Abstract
Previous studies have tested the relationship between chronic stress and sex hormones, but inconsistent results have been found. One possibility is that this association may depend on other biological factors. This study examined the relationship between stressful life events (LE) and sex hormones in men, and whether cortisol is involved in this relationship. From a total number of 2906 men who completed a screening for the early detection of prostate cancer, 139 healthy men (mean ± SD age, 57.8 ± 5.7 years) were included in this study. Participants were assessed with the Holmes and Rahe questionnaire in relation to their experience of LE during the previous 1–5 years. Salivary and serum cortisol was measured at 08:00–09:00 h, as well as luteinizing hormone (LH), total testosterone, epinephrine (E) and norepinephrine (NE). LE weight sum and LE number positively correlated with LH (r = 0.293, p = 0.004; r = 0.220, p = 0.031, respectively). In a multiple regression analysis, LE-sum explained an additional and significant 10.4% of the variance in LH levels, after statistically controlling for the effects of age, waist circumference (WC) and BMI (F(1,90) = 6.61, p < 0.05). Importantly, cortisol interacted with LE in relation to total testosterone. In men with high cortisol values (≥15.4 µg/dl), there was a statistically significant positive relationship between LE number and total testosterone levels (p = 0.05), while LE were unrelated to total testosterone in men with low cortisol. LE correlated with sex hormones, predicting LH values, and in men with high cortisol levels shows a possible moderator effect of cortisol on the relationship between LE and total testosterone.
Introduction
Stressful life events (LE) are frequent nowadays, with increasing economic hardships, natural disasters and terrorism, for example. Their relationship with the onset or prognosis of different pathologies like the metabolic syndrome (MS) (Fabre et al., Citation2013) and cardiovascular disease (Thomas et al., Citation1997) among others, is quite established. Stressful stimuli can activate neural and neuroendocrine pathways. The hypothalamic–pituitary–adrenocortical (HPA) system as well as the sympatho adrenomedullar system are considered to be mediators and regulators of the stress response (Joels et al., Citation2007; Koob, Citation1999; McEwen, Citation2007). Furthermore, a close relationship between the HPA axis and the hypothalamic pituitary–gonadal axis (HPG) exists. Studies in animal show that exogenous glucocorticoids suppress the release of gonadotropins (LH and FSH) (Breen & Karsch, Citation2006). Moreover, it has been observed that animals exposed to both acute and chronic stress show changes in plasma levels of testosterone, estradiol (E2) and progesterone (P4) (Andersen et al., Citation2004; Chichinadze & Chichinadze, Citation2008).
However, the impact of psychosocial factors has been mainly studied in women (Berga & Loucks, Citation2005; McComb et al., Citation2006) showing a decrease of HPG axis activity associated with high glucocorticoid levels, conditioning fertility (Berga & Loucks, Citation2006). On the contrary, studies in men are scarce and the impact of life events on LH has not been evaluated so far. Regarding testosterone, it has been reported that working environment produces no changes or diminution on circulating hormone levels (Hansen et al., Citation2009).
Male subjects present greater HPA axis responses to a psychological stressor than female subjects (Uhart et al., 2006). Moreover, in men, cortisol levels progressively increase with aging (Elmlinger et al., Citation2003). On the contrary, cortisol awakening response and cortisol reactivity to stressors increases during adolescence with advancing pubertal development (Quevedo et al., Citation2012).
The contradictory results found in numerous studies about the relationship of stress to sex hormones may be due to gender differences in the populations studied, the need to consider the role of the HPA axis in these situations and the different stressors evaluated (Kajantie & Phillips, Citation2006). One possibility for the inconsistent effects of LE or stress on sex hormones could be the role of the HPA in such links. The HPA axis may react in extremes (Stratakis & Chrousos, Citation1995), resulting in either hyperactivity in some individuals or conditions, such as acute stress (Bellingrath & Kudielka, Citation2008) and depression (Tafet & Smolovich, Citation2004); or hypoactivity in others such as burnout (Bellingrath et al., Citation2008) and atypical depression (Gold et al., Citation1995). Moreover, a synergistic interaction between LE and cortisol was found in relation to different metabolic situations (Fabre et al., Citation2013; Gidron et al., Citation2011). The aim of this study was to examine the relationship between LE and sex hormones, and whether this relationship is moderated by cortisol levels, in middle-aged adult men.
