Abstract
Adverse experiences early in life may sensitize the hippocampus to subsequent stressors throughout the individual's life. We analyzed in male rats, whether, the interaction between early maternal separation and chronic stress affects: (1) the volume of the dorsal hippocampus, (2) CA1, CA2/3 and dentate gyrus (DG) and () hippocampal-dependent memory in adulthood. Male Wistar rats were subjected to daily maternal separation for 4.5 h between postnatal days 1–21. From postnatal day 50, animals were exposed to a chronic unpredictable stress paradigm during 24 days. The volumes of the dorsal hippocampus, their areas or strata did not reveal significant differences between treatments. Non-maternally separated and stressed animals showed poor hippocampal performance in a contextual fear conditioning test, with a significant reduction in freezing behavior during post-conditioning compared with control and maternally separated and stressed animals. Also, memory retrieval 24 h after conditioning was significantly weaker in this group than in control animals. Memory performance in maternally separated and stressed rats was similar to control animals. Our results show an interaction between early environment experiences and chronic variable stress in young adulthood as evidence that early stressful experiences do not necessarily lead to a negative outcome but can help in maintaining brain plasticity and increase fitness when animals reach adulthood.
Introduction
There is compelling evidence that early adverse life events are associated with changes in the physiological response to stress and increase vulnerability to psychopathology in adult life (Aisa et al., Citation2009). Animal models of early life stress are based on postnatal disruption of the mother–infant interaction as in maternal separation (MS), consisting of separating mother and offspring for a prolonged period of 3 h/day during the first weeks after birth (Aisa et al., Citation2009; Daskalakis et al., Citation2013). Although it has been shown that MS in animals results in behavioral and neuroendocrine signs of elevated stress reactivity as adults (Eiland et al., Citation2012), other evidence suggests that aversive early life experiences do not inevitably lead to a negative outcome (Daskalakis et al., Citation2013; Nederhof & Schmidt, Citation2012).
Stressful conditions can activate the sympatho adrenomedullary system and the hypothalamo-pituitary-adrenal (HPA) system which form interacting signaling networks that coordinate metabolic and behavioral responses to stress as well as memory storage and retrieval (De Kloet et al., Citation2005). In the HPA system, corticosteroids act through mineralocorticoid and glucocorticoid receptors (MRs and GRs, respectively), which are co-expressed abundantly in the neurons of limbic structures as in the hippocampus (De Kloet et al., Citation2005; Herman et al., Citation2005). In this structure, which is involved in the inhibition of the HPA system and in encoding spatial information in contextual memory, elevated corticosteroid levels induce loss of complexity and decreased length of apical dendritic branches in the CA1 and CA3 areas (Dranovsky & Leonardo, Citation2012; McEwen et al., Citation2012). Chronic stress also decreases adult hippocampal neurogenesis in the dentate gyrus (DG) (Dranovsky & Leonardo, Citation2012; McEwen et al., Citation2012). Those effects depend on the magnitude, type and duration of the stress and the developmental timing (Dranovsky & Leonardo, Citation2012; McEwen et al., Citation2012). There are windows of vulnerability to stress that affect brain development early in life and insults at this stage can be detrimental (Andersen, Citation2003). The hippocampus continues to grow during the transition into the juvenile period and young adulthood, which is also a vulnerable stage for stress insults (Isgor et al., Citation2004).
It has been proposed both that stress-induced changes could be physiologically protective (McEwen et al., Citation2012) and that these could increase vulnerability to damage (Conrad, Citation2008). Exposure to either explicit stressors or to artificially elevated corticosteroid levels has been shown to disrupt hippocampal-dependent spatial memory performance in rodents (Conrad, Citation2008; Eiland & McEwen, Citation2012). Conversely, Champagne et al. (Citation2008) demonstrated that the offspring of low maternal care mothers displayed enhanced hippocampal-dependent learning under stressful conditions.
Given these controversial findings, our goals were to analyze in male rats, whether, the interaction between early maternal separation and chronic stress affects: (1) the volume of the dorsal hippocampus, (2) CA1, CA2/3 and dentate gyrus (DG) and (3) hippocampal dependent memory in adulthood.
Methods
Male Wistar rats were used in this study. All animals were maintained under standard laboratory conditions (12 h light–dark schedule; lights on from 07:00 to 19:00 h; temperature: 21 ± 2°C) with food and water ad libitum. All protocols were approved by the Animal Care and Use Committee of the National University of Córdoba, in accordance with the NIH Guide on Care and Use of Laboratory Animals.
