Abstract
A pooled database from diverse community samples was used to examine the associations of hair cortisol concentration (HCC) with self-reported stress and stress-linked mental health measures, including depression, anxiety, alcohol and drug use, disability and experiences with aggression. As part of innovative research using a mobile laboratory to study community mental health, data were pooled from five sub-studies: a random sample of the general population (n = 70), people who had received treatment for a mental health and/or substance use problem (n = 78), family members of people treated for mental health and/or substance use problems (n = 49), community volunteers who sometimes felt sad or blue or thought they drank too much (n = 83) and young adults in intimate partner relationships (n = 44). All participants completed a computerized questionnaire including standard measures of perceived stress, chronic stress, depression, anxiety, hazardous drinking, tobacco use, prescription drug use, illicit drug use, disability and intimate partner aggression. HCC was significantly associated with use of antidepressants, hazardous drinking, smoking and disability after adjusting for sub-study and potential confounders (sex, body-mass index, use of glucocorticoids and hair dyed). In addition, preliminary analyses suggest a significant curvilinear relationship between HCC and perceived stress; specifically, HCC increased with higher perceived stress but decreased at the highest level of stress. Overall, HCC was associated with mental health-related variables mainly reflecting substance use or experiencing a disability. The relationship between HCC and self-reported stress is unclear and needs further research.
Introduction
Cortisol is a well-accepted biological indicator of stress-response. Exposure to stress is associated with an activation of the hypothalamic-pituitary-adrenal (HPA) axis, which regulates the release of the glucocorticoid hormone cortisol from the adrenal cortex. Most research assesses cortisol in saliva, plasma or urine, reflecting cortisol concentrations at the time of collection rather than a long-term exposure to stress. Measurement of cortisol in hair has emerged as a promising strategy for assessing chronic stress (Russell et al., Citation2012). That is, the level of cortisol in hair reflects stress exposure over time, with each segment of hair growth reflecting prior hypothalamic-pituitary adrenal (HPA) axis activity (Wennig, 2000).
A number of studies have investigated whether hair cortisol concentration (HCC) provides a valid index for cortisol secretion by examining its association with self-reported stress. To date, results of the links between HCC and subjective appraisals of stress (i.e. perceived stress) have not been wholly consistent, with some studies finding significant relationships (Kalra et al., Citation2007; Karlén et al., Citation2011), but others finding no association (Dowlati et al., Citation2010; Stalder et al., Citation2010). Research has also examined HCC in relation to exposure to chronic life stressors; again, findings are mixed, with both significant (Stalder et al., Citation2012) and null associations found (Dettenborn et al., Citation2010). Divergent findings have been attributed to differences in samples, measures and time frames examined (Staufenbiel et al., Citation2013).
Stress is a complex and multi-faceted construct, making it important to assess how HCC relates not only to indicators of self-reported stress but also to social, psychological and behavioral measures that have been shown to be related to stress and HPA activity. Mental health and related variables may be especially important, given that experiencing a psychiatric disorder such as depression or anxiety has been linked to psychosocial stress (Kendler et al., Citation1999) as well as HPA activity and resultant cortisol secretion (Kendler et al., Citation1999; Knorr et al., Citation2010; Mantella et al., Citation2008). Stress and HPA activity have also been shown to be related to heavy drinking (Beresford et al., Citation2006) and other drug use (Piazza et al., Citation1990; Shaham & Stewart, Citation1995), psychosocial functioning and disability (Himle et al., Citation2009; Rasmussen et al., Citation2010; Sareen et al., Citation2007), as well as emotional arousal stemming from intimate partner violence (Feinberg et al., Citation2011).
As of yet, however, understanding of associations between HCC and mental health-related measures is limited. The link between HCC and mood and anxiety disorders has shown mixed findings (Dettenborn et al., Citation2012a; Dowlati et al., Citation2010; Steudte et al., Citation2011) and even less is known about the relation between HCC and substance use, disability and aggression. The aim of the present study was to examine the associations of HCC with (1) self-reported stress and (2) mental health-related factors including depression, anxiety, substance use, disability and experiences of intimate partner aggression.
Methods
Study participants
This study examines pooled data from a series of community studies conducted as part of a multidisciplinary research program, Researching Health in Ontario Communities (RHOC), which seeks to improve understanding of the inter-relations among stress, mental health, substance use/addiction and violence problems (Wells et al., 2011). Although each community study conducted as part of RHOC addressed a different aspect of these inter-relationships, they all involved collection of social, epidemiological and biological data using a mobile research laboratory. The advantage of using pooled data from these various studies is that they provide a heterogeneous sample with over-representation of persons who have mental health, substance use/addiction and violence problems. Thus, the data are ideal for exploring the extent that HCC is associated with these problems.