Methods
Participants and design
A total number of 2906 men (aged 45–70 years) completed an evaluation for prostatic diseases, at the Urology Division, Hospital de Clínicas “José de San Martín”, University of Buenos Aires, in May 2009, in the context of a population screening for the early detection of prostate cancer. For the purpose of the present study, a subsample was selected among patients who were assessed between 8:00 and 9:00AM, and who fulfilled all the inclusion and exclusion criteria. Participants with prostate cancer, under hormonal therapy as well as those with diseases that affect the HPA axis, with an alcohol intake >20 g/day, recent history of acute illness or under medication which could modify lipid levels, were excluded. This resulted in a subsample of 139 men with a mean (SD) age of 57.8 (5.7) years, without differences in age, body mass index (BMI), clinical and social characteristics from the total sample of men. All participants gave their written informed consent and the original screening study protocol was approved by the Ethics Committee of the hospital, and the study was performed in accordance with the Helsinki Declaration for medical studies in humans. The study used a cross-sectional design.
Measures
Background and biomedical
These included age, BMI, waist circumference (WC) and blood pressure. In order to calculate the BMI, weight and height were obtained from each patient. WC was measured at the middle level between the lateral lower rib margin and the superior anterior iliac crest, in a standing position and always by the same investigator. Blood pressure was measured in a sitting position. A thorough medical examination was performed in order to record general health conditions, medical disorders, lifestyle, smoking, educational level and marital status.
Biological tests
After 12 h fasting, blood samples were obtained, between 8:00 and 9:00 AM, from peripheral vein puncture after a 15-min rest. Serum samples were separated by centrifugation at 1500 × g for 5 min. A serum aliquot was stored at −70 °C for determination of cortisol, total testosterone and luteinizing hormone (LH), which were determined by a chemoluminiscent method (Immuliteautoanalyzer, Siemens, Los Angeles, CA). The intra-assay (CVi) and inter-assay (CVe) variation coefficients were <5% and <9.7% for cortisol; <5% and 10% for total testosterone and <6% and <9% for LH, respectively. Epinephrine (E) and norepinephrine (NE) were measured by radioimmunoassay (RIA KatCombi, IBL International GMBH, Hamburg, Germany). The detection limit for E and NE was 0.008 and 0.020 ng/ml respectively. The CVi and CVe were 5.8 and 12.8% for E and 8.0 and 16.9% for NE.
In parallel, saliva samples were obtained by spontaneous salivation (between 8:00 and 9:00 am) in order to measure salivary cortisol, which was measured by electrochemiluminescense Cobas e411 Roche (Mannheim, Germany). The results were expressed in nmol/l. The limit of quantification was 8.5 nmol/l (0.308 µg/dl). The CVi and CVe were 2.7% for a concentration of 11.5 nmol/l (0.417 µg/dl) and 11.5% for a concentration of 8.05 nmol /l (0. 292 µg/dl), respectively.
Life events
These were assessed using the Holmes and Rahe (Holmes & Rahe, Citation1967) Social Readjustment Rating Scale which was self-administered. This included 40 items, where men indicated which events they had experienced during the past 1–5 years. Two LE indices were derived: the total number of LE experienced by a participant, and the total weight of LE from the combined LE each participant had selected. The weights reflected their mean degree of stressfulness as perceived by people, in the development of this scale by large samples. Grant & Moores (Citation1977) found that both types of scores have similar correlations with a criterion of psychiatric symptoms.
Statistical analyses
We first tested the distribution of variables using normality tests (kurtosis and skewness), and then performed transformations in order to normalize the data. Pearson's correlations were computed between dependent and independent variables and between dependent variables and potential confounders. Linear multiple regression analysis was performed using hormones as dependent variables and LE (weight sum and number) as independent variables. In the multiple regressions, we tested whether LE contributed to explain variance in sex hormones, beyond the effects of age, BMI and WC.
We then performed an ordinary least square (OLS) regression analysis; entering cortisol and LE number as continuous variables, and the interaction term between cortisol and LE. The interaction was probed using a model-based test of significance regions (Hayes & Matthes, Citation2009), which allowed us to test at which precise level of cortisol the effect of LE on total testosterone was present.
Results
shows socio-demographic characteristics of the studied population. The mean age was slightly below 58 years; on average, men had a moderated overweight, 12.8% were smokers and approximately half of the men had hypertension.
Table 1. Sociodemographic and general characteristics of the studied population (n = 139).
Data concerning endocrine profile, as well as LE number and LE weight are presented in . The mean values of hormones were all within the normal reference and scale ranges.