The maternal separation procedure was based on a previously standardized protocol (Renard et al., Citation2007, Citation2010). Litters were culled to 10 pups (4–5 males, 5–6 females) on the day after birth [postnatal day (PND) 1]. Whole litters were randomly assigned to one of two rearing conditions: maternal separation or non-maternal separation. For maternal separation litters, rats were separated from the mother for 4.5 h every day from PND1 to PND 21. Dams were removed from the home cage and placed in an adjacent cage from 8:00 am to 12:30 pm. In control litters, pups remained with the dams undisturbed until weaning age at PND 22, except for routine cage cleaning twice a week. From PND 22 until PND 50, male offspring were group-housed with their respective littermates and handled for 3 min/day to minimize stress reactions to manipulation. At 50 days of age, male rats from each litter were subdivided into two groups, stressed and non-stressed and housed in pairs. To control litter effects, each experimental group was made up of subjects from different litters (one animal per litter per condition). In the stressed groups, rats were exposed to a 24-day chronic variable stress (CVS) paradigm: 4 h of noise produced by an alarm bell (85 dB), ether anesthesia until loss of consciousness, two intraperitoneal injections of isotonic saline, 24 h of food deprivation, 1 h of restraint inside a 6-cm diameter metal cylinder (Renard et al., Citation2007, Citation2010). To maximize the unpredictability, the stressors were applied in random order and at varying times during the light phase, except on day 24 when noise was used as the last stressor. All animals received the same sequence of stressors in each replication of the experiments. The unstressed control group were not exposed to any stressor and remained in their home cages until euthanization for brain processing.
In summary, we used a factorial (2 × 2) experimental design, with rearing condition [non-maternally separated (NMS) or maternally separated (MS)], and stress [unstressed (NCVS) or stressed (CVS)] as factors.
Twenty-four hours after the last stress session, each animal was tested for hippocampus-dependent contextual memory by a fear-conditioning test (Rudy et al., Citation2002). During the first day (acquisition of contextual memory), the animal was left in the box for 3 min, allowing it to explore freely and form a hippocampal representation of the context (preconditioning) (Rudy et al., Citation2002). Next, four electric shocks were applied (intensity 0.41 mA during 2 s) separated by intervals of 64 s (conditioning). Finally, the animal was left in the box for 3 min with no electrical stimulus (post-conditioning). Twenty-four hours later, contextual memory retrieval was assessed by re-exposing the animals to the same environment as the first day during 8 min, without applying the electric shock. As an index of contextual learning, the percentage of freezing was calculated each day during preconditioning, conditioning (discarding the first 10 s of the inter-trial period of unconditioned response to the electrical stimulus) and post-conditioning (during day 1), and retrieval of contextual memory (during day 2). All testing took place between 09:00 and 12:00 h, and the assessor was blind to the experimental condition of each animal.
The next day, each rat was deeply anesthetized with chloral hydrate (540 mg/kg) and transcardially perfused with heparinized saline followed by a 4% paraformaldehyde phosphate-buffered saline (PBS) solution. Brains were removed and left overnight in 4% paraformaldehyde–PBS solution and then stored at 4°C in 20% sucrose--PBS solution until sectioning.
Serial bilateral coronal sections (40 µm thick) were subsequently obtained from the dorsal hippocampus (from Bregma – 1.72 mm to Bregma – 3.84 mm) using a freezing microtome (Paxinos & Watson, Citation2007). The sections were systematically ordered into 1:20 series in multidish wells. Using a random starting point, all the sections in a series were mounted onto glass slides and stained with Nissl. The volumes of the hippocampal formation were estimated on the basis of the Cavalieri principle (Gundersen et al., Citation1988). As field CA2 is unidentifiable in Nissl-stained sections, this area was analyzed together with CA3 (Tata & Anderson, Citation2010). For CA1 and CA2/CA3, pyramidal cell layer volumes were measured. Apical dendrites from pyramidal cells that project through the strata lucidum, radiatum and lacunosum-moleculare were considered as the apical layer and the dendrites that project into the stratum oriens as the basal layer (Tata & Anderson, Citation2010). The coefficient of error (CE) of the individual estimates was calculated according to Slomianka and West (Citation2005) and in all cases it was <0.5.
