appropriate context. Therefore, they may experience difficulty to recognize and differentiate novel conditions from other negative conditions, which share the same context. Similar to findings in PTSD individuals (Brown et al., Citation2013; Levy-Gigi & Kéri, Citation2012; Levy-Gigi et al., Citation2012, Citation2014), such impairment may lead to inappropriate generalization of the negative outcome to the novel conditions. Alternatively, it is possible that like the stronger fear conditioning observed in stressed animals (e.g. Giachero et al., Citation2013; Rau & Fanselow, Citation2009; Rau et al., Citation2005 but see Tsoory et al., Citation2010), individuals with repeated exposure to trauma make stronger context–outcome associations when negative outcomes are involved. These stronger associations may then be more difficult to reverse. Therefore, they struggle to learn that a previously negative context becomes positive. Finally, it is possible that individuals with repeated traumatic exposure have an inherent bias to associate the context, but not the cue with behavioral outcomes. Therefore, when they see a new cue on a context previously paired with a positive outcome (e.g. a phone presented against an orange background), their bias to open the box allows modifying the behavior accordingly (i.e. the participants see a bomb inside and learn to skip this box in the future). In contrast, when they see a new object on a context previously paired with a negative outcome (soccer ball presented against a yellow background), their bias to leave the box closed does not allow learning (e.g. the participants receive no feedback and do not know that their choice was “wrong”) and therefore they continue to leave the box closed.
Although all these alternatives are plausible explanations of the current data, it is important to note that individuals from both groups did not differ in their tendency to open new reversal boxes when they first presented. This fact may suggest that individuals with repeated exposure to trauma recognize new boxes, even if they share context with a negative box, and have an opportunity to learn it predicts positive outcome. Yet, they struggle to reverse the negative outcome of these boxes compared to unexposed controls. Future studies may aim to use a revised task, in which participants get feedback even if they leave a box closed (e.g. by showing a transparent image of the closed box with the gold/bomb inside). The results from such a task may help to better understand the mechanisms beyond the impaired ability of individuals with repeated traumatic exposure to reverse the negative outcome of contextual information.
In a previous study, we reported that a deficit in reversing the negative outcome of contextual information was associated with reduced hippocampal volume (Levy-Gigi et al., Citation2014). Therefore, the results of this study may reflect a reduction in hippocampal volume among individuals with repeated traumatic exposure and provide further support for imaging studies that described similar structural abnormalities in trauma-exposed individuals independent of PTSD diagnosis (for meta analyses, see Karl et al., Citation2006; Kitayama et al., Citation2005; Smith, Citation2005; Woon et al., Citation2010).
Although intuitively it seems that a deficit in reversing the negative outcome of contextual information may contribute to the development of PTSD symptoms, the results revealed no significant correlations between these variables. Leaning on this set of data as proof of concept, future cross-sectional studies may aim to test a larger sample of individuals with repeated traumatic exposure in order to further understand the link between PTSD symptoms and negative and positive reversal learning. Moreover, a larger sample may allow further testing of individual differences within this group (see ) and enable looking at associations between specific response patterns (e.g. intact performance, slower learning or impaired overall performance) and different types of PTSD symptoms.
Similar to our previous findings in individuals with PTSD (Levy-Gigi et al., Citation2014), the impairment of individuals with repeated traumatic exposure was selective to conditions of reversing negative, but not positive outcome of context-related information. These results may suggest that the hippocampus–amygdala connectivity in individuals who repeatedly exposed to trauma facilitates learning in conditions of negative feedback (LaBar & Cabeza, Citation2006). Specifically, although they struggle to learn when negative context becomes positive, they can successfully learn that a previously positive context becomes negative. Support for such claim can be found in neuroimaging studies, which observed enhanced amygdala response in threatening and aversive contextual conditions (Buchel et al., Citation1999; Phelps et al., Citation2001; Smith et al., Citation2004, Citation2006; Stevens et al., Citation2013) and advantage in attending and processing aversive stimuli in trauma-exposed individuals (Fani et al., Citation2012; Kleim et al., Citation2012; Vythilingam et al., Citation2007; Wald et al., Citation2013). Future fMRI study, which assesses hippocampus–amygdala connectivity in highly exposed individuals during context reversal-learning, is needed in order to clarify this point.