Table 2. Hormone profile and life events in the studied population.
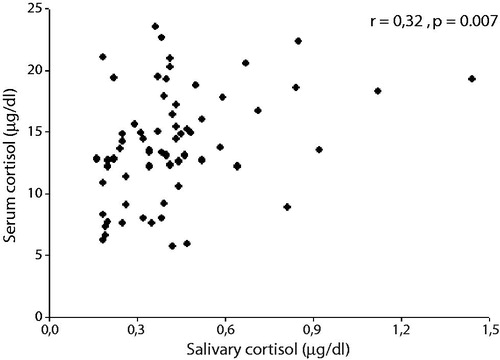
Correlations between salivary cortisol and serum cortisol
The correlation between these two measurements can be seen in . The linearity between the two assays decreased at high concentrations of salivary cortisol (0.39 µg/dl or 10.5 nmol/l; r = 0.148, p = 0.426).
Correlations between hormones and life events
LE weight and LE number positively correlated with LH (r = 0.293, p = 0.004; r = 0.220, p = 0.031, respectively). In reference to stress hormones, a significant correlation was observed between serum cortisol and LH (r = 0.215, p = 0.034). No correlations between LE and total testosterone were observed (p > 0.05), as well as between LE and cortisol (p > 0.05 for both). Likewise no association was observed between cortisol and total testosterone (r = 0.14, p = 0.12), neither between LE and E (r = 0.16, p = 0.35) nor LE and NE (r = 0.14, p = 0.20). Among the potential confounders, BMI and WC negatively correlated with total testosterone (r = −0.382, p = 0.001; r = −0.367, p = 0.001, respectively).
We then evaluated the ability of LE to predict sex hormones concentrations; adjusting for different covariates. In a multiple regression analysis, LE weight explained an additional and significant 10.4% of the variance in LH levels, after statistically adjusting for age, WC, BMI and smoking [F (1,90) = 6.61, p < 0.05]. No effect of LE on total testosterone was observed.
Study of the possible “moderator” role of serum cortisol in the relationship between LE number and total testosterone
Given the lack of association between serum cortisol and LE, as well as between serum cortisol and total testosterone, and considering that the HPA axis responds with hyperactivity in some individuals or conditions (e.g. chronic stress, depression) or hypoactivity in others (e.g. atypical depression) we decided to perform an OLS regression analysis considering cortisol and LE as continuous variables and including age, BMI, WC and smocking as confounders (). The goal of this analysis was to estimate the effect of LE number on total testosterone levels, and how much, if at all, that effect depends on cortisol levels. depicts the complete model of regression summary and shows the correlations between LE number and total testosterone at different cortisol values (mean and mean ± 1SD). We found an interaction between LE and cortisol, conditioning total testosterone values [β = 0.022; t(112) = 2.42, p < 0.02].
Figure 2. Interaction between life events, cortisol and total testosterone.
An ordinary least square (OLS) regression analysis considering cortisol and life events (LE) as continuous variables and including age, body mass index, waist circumference and smocking, as confounders, was performed. Positive correlation between LE number and total testosterone at high cortisol values (mean and mean + 1SD) was obtained.
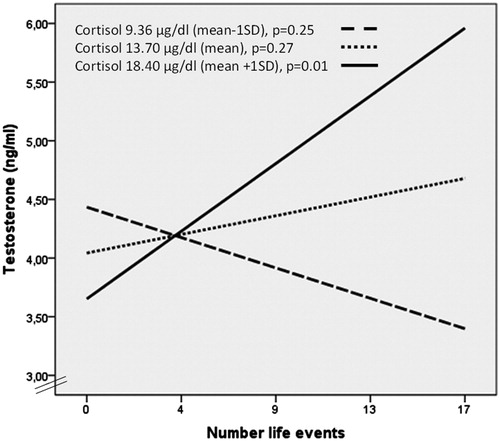
Table 3. OLS regression model with LE number × cortisol interaction for testosterone.
When applying the Johnson–Neyman technique to identify the ranges of concentration of the moderator variable (cortisol), we found that the effect of cortisol is observed from 15.4 µg/dl [t(112] = 1.98, p = 0.05]. These results confirm that cortisol is a moderator of the relationship between LE number and total testosterone. Moreover, only in men with high cortisol levels we found that LE number positively correlates with total testosterone (r = 0.284, p = 0.03) and a positive correlation between LH and NE (r = 0.398, p = 0.05) was observed.