Pooled data from five sub-studies were collected in four communities of varying sizes (populations of approximately 18,000 to over 200,000) in Southern Ontario. The original sample from the five sub-studies consisted of 462 participants but was reduced to 324 when only those eligible and willing to provide hair samples were included (see description of hair sampling below). Although the target population and recruitment strategy varied across these five sub-studies, as described below, all participants completed a common set of core measures at the mobile research lab, with the a priori goal of combining samples in order to explore overarching issues such as the inter-relations among biological and self-reported measures of stress and mental health. In particular, all participants completed a self-administered computerized questionnaire with standard measures of stress, mental health, substance use and violence problems and were asked to provide biological samples, including hair (to assess cortisol).
The Random Walk sub-study used a modified “random walk” strategy using door-to-door recruitment to generate a random sample of the general population in one community (Flynn et al., Citation2013). This involved a two-stage cluster sampling design, with the random selection of blocks using Canadian census data and the random selection of households within blocks. The Consumer Journey (CJ) – Consumer sub-study recruited adults (aged 18 and over) who had mental health and/or substance use problems and had sought treatment for either type of problem (or both) (i.e. consumers) through posters placed in local treatment agencies and in various public locations within the communities. The Consumer Journey (CJ) – Family Member sub-study included family members of persons with mental health and/or substance use problems who were similarly recruited through posters placed in treatment agencies and in community settings. In both CJ studies, interviews were conducted to better understand, from a qualitative perspective, experiences of seeking and receiving care for people who have mental health and substance use problems. The Valuations of Health States sub-study assessed perceptions regarding the disabling effects of different health conditions. Adults (aged 18 and over) were recruited using advertisements and posters placed at various locations in the community requesting volunteers who “sometimes feel sad or blue or think you drink too much”. They completed ratings of disability for different combinations of health conditions. The Communication and Conflict sub-study recruited young adults (aged 18 to 29) to examine conflict in intimate partner relationships. Participants were recruited using Respondent Driven Sampling (RDS), a form of chain referral or “snowball sampling” involving recruitment of a small number of initial participants known as “seeds” who then recruit additional eligible participants. The “seeds” were recruited using posters in public places or were approached in a local shopping mall and asked to participate. Participants completed an in-depth interview about their experiences of conflict and aggression in intimate partner relationships.
The overarching RHOC project and all sub-studies were reviewed and approved by the Research Ethics Boards at the Centre for Addiction and Mental Health and Western University. For each sub-study, participants were given a description of the study and were advised of the voluntary nature of the study, data confidentiality and their rights as research participants. All participants provided written consent.
Hair sample collection and analysis
Participants in each of the five sub-studies were asked to provide a sample of their hair if they had a minimum hair length of 3 cm. Research assistants were trained to cut a sample of hair from the vertex posterior of the scalp. Hair samples were handled with gloves at all times. The most proximal 2 cm segment, reflecting systemic cortisol exposure over the previous two months, was selected for analysis. Given that a minimum hair length of 3 cm was required (to obtain the proximal sample of 2 cm), only 97 (48%) of the 202 men who participated in the five sub-studies were eligible and, of these, 93 provided a sample. Of the 260 female participants, 247 women (95%) were eligible to provide a hair sample and 237 women did so. Six cases were removed due to HCC exceeding 1500 ng/g, resulting in a final sample of 324 participants (91 males and 233 females).
Due to the large number of men who were ineligible to provide a hair sample, analyses (t-tests, Analysis of Variance and chi-square tests) were conducted to determine whether men who provided a hair sample differed from those who did not in terms of the main variables of interest (i.e. sociodemographic variables and measures of self-reported stress and mental health-related variables). The only significant difference found between these two groups was that those who used illicit drugs were less likely to provide a hair sample (39.2%) compared with individuals who did not use illicit drugs (54.8%) (chi-square = 4.382, df = 1, p = 0.036).
Hair cortisol was measured according to a standard procedure employed by the hair cortisol analysis research group at Western University (Russell et al., Citation2012). For each measurement, 10–15 mg of the hair collected was used. Hair samples were washed twice with isopropanol, cut finely with surgical scissors, and incubated in 1 ml of methanol for 16 h. Following this period, samples were evaporated with nitrogen gas on a test tube hot plate set to 50 °C. Samples were then reconstituted with PBS (pH 8.0) and analyzed on a salivary ELISA kit manufactured by Alpco Diagnostics for quantification. Intra- and inter-assay coefficients of variation were 6.7 and 11.3%, respectively. The ELISA kits also include reports of sensitivity of 1 ng/ml. Participants with HCC above 1500 ng/g were excluded, as is standard practice given that extremely high HCC may be reflective of Cushing's syndrome or contamination by corticosteroids (Thomson et al., 2010).
Self-report measures
Stress
Stress was measured with two instruments, one measuring past month perceived stress (Perceived Stress Scale – PSS; Cohen et al., Citation1983), the other, current exposure to chronic stress (Chronic Stress Scale; Turner & Turner, 2005). The PSS is a 10-item measure with good psychometric properties (Lee, 2012) that includes questions on how often in the previous month people view their lives as stressful and their ability to cope with stress (example items: in the last month, how often have you felt nervous and “stressed?” How often have you found that you could not cope with all the things that you had to do?). Response options range from never (0) to very often (4), with total scores on the scale ranging from 0 to 40 (Cronbach's alpha = 0.90, current sample).