Finally, the results may shed new light on recent studies of PTSD in first responders. A large number of these studies reported relatively low PTSD prevalence in firefighters (e.g. Chang et al., Citation2008; Del Ben et al., Citation2006; Fushimi, Citation2012; Meyer et al., Citation2012; Soo et al., Citation2011). Furthermore, a number of prospective studies, which aimed to predict PTSD symptoms in active-duty firefighters and police after exposure to traumatic events, revealed low rates of PTSD symptoms (Guthrie & Bryant, Citation2006; Orr et al., Citation2012; Pole et al., Citation2009). This study highlights the importance of behavioral measures, showing that repeated traumatic exposure has a hidden price even in non-PTSD individuals, which may affect the way these individuals interpret and react to their environment. Moreover, the fact that our cue–context reversal paradigm uses neutral stimuli suggests that such price is not limited to trauma-related conditions and might reflect a more general impairment.
A possible limitation of this study may relate to the nature of the cue–context reversal paradigm. The basic assumption in this and other similar paradigms (e.g. Fellows & Farah, Citation2003; Foerde & Shohamy, Citation2011; Rogers et al., Citation2000) is that the participants are rational learners. However, it is possible that decision makers have expectancies and inner values and representations on acts, outcomes and contingencies (Tversky & Kehneman, Citation1981). Therefore, decisions are often guided by biases and heuristics rather than stimulus–response mechanisms. Accordingly, it may be claimed that factors such as expectations, risk taking and loss aversion would affect the performance on the cue–context reversal paradigm. If this were the case, we would expect to see a robust effect of negative or positive outcome. For example, participants who avoid risk would struggle to learn that a previously negative stimulus becomes positive in conditions of both cue and context reversal. Moreover, since this tendency represents inner values and expectations, and is not necessarily a result of traumatic exposure, such effects would be expected in both trauma exposed and unexposed groups. However, the results show that only trauma-exposed individuals have impaired learning. This impairment is unique to reversal trials and was not observed during positive and negative acquisition trials. Furthermore, it was observed exclusively in conditions of negative context (but not negative cue) reversal trials. Although the selectivity of the observed effect support a dominant effect of traumatic exposure, future studies may aim to test whether expectancies and different attitudes toward reward and punishment mediate individual differences in reversal learning within each group.
Another possible limitation is that we tested only firefighters without comparing them to other first responders. It can be claimed that since firefighters are trained to focus and react to aversive environmental conditions, they center their attention on the context and ignore other elements, and therefore display impaired reversal of negative context. One way to test this claim is by evaluating cue–context reversal learning of firefighters at the end of their training course and before trauma exposure. In addition, it might be informative to compare cue–context reversal learning of first responders from different occupations, for example, firefighters who are trained to attend the general context and criminal scene investigators who are trained to look for evidences and therefore may focus their attention on different cues in the environment.
Conclusions
In conclusion, this study showed that repeated traumatic exposure might have a hidden price independent of PTSD symptoms and other psychiatric diagnosis. Specifically, firefighters who are repeatedly exposed to traumatic events as part of their daily routine are impaired in reversing the negative outcome of contextual information. This impairment is not restricted to trauma-related situations and may affect the way these individuals interpret and react to their environment.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
We wish to thank Szabolcs Keri for his thoughtful comments and contribution for the design of this study.