Discussion
This study investigated the relationship between LE and sex hormones, as well as their association with the stress hormones, such as cortisol, epinephrine and norepinephrine. We found that LE weight and number positively correlated with LH. No association was observed between LE and cortisol or with total testosterone. However, LE number interacted with cortisol levels in relation to total testosterone. Moreover, LE number showed a significant positive relationship with total testosterone only in men with high cortisol levels.
Regarding the effect of psychosocial stress on gonadal profile, the results of different studies are contradictory. These contradictions may be due to gender differences in the populations studied, as well as differences in the type of stressors evaluated. Studies in animals showed that intravenous injection of corticotrophin-releasing hormone caused a statistically significant increase of LH in stressed macaques (Norman, Citation1993); however, the same author, a year earlier, showed a decrease in both LH and testosterone levels in stressed rhesus monkeys (Norman & Smith, Citation1992). Meanwhile in humans, no conclusive results were found between the impact of different stressors and LH (Chichinadze & Chichinadze, Citation2008). Some studies have found that social stress, including job stress, inhibit gonadal axis and are associated negatively with testosterone levels (Chandola et al., Citation2010; Hansen et al., Citation2009; Rivier & Rivest, Citation1991). In our study, we observed that LE predicted LH levels independently of other confounding factors (age, BMI, smoking and waist circumference). The mechanisms by which CRH influences GnRH release are not fully clarified (Chichinadze & Chichinadze, Citation2008) and there are no previous results about the relationship between LE and LH. In women, it has been reported that the inhibition of reproductive function following chronic stress as a result of the inhibition of GnRH (Berga & Loucks, Citation2006; Van den Berghe, Citation2001). However, in men results are contradictory.
Given the lack of association between LE and the stress hormones with total testosterone in our population, and knowing that the HPA axis may react at times with hyper or hypoactivity (Bellingrath & Kudielka, Citation2008; Bellingrath et al., Citation2008; Gold et al., Citation1995; Stratakis & Chrousos, Citation1995; Tafet & Smolovich, Citation2004), we evaluated the impact of different cortisol levels on testosterone, resembling previous findings in other studies (Fabre et al., Citation2013; Gidron et al., Citation2011; Tomei et al., Citation2003). It is possible that both environmental factors (e.g. LE) and biological vulnerability (e.g. HPA hyper/hypoactivity) might be needed to induce alterations in sex hormones (Wingfield & Sapolsky, Citation2003). In addition, it is possible that cortisol is related to sex hormones in a more complex manner than initially thought. Our results indeed support a permissive interaction between LE and cortisol in relation to total testosterone only in men with high cortisol values. These results point to the possibility that the HPA axis, through cortisol, may moderate the relationship between LE and sex hormones specifically and possibly between LE and health outcomes in general. This is an important conclusion given that previous studies have shown controversial results concerning the impact of different behavioral situations on testosterone levels. Popma et al. (Citation2007) showed that cortisol moderates the relationship between overt aggression and testosterone, only at low cortisol levels.
Moreover, the direct association observed between NE and LH in individuals with high levels of cortisol, could be one of the mechanisms that leads to an increase of testosterone observed during stress as described by other authors (Chichinadze & Chichinadze, Citation2008). It is known that NE stimulates GnRH secretion in both men and women (Chrousos et al., Citation1998). In vivo (Chiocchio et al., Citation1999) and in vitro studies (Mayerhofer et al., Citation1993) have shown that NE stimulates testosterone production. However, other studies have reported the inhibitory effect of glucocorticoids on the synthesis of gonadotrophic hormones (Hardy et al., Citation2002). Our results show that higher total testosterone levels in response to LE were observed in those subjects with high cortisol levels and a tendency to higher NE levels. Notably, the association between NE and LH described in this paper was also evident at high cortisol levels.
In turn it should also be taken into account, the psychological characteristics of the studied populations. It is known that dominant and aggressive subjects present an increased sympathetic activity (Arregi et al., Citation2006) as response to stress, with higher testosterone levels (Batrinos, Citation2012; Giotakos et al., Citation2003). Unfortunately, we were not able to evaluate these characteristics with the Holmes–Rahe questionnaire.