Chronic stress was assessed with a 17-item measure reflecting current exposure to specific stressors, including financial, work, relationship, parenting and family stress. Respondents were provided with a list of stressors (example items: your neighborhood or community is too noisy or too polluted; you do not have enough money to buy the things you need) and asked whether they were true (1) or false (0) for the person at this time (scores range from 0 to 17). Used widely in community-based surveys in Canada and the US (e.g. Turner et al., 1995), the Chronic Stress Scale has been shown to have excellent measurement properties (Cronbach's alpha > 0.85), with an evidence of convergent and discriminant validity (Wheaton, 1994).
Depression and anxiety
The DSM-IV University of Michigan version of the Composite International Diagnostic Interview Short Form (CIDI-SF) was used to measure major depressive disorder and anxiety. The CIDI-SF was developed and validated against the full version of CIDI and demonstrated good classification accuracy (Kessler et al., Citation1998). Individuals met the criteria for depression if they endorsed screening questions for depression and anhedonia as well as three out of seven depressive symptoms (World Health Organization (WHO), 1990). For anxiety, two screener items from the CIDI-SF were used, with endorsement of either indicating anxiety problems.
Substance use
Standard measures of substance use were employed. For prescription drug use, respondents were asked whether they had used antidepressants in the past 12 months and given the following list of common brand names in Canada (generic names in parentheses): Prozac (fluoxetine), Paxil (paroxetine), Zoloft (sertraline), Celexa (citalopram), Cipralex (escitalopram), Effexor (venlafaxine) and Wellbutrin (bupropion). They were also asked whether they had used tranquilizers or anti-anxiety medications (examples included: Valium (diazepam), Ativan (lorazepam), Xanax (alprazolam), also known as tranqs, downers) in the previous 12 months. These two variables were scored dichotomously (any use versus no use).
For alcohol use, the Alcohol Use Disorders Identification Test (AUDIT) was used (Saunders et al., Citation1993), which is a 10-item measure that has good internal consistency and has been shown to be a valid screening tool for alcohol use disorders in diverse populations (Reinert & Allen, Citation2007). Scores on the AUDIT vary from 0 to 40 (Cronbach's alpha = 0.91, current sample). For smoking, we asked whether the person was a current smoker and usual number of cigarettes smoked per day, both standard questions (Heatherton et al., Citation1989). We also asked about any use of marijuana and other illicit drugs in the previous 12 months. Measures of illicit and prescription drug use were based on the Centre for Addiction and Mental Health (CAMH) Monitor (Ialomiteanu & Adlaf, Citation2010), an on-going monitoring survey of the Ontario general population, reflecting common drugs and their classifications in this province.
Disability
The World Health Organization Disability Assessment Schedule 2.0 (WHO-DAS II; short 12-item version) was used to assess limitations in activities and daily functioning (Üstün et al., 2010a). This instrument has been shown to be valid and reliable (Üstün et al., 2010b). Participants were asked how much difficulty they had doing various activities, with response options of “none”, “mild”, “moderate”, “extreme” and “severe, cannot do” (scores range from 12 to 52, Cronbach's alpha = 0.90, current sample).
Intimate partner aggression
We employed a measure adapted from Harris (Citation1992) and used in previous international research (Graham et al., Citation2011) that asks participants in recent intimate relationships (i.e. people who had been involved in an intimate relationship in the previous 2 years) to report the most physically aggressive thing done by their partner or toward their partner in the previous 2 years. Two dichotomous variables were constructed: whether participants had been the victim of aggression by a partner and whether the participant had been aggressive toward a partner.
Potential confounders
Understanding associations of HCC with stress and mental health-related variables requires consideration of potential confounders that may influence HCC, including sex, age, body-mass index (BMI), use of corticosteroids, hair color and whether hair has been treated. Sex and age were asked directly in the core questionnaire. BMI was calculated from self-reported weight and height as kg/m2. For use of glucocorticoids, participants were asked whether they had taken oral or parenteral glucocorticoids in the previous three months or whether they used any creams containing hydrocortisone in the previous month. For hair color, participants were asked to report their natural hair color. Whether or not hair had been treated with color was determined through laboratory observation. In particular, following the methanol incubation, if the solution became colored, this was an indication that the hair had been dyed.
Demographic variables
We also included standard measures of education, employment and income to describe the characteristics of the overall sample and samples in each sub-study.