References
- Acheson DT, Gresack JE, Risbrough VB. (2012). Hippocampal dysfunction effects on context memory: possible etiology for post-traumatic stress disorder. Neuropharmacology 62:674–85
- Beck AT, Steer RA, Brown GK. (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation
- Brown AD, Root JC, Romano TA, Chang LJ, Bryant RA, Hirst W. (2013). Overgeneralized autobiographical memory and future thinking in combat veterans with post-traumatic stress disorder. J Behav Ther Exp Psychiatry 44:129–34
- Buchel C, Dolan RJ, Armony JL, Friston KJ. (1999). Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related fMRI. J Neurosci 19:10869–76
- Chang CM, Lee LC, Connor KM, Davidson JR, Lai TJ. (2008). Modification effects of coping on post-traumatic morbidity among earthquake rescuers. Psychiatry Res 158:164–71
- Davachi L. (2006). Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16:693–700
- Del Ben KS, Scotti JR, Chen Y, Fortson BL. (2006). Prevalence of posttraumatic stress disorder symptoms in firefighters. Work Stress 20:37–48
- Diana RA, Yonelinas AP, Ranganath C. (2012). Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia 50:3062–9
- Dickerson BC, Eichenbaum H. (2010). The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 35:86–104
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ, Kamkwalala A, Jovanovic T. (2012). Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med 42:533–43
- Fellows LK, Farah MJ. (2003). Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain 126:1830–7
- First MB, Gibbon M, Spitzer RL, Williams JBW. (1996). User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders-Research Version. New York: Biometrics Research Department, New York State Psychiatric
- Foerde K, Shohamy D. (2011). Feedback timing modulates brain systems for learning in humans. J Neurosci 31:13157–67
- Fushimi M. (2012). Posttraumatic stress in professional firefighters in japan: rescue efforts after the Great East Japan Earthquake (higashi nihon dai-shinsai). Prehosp Disaster Med 27:416–18
- Giachero M, Calfa GD, Molina VA. (2013). Hippocampal structural plasticity accompanies the resulting contextual fear memory following stress and fear conditioning. Learn Mem 20:611–16
- Goosens KA. (2011). Hippocampal regulation of aversive memories. Curr Opin Neurobiol 21:460–6
- Guthrie RM, Bryant RA. (2006). Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med 68:307–11
- Hockley WE. (2008). The effects of environmental context on recognition memory and claims of remembering. J Exp Psychol Learn Mem Cogn 34:1412–29
- Isarida T, Isarin TK. (2007). Environmental context effects of background color in free recall. Mem Cognit 35:1620–9
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. (2006). A meta analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 30:1004–31
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. (2005). Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord 88:79–86
- Kleim B, Ehring T, Ehlers A. (2012). Perceptual processing advantages for trauma-related visual cues in post-traumatic stress disorder. Psychol Med 42:173–81
- LaBar KS, Cabeza R. (2006). Cognitive neuroscience of emotional memory. Nat Rev Neurosci 7:54–64
- Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. (2009). Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci 29:823–32
- Levy-Gigi E, Kelemen O, Gluck MA, Kéri S. (2011). Impaired context reversal learning, but not cue reversal learning, in patients with amnestic mild cognitive impairment. Neuropsychologia 49:3320–6
- Levy-Gigi E, Kéri S. (2012). Falling out of time: enhanced memory for scenes presented at behaviorally irrelevant points in time in Posttraumatic Stress Disorder (PTSD). PLoS One 7:e42502
- Levy-Gigi E, Kéri S, Myers CE, Lencovsky Z, Sharvit-Benbaji H, Orr SP, Gilbertson MW, et al. (2012). Individuals with post-traumatic stress disorder show a selective deficit in generalization of associative learning. Neuropsychology 26:758–67
- Levy-Gigi E, Szabo C, Richter-Levin G, Kéri S. (2014). Reduced hippocampal volume is associated with impaired generalization of negative context in individuals with PTSD. [Epub ahead of print]
- Macken WJ. (2002). Environmental context and recognition: the role of recollection and familiarity. J Exp Psychol Learn Memory Cogn 28:153–61