In this work as well as Vining et al.'s (Citation1983), we observed the lack of linearity between salivary and serum cortisol in concentrations above 10 nmol/l. Hellhammer et al. (Citation2009) argued that the ratio of concentration of salivary cortisol to serum cortisol is 1–2% in the low range of cortisol, but rises to 8–9% in high ranges. Moreover, it is known that 30% of the free cortisol is enzymatically converted to cortisone in saliva (Hellhammer et al., Citation2009), resulting in lower levels of salivary cortisol compared to serum cortisol. In this study, the findings reported between serum cortisol and LE could not be reproduced when considering the salivary cortisol.
One limitation of this study is that the use of the Holmes and Rahe questionnaire did not enable us to examine the subjective appraisal of LE by the participants, which could have potentially added predictive value beyond the mere number or population-based LE weights. The use of other scales with the ability to study the impact of chronic stress/life events on neuroendocrine systems will also be helpful to assess chronic stress, depression, anxiety and type of personality and to replicate our results. Other point to consider is that only one single measure of morning cortisol was obtained, which is not the optimal way to evaluate links between HPA activity and the HPG axis. Nevertheless, it is a valid and feasible approach for screening studies and has been previously applied (Fabre et al., Citation2013; Gidron et al., Citation2011; Tomei et al., Citation2003). Moreover, given variances in stress response, it could be that hormones respond differently to diverse types of stressors (Zitzmann & Nieschlag, 2001), future studies should broaden this hypothesis. Finally, testosterone decreases significantly with age, and is substantially lower in middle age than earlier in life. It is possible that the inclusion of middle-aged men could limit the interpretation of the variables under study in this population.
Our results indeed support a direct effect of LE on LH and a permissive interaction between LE and cortisol in relation to total testosterone only in men with high cortisol values. These results point to the possibility that the HPA axis, through cortisol and NE, may moderate the relationship between life events and sex hormones.
Declaration of interest
All authors declare that they have no conflicts of interest.
This work was supported by a grant from University of Buenos Aires (N°100041, 2012–2015). Bibiana Fabre received a Carrillo-Oñativia doctoral fellowship from the Health Ministry, Argentina.
References
- Andersen ML, Bignotto M, Machado RB, Tufik S. (2004). Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res 37:791–7
- Arregi A, Azpiroz A, Fano E, Garmendia L. (2006). Aggressive behavior: implications of dominance and subordination for the study of mental disorders. Aggress Violent Behav 11:394–413
- Batrinos M. (2012). Testosterone and aggressive behavior in man. Int J Endocrinol Metab 10:563–8
- Bellingrath S, Kudielka BM. (2008). Effort-reward-imbalance and over commitment are associated with hypothalamus-pituitary-adrenal (HPA) axis responses to acute psychosocial stress in healthy working school teachers. Psychoneuroendocrinology 33:1335–43
- Bellingrath S, Weigl T, Kudielka BM. (2008). Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort-reward-imbalance. Biol Psychol 78:104–13
- Berga SL, Loucks TL. (2005). The diagnosis and treatment of stress-induced anovulation. Minerva Gynecol 57(1):45–54 (Review)
- Berga SL, Loucks TL. (2006). Use of cognitive behavior amenorrhea therapy for functional hypothalamic. Ann NY Acad Sci 1092:114–29
- Breen KM, Karsch FJ. (2006). New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrinol 27:233–45
- Chandola T, Heraclides A, Kumari M. (2010). Psychophysiological biomarkers of work place stressors. Neurosci Biobehav Rev 35:51–7
- Chichinadze K, Chichinadze N. (2008). Stress-induced increase of testosterone: contributions of social status and sympathetic reactivity. Physiol Behav 94:595–603
- Chiocchio SR, Suburo AM, Vladucic E, Zhu BC, Charreau E, Décima EE, Tramezzani JH. (1999). Differential effects of superior and inferior spermatic nerves on testosterone secretion and spermatic blood flow in cats. Endocrinology 40:1036–43
- Chrousos G, Torpy D, Gold P. (1998). Interactions between the hypothalamic-pituitary adrenal axis and the female reproductive system: clinical implications. Ann Intern Med 129:229–40