To test whether hippocampal volume variation was correlated with body weight, group differences were determined by two-way analyses of covariance, with rearing condition and stress as factors and body weight as a covariate. As covariance was not significant in any group, two-way ANOVA (with rearing condition and stress as factors) was used for statistical comparisons. For the contextual fear memory test, a two-way ANOVA with the same factors was also performed. Significant results were further analyzed using Fisher's least significant difference (LSD) post hoc test. Significance was set at p < 0.05. All analyses were conducted by using Infostat software (www.infostat.com.ar).
Results
There was no effect of either MS or CVS on dorsal hippocampal volume () or volumes of individual subregions (). In addition, there were no MS by CVS interactions ().
Figure 1. Effect of early maternal separation and variable chronic stress on the total volume (in mm3, mean ± SE) of the dorsal hippocampus. The number of animals for each treatment is included inside each bar.
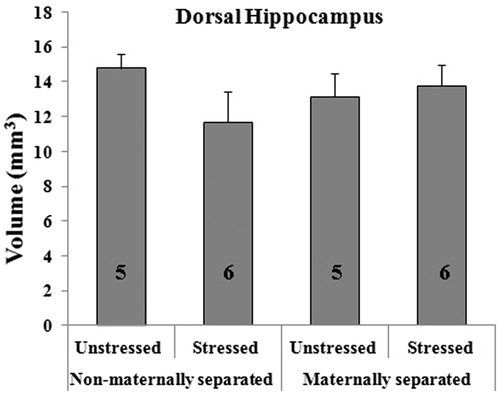
Figure 2. Effect of early maternal separation and variable chronic stress on the volume (in mm3, mean ± SE) of the CA1 (A), CA2/CA3 (B) and DG (C) areas of the dorsal hippocampus and their respective strata (D–F). The number of animals for each treatment is included inside each bar.
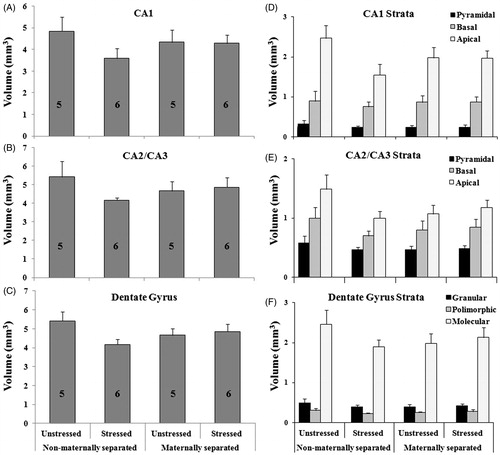
Figure 3. Effect of early maternal separation and variable chronic stress on freezing behavior recorded on the first day of the test, during the conditioning and post-conditioning stages (A) and during memory retrieval 24 h after conditioning (B). Values represent the percentage time of freezing (mean ± SE) for animals tested in the same context. The number of cases for each treatment is included inside each bar. (*) Means significant difference between groups (p < 0.05). Post hoc analysis showed significant difference between NMS-CVS and controls (NMS-NCVS) and MS-CVS during post-conditioning (A) and between NMS-CVS and controls (NMS-NCVS) during memory retrieval (B).
Table 1. Results of the two-way ANOVA analysis from dorsal hippocampal, its areas and strata volumes.
With regard to contextual memory, no experimental group showed freezing behavior during the preconditioning. All animals showed exploratory behavior, indicating the absence of alterations in their ability to explore the context.
During the conditioning stage, MS (F1,42 = 0.004; p = 0.947), CVS (F1,42 = 0.010; p = 0.923) and their interaction (F1,42 = 1.81; p = 0.186) did not have significant effects on freezing behavior ().
There were no main effects of MS (F1,42 = 0.004; p = 0.947) or CVS (F1,42 = 0.010; p = 0.923) on freezing behavior during the conditioning stage. Also, there were no MS by CVS interactions (F1,42 = 1.81; p = 0.186).
During post-conditioning, an increased percentage of freezing was observed in all groups compared with that during conditioning (). There was a significant MS × CVS interaction effect on the percentage of freezing during post-conditioning. Analysis of percentage of freezing in post-conditioning revealed a significant MS × CVS interaction effect (F1,42 = 6.22; p = 0.01).
Post hoc test showed significantly less freezing behavior in NMS-CVS animals than in NMS-NCVS (controls) and MS-CVS animals ().