- Maren S, Phan KL, Liberzon I. (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14:417–28
- Mayes AR, MacDonald C, Donlan L, Pears J, Meudell PR. (1992). Amnesics have a disproportionately severe memory deficit for interactive context. Q J Exp Psychol 45:265–97
- Meyer EC, Zimering R, Daly E, Knight J, Kamholz BW, Gulliver SB. (2012). Predictors of posttraumatic stress disorder and other psychological symptoms in trauma-exposed firefighters. Psychol Serv 9:1–15
- Moustafa AA, Gilbertson MW, Orr SP, Herzallah MM, Servatius RJ, Myers CE. (2013). A model of amygdala-hippocampal-prefrontal interaction in fear conditioning and extinction in animals. Brain Cogn 81:29–43
- Murnane K, Phelps M, Malmberg K. (1999). Context-dependent recognition memory: the ICE theory. J Exp Psychol 128:403–15
- Orr SP, Lasko NB, Macklin ML, Pineles SL, Chang Y, Pitman RK. (2012). Predicting post-trauma stress symptoms from pre-trauma psychophysiologic reactivity, personality traits and measures of psychopathology. Biol Mood Anxiety Disord 2:8. doi: 10.1186/2045-5380-2-8
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. (2001). Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4:437–41
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. (2009). Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biol Psychiatry 65:235–40
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. (2000). Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci 12:142–62
- Rau V, DeCola JP, Fanselow MS. (2005). Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev 29:1207–23
- Rau V, Fanselow MS. (2009). Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress 12:125–33
- Rudy JW. (2009). Context representations, context functions, and the parahippocampale-hippocampal system. Learn Mem 16:573–85
- Rutherford A. (2004). Environmental context-dependent recognition memory effects: an examination of ICE model and cue-overload hypotheses. Q J Exp Psychol A 57:107–27
- Smith AP, Henson RN, Dolan RJ, Rugg MD. (2004). fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage 22:868–78
- Smith AP, Stephan KE, Rugg MD, Dolan RJ. (2006). Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron 49:631–8
- Smith ME. (2005). Bilateral hippocampal volume reduction in adults with posttraumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus 15:798–807
- Soo J, Webber MP, Gustave J, Lee R, Hall CB, Cohen HW, Kelly KJ, Prezant DJ. (2011). Trends in probable PTSD in firefighters exposed to the World Trade Center disaster, 2001–2010. Disaster Med Public Health Prep 2:197–203
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR. (1983). State-Trait Anxiety Inventory (STAI). Bib 2010:180
- Spitzer RL, Williams JB, Gibbon M, First MB. (1990). User’s guide for the structured clinical interview for DSM-III-R: SCID. Washington, DC: American Psychiatric Press
- Spreen OA. Compendium of neuropsychological tests: administration, norms, and commentary. NY: Oxford University Press; 1998
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ. (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 47:1469–78
- Tsoory MM, Guterman A, Richter-Levin G. (2010). “Juvenile stress” alters maturation-related changes in expression of the neural cell adhesion molecule L1 in the limbic system: relevance for stress-related psychopathologies. J Neurosci Res 88:369–80
- Tversky A, Kahneman D, Choice R. (1981). The framing of decisions. Science 211:453–8
- Vythilingam M, Blair KS, McCaffrey D, Scaramozza M, Jones M, Nakic M, Mondillo K, et al. (2007). Biased emotional attention in post traumatic stress disorder: a help as well as a hindrance? Psychol Med 37:1445–55
- Wald I, Degnan KA, Gorodetsky E, Charney DS, Fox NA, Fruchter E, Goldman D, et al. (2013). Attention to threats and combat-related posttraumatic stress symptoms: prospective associations and moderation by the serotonin transporter gene. JAMA Psychiatry 70:401–8
- Wechsler D. (1997). Wechsler Adult Intelligence Scale – Third addition. San Antonio: The Psychological Corporation
- Woon FL, Sood S, Hedges DW. (2010). Hippocampal volume deficits associated with exposure to psychological trauma and post-traumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 34:1181–8