Statistical analyses
The HCC measure was positively skewed (skewness of 2.78 and a kurtosis of 8.82). A log transformation applied to the data reduced the skewness to 0.68 and the kurtosis to 0.70, indicating that the transformed data did not depart substantially from normality. Therefore, all analyses were conducted using the log-transformed data. Significance of relationships was assessed using t-tests, Analysis of Variance, Pearson's r correlations and chi-square tests where appropriate and depending on whether variables were dichotomous, categorical or continuous. Linear regression analyses were also conducted, with HCC regressed onto each explanatory variable and multiple regression models computed controlling for identified confounders, including sex, age, BMI, dyed hair and use of glucocorticoids. The analyses also control for sub-study (with dummy variables entered to contrast the studies), thereby adjusting for the potential impact that each sub-study may have on the findings. In order to test for non-linear relationships between HCC and stress, multiple regression models were computed that included linear and quadratic components for the measures of stress (Perceived Stress Scale and Chronic Stress) based on the procedure presented by Cohen (Citation1978).
Results
Sample characteristics and assessment of potential confounders
presents demographic characteristics of study participants for the total sample and by sub-study. As is evident from the table, the pooled sample represented a wide range of ages, marital status groups, and education and income levels. The demographic characteristics were found to differ by sub-study, as expected given the different focus of each sub-study. Because of the heterogeneity in samples across the five sub-studies, a variable reflecting sub-study was included in all multivariate analyses of the relationships of HCC with self-reported stress and mental health-related measures.
Table 1. Sample characteristics by sub-study.
The overall descriptive data for the study variables were as follows. The mean HCC for the sample of 324 individuals was 274.42 ng/g, with a SD of 221.97 ng/g (log transformed mean = 5.41, SD = 0.60). The Perceived Stress Scale and the Chronic Stress scales had means of 20.03 (SD = 9.05) and 5.41 (SD = 4.05), respectively. Based on the CIDI-SF, 43.1% of respondents reported depression and 33.7% reported anxiety. In terms of substance use, 34.1% reported use of antidepressants, 35.4% tranquilizers, 49.1% smoking, 41.5% marijuana and 29.2% other illicit drugs (excluding marijuana). The mean score for hazardous drinking (AUDIT) was 7.44 (SD = 8.07) and the mean disability score (WHO-DAS II) was 22.72 (SD = 9.52). About 22.2% of respondents reported aggression by partner and 18.0% reported that they had been aggressive toward their partner in the past two years.
Associations between HCC and potential confounders of sex, age, use of glucocorticoids, natural hair color and dyed (treated) hair were tested: men had significantly higher HCC than did women (mean for men = 5.61, SD = 0.58; mean for women = 5.33, SD = 0.59; t = 3.76, df = 322, p < 0.001); BMI was significantly and positively correlated with HCC (r = 0.15, p = 0.010); HCC was significantly higher for those who used glucocorticoids (mean = 5.65, SD = 0.71) compared with those who had not (mean = 5.35, SD = 0.56; t = 3.12, df = 78, p < 0.001); HCC was lower for dyed hair (mean = 5.11, SD = 0.54) compared with untreated hair (mean = 5.48, SD = 0.59; t = 4.64, df = 322, p < 0.001) but was not significantly associated with natural hair color (F(5, 313) = 1.56, p = 0.172). All confounders with p values of p < 0.10 for associations with HCC (i.e. sex, age, BMI, use of glucocorticoids and hair treatment) were included in subsequent analyses as control variables (Mickey & Greenland, Citation1989).
Associations of HCC with self-reported stress, mental health, substance use, disability and intimate partner aggression
presents associations of HCC with self-reported stress and mental health-related measures. Standardized regression coefficients are reported in the table, with the first column showing unadjusted coefficients and the second column showing adjusted coefficients, controlling for identified confounders (based on analyses described above). Significant positive relationships with HCC in both unadjusted and adjusted analyses were identified for use of antidepressants (unadjusted β = 0.213, p < 0.001; adjusted β = 0.238, p < 0.001), level of hazardous drinking (unadjusted β = 0.145, p = 0.010; adjusted β = 0.129, p = 0.022), smoking (unadjusted β = 0.228, p < 0.001; adjusted β = 0.242, p < 0.001) and disability (unadjusted β = 0.202, p < 0.001; adjusted β = 0.159, p = 0.009). Associations between HCC and measures of perceived and chronic stress were weak, with a significant positive unadjusted relationship for chronic stress (unadjusted β = 0.114, p = 0.045) that became non-significant controlling for confounders and a non-significant relationship with perceived stress in both unadjusted and adjusted analyses. Anxiety (unadjusted β = 0.121, p = 0.032), use of tranquilizers (unadjusted β = 0.115, p = 0.046) and number of cigarettes (among smokers; unadjusted β = 0.168, p = 0.037) were significantly and positively associated with HCC in unadjusted analyses but not when controlling for confounders. Aggression by partner, on the other hand, was found to be significantly and positively associated with HCC controlling for confounders (β = 0.170, p = 0.011), but not in the unadjusted analysis.
Table 2. Associations between HCC and indices of stress, mental health, substance use/problems, disability and aggression.