During retrieval, the ANOVA for the percentage of freezing showed a significant MS × CVS interaction effect (F1,42 = 3.78; p = 0.05). Post hoc testing revealed that freezing behavior was significantly less in NMS-CVS animals than in controls ().
In all cases, a lower percentage of freezing means worse contextual memory performance.
Discussion
This study of the interaction between early maternal separation and chronic stress on hippocampal-dependent memory revealed that NMS-CVS animals have a significantly lower percentage of freezing than both controls (NMS-NCVS) and MS-CVS animals, in the post-conditioning period of the fear conditioning test. During retrieval, this decrease is evidenced only in NMS-CVS animals compared to controls.
In contrast, other authors have shown that chronic restraint stress in rats enhanced contextual freezing in the fear conditioning (Conrad et al., Citation1999; Sandi et al., Citation2001). A possible explanation of these contradictory results might be the difference in the stress protocol used, i.e. chronic restraint versus chronic unpredictable variable stressors. This is important, since the impact of stressors depends on the coping abilities of the organisms – i.e. their ability to master the situation – and successful coping will depend on the controllability and predictability of the stressor (Koolhaas et al., Citation2011). Indeed, Das et al. (Citation2005) showed that rats subjected to chronic predictable stress exhibit increased memory performance in an aversive test (passive avoidance), while rats subjected to chronic unpredictable stress showed no significant learning. Ricon et al. (Citation2012) demonstrated that exposure to unpredictable chronic stress in adult rats produces deficits in learning and memory in the two-way shuttle avoidance task. These authors emphasize that the unpredictability rather than the severity of the stress protocol induces impaired cognitive function in adulthood (Ricon et al., Citation2012).
However, in our investigation, chronic variable stress in maternally separated animals did not impair learning but, in fact, they showed significantly more freezing during post-conditioning than the NMS-CVS, and a similar retrieval ability to that of the control group. Thus, chronic variable stress in our study had two different outcomes in learning and memory abilities depending on the interaction between the early and the adult environment.
In the same line, previous studies in our laboratory indicated that male rats subjected to early maternal separation are better able to cope with chronic stress in adulthood (Renard et al., Citation2007, Citation2010). In these animals, lower levels of ACTH and plasmatic corticosterone were detected compared with NMS-CVS (Renard et al., Citation2007). They also showed a greater number of GR immunoreactive cells in CA areas compared to controls (Renard et al., Citation2010).
Interpretation of the reported effects of MS and adult stress interactions on memory is complicated because of the substantial variations between strains of animals, duration, frequency and age of application of MS and stressors and the tests employed for measuring memory (Claessens et al., Citation2011). Ultimately, the lasting consequences of maternal separation will depend on specific genetic vulnerability and on the combination with a specific environment in adulthood (Schmidt et al., Citation2011).
It has been suggested that adverse experiences in early developmental stages trigger adaptive responses that increase individual fitness under stressful conditions in adulthood. When the adult environment matches the early environment, it promotes a healthy and resilient phenotype, while when there is a mismatch between both environments it is conducive to illness (Nederhof & Schmidt, Citation2012). This has been proposed as the match–mismatch hypothesis (Daskalakis et al., Citation2013; Nederhof & Schmidt, Citation2012).
In our work, NMS-CVS animals may be considered as the most vulnerable group, probably as a result of the mismatch between the early and adult environment (considering NMS as a non-adverse environment and stress as an adverse environment).
Animals maternally separated and without exposure to CVS showed no significant differences, even though there was a mismatch between the early and late environment. This discrepancy with the match–mismatch hypothesis may be attributed to our separation protocol. The importance of leaving the offspring in the home-cage should be highlighted, since it has been reported that, when they are separated from their mother and placed in a novel cage, they maintain hyper-responsiveness to stress throughout life (Daskalakis et al., Citation2013). In our protocol, this adaptive response would enable the young animals to maintain homeostasis during this early stage of development by predicting the return of the mother when they are exposed again to maternal separation (Claessens et al., Citation2011). In addition, the mother's proximity in an adjacent cage in the breeding room would allow communication by vocalizations between dam and offspring (Oomen et al., Citation2010).
It has been proposed that chronic stress can induce morphological changes in the hippocampus which cause subsequent poor performance in learning and memory tasks (Conrad, Citation2006). Contrary to our expectations, in our work there were not significant differences in hippocampal volume between the experimental groups.