- Elmlinger M, Dengler T, Weinstock C, Kuehnel W. (2003). Endocrine alterations in the aging male. Clin Chem Lab Med 41:934–41
- Fabre B, Grosman H, Mazza O, Nolazco C, Fernandez Machulsky N, Mesch V, Schreier L, et al. (2013). Relationship of cortisol and life events to the metabolic syndrome in men. Stress 16:16–23
- Gidron Y, Fabre B, Grosman H, Nolazco C, Mesch V, Mazza O, Berg G. (2011). Life events, cortisol and levels of prostate specific antigen: a story of synergism. Psychoneuroendocrinology 36:874–80
- Giotakos O, Markianos M, Vaidakis N, Christodoulou GN. (2003). Aggression, impulsivity, plasma sex hormones, and biogenic amine turnover in a forensic population of rapists. J Sex Marital Ther 29:215–25
- Gold PW, Licinio J, Wong ML, Chrousos GP. (1995). Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Ann NY Acad Sci 29:716–29
- Grant GW, Moores B. (1977). Resident characteristics and staff behavior in two hospitals for mentally retarded adults. Am J Mental Deficiency 82:259–65
- Hansen AM, Larsen AD, Rugulies R, Garde AH, Knudsen LE. (2009). A review of the effect of the psychosocial working environment on physiological changes in blood and urine. Basic Clin Pharmacol Toxicol 105(2):73–83
- Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, et al. (2002). Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod 67:1750–5
- Hayes AF, Matthes J. (2009). Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Meth 41:924–36
- Hellhammer D, Wust S, Kudielka B. (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34:163–71
- Holmes TH, Rahe RH. (1967). The social readjustment rating scale. J Psychosom Res 11:213–18
- Joels M, Karst H, Krugers J, Lucassen PJ. (2007). Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol 28:72–96
- Kajantie E, Phillips DI. (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31:151–78
- Koob GF. (1999). Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 46:1167–80
- Mayerhofer A, Bartke A, Began T. (1993). Catecholamines stimulate testicular steroidogenesis in vitro in the Siberian hamster, Phodopussungorus. Biol Reprod 48:883–8
- McComb JJ, Qian XP, Veldhuis JD, McGlone J, Norman RL. (2006). Neuroendocrine responses to psychological stress in eumenorrheic and oligomenorrheic women. Stress 9(1):41–51
- McEwen BS. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904
- Norman RL. (1993). Effects of corticotropin-releasing hormone on luteinizing hormone, testosterone, and cortisol secretion in intact male rhesus macaques. Biol Reprod 49:148–53
- Norman RL, Smith CJ. (1992). Restraint inhibits luteinizing hormone and testosterone secretion in intact male rhesus macaques: effects of concurrent naloxone administration. Neuroendocrinology 55:405–15
- Popma A, Vermeiren R, Geluk CA, Rinne T, van den Brink W, Knol DL, Jansen LM, et al. (2007). Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biol Psychiatry 61:405–11
- Quevedo K, Johnson A, Loman M, Lafavor T, Gunnar M. (2012). The confluence of adverse early experience and puberty on the cortisol awakening response. Int J Behav Dev 36:19–28
- Rivier C, Rivest S. (1991). Effect of stress on the activity of the hypothalamic-pituitary gonadal axis: peripheral and central mechanisms. Biol Reprod 45:523–32
- Stratakis CA, Chrousos GP. (1995). Neuroendocrinology and pathophysiology of the stress system. Ann NY Acad Sci 29:1–18
- Tafet GE, Smolovich J. (2004). Psychoneuroendocrinological studies on chronic stress and depression. Ann NY Acad Sci 1032:276–8
- Thomas SA, Friedmann E, Wimbush F, Schron E. (1997). Psychological factors and survival in the cardiac arrhythmia suppression trial (CAST): a reexamination. Am J Crit Care 6:116–26
- Tomei F, Rosati MV, Ciarrocca M, Baccolo TP, Gaballo M, Caciari T, Tomao E. (2003). Plasma cortisol levels and workers exposed to urban pollutants. Ind Health 41:320–6
- Uhart M, Chong R, Oswald L, Linc P, Wand G. (2006). Gender differences in hypothalamic–pituitary–adrenal (HPA) axis reactivity. Psychoneuroendocrinology 31:642–52
- Van den Berghe G. (2001). The neuroendocrine response to stress is a dynamic process. Best Pract Res Clin Endocrinol Metab 15:405–19
- Vining RF, McGinley RA, Maksvytis JJ, Ho KY. (1983). Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem 20(Pt 6):329–35
- Wingfield JC, Sapolsky RM. (2003). Reproduction and resistance to stress: when and how. J Neuroendocrinol 15:711–24
- Zitzmann M, Nieschlag E. (2001). Testosterone levels in healthy men and the relation to behavioural and physical characteristics: facts and constructs. Eur J Endocrinol 144:183–97