Tests for curvilinear relation between stress and cortisol
Decreased cortisol secretion, or hypocortisolism, has been identified among people who have experienced trauma or developed post-traumatic stress disorder (PTSD) (Yehuda, 1997) possibly due to dysregulation in the HPA axis activity due to prolonged periods of stress (Heim et al., Citation2000) or stress severity. Therefore, to further explore the nature of relationships between self-reported stress and HCC, curvilinear associations of perceived stress and chronic stress with HCC were tested in regression analyses. Both measures have continuous scales (ranging from 0 to 40 and 0 to 16, respectively) and thus are suitable for assessing curvilinear relations. presents a series of three models for both measures. The first model includes only the linear component, the second model includes the quadratic component to test whether there is a significant non-linear relation, and the third model includes the covariates to assess whether a curvilinear relation remains controlling for other variables. As can be seen in , analyses for perceived stress show no significant linear association with HCC in model 1, but the analyses revealed a significant negative quadratic association in model 2 (β = −0.139, p = 0.018), indicating a possible inverse U relationship. The quadratic component (β = −0.127, p = 0.022) remained significant when the covariates were included in the third model. For chronic stress, the quadratic component was significant in model 2 (β = −0.129, p = 0.046) but became non-significant in the third model controlling for covariates.
Table 3. Test of quadratic associations of Perceived Stress Scale and Chronic Stress with HCC (transformed).
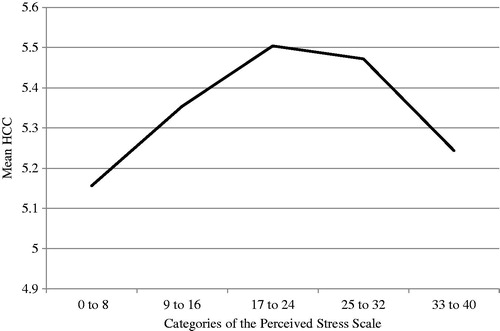
To further clarify the nature of relationship of HCC and perceived stress, mean HCC was compared across five levels of perceived stress (0–8, 9–16, 17–24, 25–32, and 33–40) adjusting for covariates. Significant overall mean differences among the levels of stress (F(4,288) = 2.96, p < 0.01) were found. As shown in , these means suggest an inverse U relationship, with HCC increasing with higher levels of perceived stress but decreasing at the highest level of perceived stress. The effect size for the largest group difference in HCC (i.e. between individuals in the lowest category of the perceived stress scale (scores of 0 to 8) who had the lowest level of HCC and those in the middle category (scores of 17 to 24) who had the highest level of HCC) was calculated to be 0.58, which reflects a medium effect size (Cohen, Citation1988). Thus, although some differences in HCC across categories of perceived stress appear to be small, differences between those at the lowest and highest level indicated a medium effect size that may have biological significance.
Figure 1. Mean HCC across categories of the Perceived Stress Scale. The Perceived Stress Scale was categorized as follows: 0–8, 9–16, 17–24, 25–32 and 33–40. Significant overall mean differences among the levels of stress (F(4,288) = 2.96, p < 0.01) were found, with means and standard deviations (SD) for each category of stress as follows: 0–8, mean = 5.16, SD = 0.59; 9–16, mean = 5.35, SD = 0.52; 17–24, mean = 5.50, SD = 0.60; 25–32, mean = 5.47, SD = 0.63; and 33–40, mean = 5.24, SD = 0.49.
Discussion
Using pooled data from five diverse community samples, we tested associations between HCC and measures of self-reported stress as well as mental health-related measures found to be linked to stress in previous research, including depression, anxiety, alcohol and drug use, disability and experiences with aggression. Correlations of HCC with self-reported chronic stress and perceived stress were small and both were non-significant controlling for confounders. Of the mental health-related measures, HCC was significantly related to the use of antidepressants, hazardous drinking, smoking, disability and aggression by partner, controlling for confounders.
The lack of significant relationships between HCC and self-reported stress is consistent with other studies that have shown null findings (e.g. Dettenborn et al., Citation2010; Dowlati et al., Citation2010; Stalder et al., Citation2010). This may indicate that there is no relationship between HCC and self-reported stress. However, methodological explanations are also possible. One problem in assessments of these associations, in the present and previous studies, is the mismatch between time frames for self-reported stress and HCC (Staufenbiel et al., Citation2013); that is, hair cortisol was estimated for the past two months (i.e. 2 cm segments of hair) while perceived stress was measured for the past month and the chronic stress items, though reflecting exposure to enduring stressors (e.g. financial problems), referred to current stress. Thus, future research needs to include measures of stress that better match the time frame for HCC. Null findings may also be due to what has been termed a “lack of psychoendocrine covariance”, with endocrine responses not coinciding with psychological stress responses (Staufenbiel et al., Citation2013). Additionally, the lack of extreme scores on self-reported stress, particularly for the chronic stress measure, may have reduced the ability to detect an association.