As NMS-CVS animals are the group that showed the worst contextual memory, we still consider that there must be an anatomical substrate mediating such differences between the groups. In the NMS-CVS group, there was a 26% and 23% reduction in the volume occupied by apical dendrites from areas CA1 and CA3, respectively, compared with controls. We think that statistical significance could not be reached for reasons of variability in the data set, since the reductions in hippocampal volume that we calculated were larger than those reported by other authors as a result of chronic stress protocols (Donohue et al., Citation2006; Stewart et al., Citation2005).
This study showed that chronic variable stress in adulthood can impact hippocampal functioning depending on the early postnatal experiences and that this interaction does not always involve deleterious or pathogenic consequences in individuals. We saw that chronic variable stress in animals reared with their mothers produced a significantly lower percentage of freezing in the post-conditioning period than in either animals that were also exposed to stress but maternally separated early in life, or in controls. During the retrieval of contextual memory, this decrease is seen only in non-maternally separated animals exposed to chronic stress compared with controls. Thus, the consequences seen in hippocampal-dependent performance could be the result of a mismatch between early and adult experiences. Due to the complex interaction between early and late stressful conditions in life, further understanding is needed of how this environmental interplay influences individual stress sensitivity.
Declaration of interest
This work was supported by SECyT Grants 214/10 and 26/11, MINCyT PID 2008-Resol 82/09. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Aisa B, Gil-Bea FJ, Marcos B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. (2009). Neonatal stress affects vulnerability of cholinergic neurons and cognition in the rat: involvement of the HPA axis. Psychoneuroendocrinology 34(10):1495–505
- Andersen SL. (2003). Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27(1):3–18
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. (2008). Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci 28(23):6037–45
- Claessens SE, Daskalaki NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. (2011). Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology 214(1):141–54
- Conrad CD. (2006). What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev 5(1):41–60
- Conrad CD. (2008). Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci 19(6):395–412
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS. (1999). Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113(5):902–13
- Das A, Rai D, Dikshit M, Palit G, Nath C. (2005). Nature of stress: differential effects on brain acetylcholinesterase activity and memory in rats. Life Sci 77(18):2299–311
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER. (2013). The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38(9):1858–73
- De Kloet ER, Joëls M, Holsboer F. (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6(6):463–75
- Donohue HS, Gabbott PL, Davies HA, Rodríguez JJ, Cordero MI, Sandi C, Medvedev NI, et al. (2006). Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience 140(2):597–606
- Dranovsky A, Leonardo ED. (2012). Is there a role for young hippocampal neurons in adaptation to stress? Behav Brain Res 227(2):371–5
- Eiland L, McEwen BS. (2012). Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus 22(1):82–91
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. (2012). Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology 37(1):39–47
- Gundersen H, Bendtsen T, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard J, et al. (1988). Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96(1–6):379–94
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29(8):1201–13
- Isgor C, Kabbaj M, Akil H, Watson SJ. (2004). Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 14(5):636–48
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, et al. (2011). Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35(5):1291–301
- McEwen BS, Eiland L, Hunter RG, Miller MM. (2012). Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62(1):3–12
- Nederhof E, Schmidt MV. (2012). Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol Behav 106(5):691–700
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joels M, et al. (2010). Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci 30(19):6635–45
- Paxinos G, Watson C. (2007). The rat brain in stereotaxic coordinates. 6th ed. New York: Elsevier/Academic Press. 456p
- Renard GM, Rivarola MA, Suárez MM. (2010). Gender-dependent effects of early maternal separation and variable chronic stress on vasopressinergic activity and glucocorticoid receptor expression in adult rats. Dev Neurosci 32(1):71–80
- Renard GM, Rivarola MA, Suárez MM. (2007). Sexual dimorphism in rats: effects of early maternal separation and variable chronic stress on pituitary-adrenal axis and behavior. Int J Dev Neurosci 25(6):373–9
- Ricon T, Toth E, Leshem M, Braun K, Richter-Levin G. (2012). Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress, 15(1):11–20
- Rudy JW, Barrientos RM, O'Reilly RC. (2002). Hippocampal formation supports conditioning to memory of a context. Behav Neurosci 116(4):530–8
- Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. (2001). Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience 102(2):329–39
- Schmidt MV, Wang XD, Meijer OC. (2011). Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology 214(1):131–40
- Slomianka L, West MJ. (2005). Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience 136(3):757–67
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, Rodriguez JJ, et al. (2005). Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience 131(1):43–54
- Tata DA, Anderson BJ. (2010). The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol Behav 99(2):186–93