Another possible explanation for null associations between HCC and self-reported stress is that such associations may be non-linear. In the present study, exploratory analyses suggested a possible curvilinear association between HCC and perceived stress, with cortisol increasing with higher perceived stress, but dampened at the highest level. This finding, though preliminary, appears to be consistent with evidence for hypocortisolism found among people with PTSD (Heim et al., Citation2000; Yehuda, 1997). Given the preliminary nature of this finding, however, further research is needed on the nature of the relationship between HCC and self-reported stress, incorporating measures of stress that better capture the timing, duration, intensity and chronicity of stress in relation to cortisol secretion.
Consistent with Dowlati et al. (Citation2010), no association was found between HCC and depression. This finding, however, appears to be inconsistent with Dettenborn et al. (Citation2012a), who found higher HCC among patients with depression compared with healthy controls. It is important to note that participants in the Dettenborn et al. (Citation2012a) study were medicated inpatients who had severe forms of depression, while Dowlati et al. (Citation2010) measured depression among persons attending a cardiac rehabilitation centre. Therefore, associations may depend on the severity of the disorder or, as suggested by Dettenborn et al. (Citation2012a), on whether individuals are using antidepressant medications. Importantly, the present study found a significant positive association between HCC and antidepressant use. This finding supports a possible biochemical association between antidepressants and cortisol, as has been found in previous research (Bschor et al., Citation2002). However, the link between cortisol and antidepressant use may depend on the type of antidepressants used. For example, lithium has been found to be associated with an increase in cortisol (Bschor et al., Citation2011), whereas citalopram has been shown to decrease HPA axis activity (Bschor et al., Citation2012). Therefore, further research is needed on the type of antidepressants used and HCC.
The significant positive association between HCC and smoking, although inconsistent with findings of Dettenborn et al. (Citation2012b) is consistent with evidence of higher salivary cortisol concentrations in smokers than in non-smokers (Steptoe & Ussher, Citation2006). We also found a significant positive association between HCC and level of hazardous/harmful drinking. Some evidence suggests that stress may trigger substance use or relapse (Piazza et al., Citation1990; Shaham et al., Citation2000). Stalder et al. (Citation2010) found that alcoholics in acute alcohol withdrawal (whose hair samples likely reflected a period of heavy alcohol use) had higher HCC compared with alcoholics who had been abstinent as well as healthy controls (Stalder et al., Citation2010). Therefore, it is possible that alcohol use or intoxication produces alterations in HPA activity (Adinoff et al., Citation2003). Overall, although further research is needed to assess the exact mechanisms linking use of various substances and cortisol, antidepressants, tobacco and alcohol may be viewed as potential confounders of HCC.
The association of HCC with disability suggests that persons with disabilities may be at a high risk of additional negative health effects from high cortisol levels. Mental illness and functional disability have been shown to be highly correlated and to vary together over time (Von Korff et al., 1992). Therefore, those most severely affected by mental health problems, such that their daily lives and physical functioning are impaired, may have high cortisol levels.
Our results also offer partial evidence to suggest an association between HCC and partner aggression, with a significant association found for victimization but only in the model controlling for confounders. Previous evidence indicates that salivary cortisol is associated with experiencing intimate partner violence (Feinberg et al., Citation2011). The link between HCC and partner aggression is likely to vary depending on a number of factors, including the gender of the person, whether aggression is done with the intent to harm or in self-defense and the severity of aggression. It follows that understanding the relation between HCC and partner aggression may require additional information about study participants and the nature of their conflicts.
We also identified variables associated with HCC that may need to be controlled for when examining links between HCC and indicators of stress. Consistent with previous research (Dettenborn et al., Citation2012b; O'Brien et al., Citation2013), males were found to have higher HCC than females. As shown previously, use of glucocorticoids (O'Brien et al., Citation2013) and BMI (Stalder et al., Citation2012) were found to be positively associated with HCC. Additionally, consistent with Sauvé et al. (Citation2007), HCC was found to be lower for people with dyed hair than for people with untreated hair.
The present findings should be interpreted in light of the following limitations. Fewer males than females were eligible to participate, given that eligibility for hair samples required a minimum hair length of 3 cm (representing 3 months of hair growth). However, few significant differences on key variables were found between men who gave a hair sample and those who did not. Nevertheless, future research should examine potential bias in results for males related to eligibility to provide a hair sample. The assessment of hair dye (i.e. addition of color) did not account for the effects of other hair treatments such as bleaching, highlights or perms. Therefore, self-report data capturing various hair treatment practices is needed to fully assess the effects of hair treatment on HCC. BMI was based on self-reported height and weight; thus, findings for BMI need to be confirmed with objective assessments of BMI. The present data are cross-sectional, precluding interpretation of the temporal ordering of relationships. Also, as noted above, our analysis of cortisol was targeted to the previous two months based on the most proximal 2 cm of hair that was collected, but some of the self-report measures assess symptoms occurring in different time periods. Given evidence that the link between stress and cortisol release appears to be time dependent (Miller et al., Citation2007), longitudinal research is needed to map out the timing and impact of stress on cortisol. Another limitation is that we were not able to take exercise level into account, which has been shown to be linked to salivary and serum cortisol (Hansen et al., Citation2008). Finally, as noted above, we were unable to distinguish between different types of antidepressants used, which may influence cortisol in different ways.
An important strength of the current study was its examination of both self-reported stress and a number of mental health-related measures using a database consisting of high-risk individuals as well as people from the general population. Another key strength of the current study was its large total sample size, which exceeds that of most previous research on self-reported stress and HCC, with the majority of previous studies having samples of less than 100 subjects from mostly student and clinical samples (Staufenbiel et al., Citation2013).
In conclusion, HCC was found to be associated with mostly substance use variables, including use of antidepressants, smoking and hazardous drinking. Further research is needed to examine the nature of relationships between HCC and self-reported stress, as well as to explore in greater depth associations of HCC with mental health, substance use, disability and aggression. Additionally, further research is needed to clarify the extent that cortisol as measured in hair is a valid biological marker of stress and the possibility that HCC may actually decrease at the highest levels of self-reported stress.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
The research for this article was supported by the Canadian Institutes for Health Research (CIHR), Emerging Team Grant (CBG – 101926) and by the Canada Foundation for Innovation (#20289) and the Ontario Ministry of Research and Innovation. The views expressed here reflect those of the authors and do not necessarily reflect those of CIHR.
Acknowledgements
We would like to thank Sue Steinback for editorial assistance, the research staff who played an important role in recruiting study participants and collecting data at the mobile lab, and Roseanne Pulford who assisted in preparing and processing study materials.
References
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. (2003). Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res 27:1420–7
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Beresford HF, Du Y, et al. (2006). Hypercortisolism in alcohol dependence and its relation to hippocampal volume loss. J Stud Alcohol Drugs 67:861–7
- Bschor T, Adli M, Baethge C, Eichmann U, Ising M, Uhr M, Model LS, et al. (2002). Lithium augmentation increases the ACTH and cortisol response in the combined DEX/CRH test in unipolar major depression. Neuropsychopharmacology 27:470–8
- Bschor T, Ising M, Erbe S, Winkelmann P, Ritter D, Uhr M, Lewitzka U. (2012). Impact of citalopram on the HPA system: a study of the combined DEX/CRH test in 30 unipolar depressed patients. J Psychiatr Res 46:111–17
- Bschor T, Ritter D, Winkelmann P, Erbe S, Uhr M, Ising M, Lewitzka U. (2011). Lithium monotheraphy increases ACTH and cortisol responses in the Dex/CRH Test in unipolar depressed subjects. A study with 30 treatment-naive patients. PLoS Medicine 6:e27613
- Cohen J. (1978). Partialed products are interactions; partialed powers are curve components. Psychol Bull 85:858–66
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates
- Cohen S, Kamarck T, Mermelstein R. (1983). A global measure of perceived stress. J Health Soc Behav 24:385–96
- Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, Kirschbaum C, Otte C. (2012a). Introducing a novel method to assess cumulative steriod concentrations: increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress 15:348–53
- Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. (2010). Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology 35:1404–9
- Dettenborn L, Tietze A, Kirschbaum C, Stalder T. (2012b). The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress 15:578–88
- Dowlati Y, Herrmann N, Swardfager W, Thomson S, Oh PI, Van Uum S, Koren G, Lanctôt KL. (2010). Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsych Dis Treat 6:393–400
- Feinberg ME, Jones DE, Granger DA, Bontempo D. (2011). Relation of intimate partner violence to salivary cortisol among couples expecting a first child. Aggress Behav 37:492–502
- Flynn A, Tremblay PF, Rehm J, Wells S. (2013). A modified random walk door-to-door recruitment strategy for collecting social and biological data relating to mental health, substance use/addictions and violence problems in a Canadian community. IJADR 2:7–16
- Graham K, Bernards S, Wilsnack S, Gmel G. (2011). Alcohol may not cause partner violence but it seems to make it worse: a cross national comparison of the relationship between alcohol and severity of partner violence. J Interpers Violence 26:1503–23
- Hansen AM, Garde AH, Persson R. (2008). Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: a review. Scand J Clin Lab Investig 68:448–58
- Harris MB. (1992). Sex, race, and experiences of aggression. Aggress Behav 18:201–17
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. (1989). Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict 84:791–9
- Heim C, Ehlert U, Hellhammer DH. (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25:1–35
- Himle JA, Baser RE, Taylor RJ, Campbell RD, Jackson JS. (2009). Anxiety disorders among African Americans, blacks of Caribbean descent, and non-Hispanic whites in the United States. J Anxiety Disord 23:578–90
- Ialomiteanu A, Adlaf EM. (2010). CAMH Monitor 2009: technical guide, in Series CAMH Monitor 2009: technical guide, 2010. Toronto: Centre for Addiction and Mental Health
- Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. (2007). The relationship between stress and hair corisol in healthy pregnant women. Clin Invest Med 30:E103–7
- Karlén J, Ludvigsson J, Frostell A, Theodorsson E, Faresjö T. (2011). Cortisol in hair measured in young adults – a biomarker of major life stressors? BMC Clin Pathol 11:12
- Kendler KS, Karkowski LM, Prescott CA. (1999). Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156:837–41
- Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen H-U. (1998). The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF). Int J Methods Psychiatr Res 7:171–85
- Knorr U, Vinberg M, Kessing LV, Wetterslev J. (2010). Salivary cortisol in depressed patients versus control persons: a systematic review and meta-analysis. Psychoeuroendocrinology 35:1275–86
- Lee E-H. (2012). Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res 6:121–7
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Reynolds CF, Lenze EJ. (2008). Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology 33:773–81
- Mickey J, Greenland S. (1989). A study of the impact of confounder-selection criteria on effect estimation. Am J Epidemiol 129:125–37
- Miller GE, Chen E, Zhou ES. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 133:25–45
- O'Brien KM, Tronick EA, Moore CL. (2013). Relationship between hair cortisol and perceived chronic stress in a diverse sample. Stress Health 29:337–44
- Piazza PV, Deminiere JM, Le Moal M, Simon H. (1990). Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res 514:22–6
- Rasmussen A, Nguyen L, Wilkinson J, Vundla S, Raghavan S, Miller KE, Keller AS. (2010). Rates and impact of trauma and current stressors among Darfuri refugees in Eastern Chad. Am J Orthopsychiatry 80:227–36
- Reinert DF, Allen JP. (2007). The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 31:185–99
- Russell EM, Koren G, Rieder M, Van Uum S. (2012). Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Pychoneuroendocrinology 37:589–601
- Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, Asmundson GJG. (2007). Physical and mental comorbidity, disability, and suicidal behavior associated with Posttraumatic Stress Disorder in a large community sample. Psychosom Med 69:242–8
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88:791–804
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. (2007). Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 30:E183–91
- Shaham Y, Erb S, Stewart J. (2000). Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev 33:13–33
- Shaham Y, Stewart J. (1995). Stress reinstates heroin-seeking in drug free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 199:334–41
- Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L. (2010). Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol 85:357–60
- Stalder T, Steudte S, Alexander N, Miller RE, Gao W, Dettenborn L, Kirschbaum C. (2012). Cortisol in hair, body mass index and stress-related measures. Biol Psychol 90:218–23
- Staufenbiel SM, Penninx BWJH, Spijker AT, Elzinga BM, Van Rossum EFC. (2013). Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology ;38:1220–35
- Steptoe A, Ussher M. (2006). Smoking, cortisol and nicotine. Int J Psychophysiol ;59:228–35
- Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. (2011). Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res 186:310–14
- Thomson S, Koren G, Fraser L-A, Rieder M, Friedman TC, Van Uum SHM. (2010). Hair analysis provides a historical record of cortisol levels in Cushing's Syndrome. Exp Clin Endocrinol Diabetes. 118:133–8
- Turner HA, Turner RJ. (2005). Understanding variations in exposure to social stress. Health: An Interdisciplinary Journal for the Social Study of Health, Illness and Medicine 9:209–40
- Turner RJ, Wheaton B, Lloyd DA. (1995). The epidemiology of social stress. Am Sociol Rev 60:104–25
- Üstün TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, Saxena S, et al.; In collaboration with WHO/NIH Joint Project. (2010a). Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ 815–823
- Üstün TB, Kostanjsek N, Chatterji S, Rehm J. (2010b). Measuring health and disability. Manual for WHO Disability Assessment Schedule. WHODAS 2.0., in Series Measuring health and disability. Manual for WHO Disability Assessment Schedule. WHODAS 2.0., Geneva Switzerland: World Health Organization
- Von Korff M, Ormel J, Katon W, Lin EHF. (1992). Disability and depression among high utilizers of health care. A longitudinal analysis. Arch Gen Psychiatry. 49:91–100
- Wells S, Flynn A, Graham K, Rehm J, Cairney J, Kates N, Kennedy JL, et al. (2011). Using a mobile laboratory to study mental health, addictions, and violence: a research plan. Challenges 2:1–18
- Wennig R. (2000). Potential problems with the interpretation of hair analysis results. Forensic Sci Int 107:5–12
- Wheaton B. (1994). Sampling the stress universe, in Stress and Mental Health: Contemporary Issues and Prospects for the Future, Stress and Mental Health: Contemporary Issues and Prospects for the Future (Avison WR, Gotlib IH eds), pp 77–114, Plenum Press, New York
- World Health Organization (WHO). (1990). Composite International Diagnostic Interview (CIDI, Version 1.0). Geneva, Switzerland: World Health Organization
- Yehuda R. (1997). Sensitization of the hypothalamic-pituitary-adrenal axis in posttraumatic stress disorder. Ann NY Acad Sci. 821:57–75

