Abstract
The analysis of hair cortisol concentrations (HCC) is a promising new biomarker for retrospective measurement of chronic stress. The effect of basic military training (BMT) on chronic stress has not yet been reported. The aim of this study was to investigate the effect of 10-week BMT on HCC, while further exploring the role of known and novel covariates. Young healthy male recruits of the Swiss Army participated twice, 10 weeks apart, in data collection (1st examination: n = 177; 2nd examination: n = 105). On two occasions, we assessed HCC, perceived stress and different candidate variables that may affect HCC (e.g. socioeconomic status, meteorological data). Military training increased perceived stress from the first to the second examination, but did not affect HCC. In line with this, there was no correlation between HCC and perceived stress ratings. This could be interpreted as a missing influence of mainly physical stress (e.g. exercise) on HCC. In contrast, significant correlations were found between HCC and ambient temperature, humidity and education. Future studies should control for meteorological data and educational status when examining HCC.
Introduction
Stress has become an omnipresent phenomenon in our everyday life. One important pathway linking stress with morbidity is the hypothalamic–pituitary–adrenal (HPA) axis. Cortisol, which is secreted during activation of the HPA axis, is an important hormone in the bodily stress response. Hair cortisol represents cumulative stress hormone reactivity, providing the ability to retrospectively index stress levels extending back months or even years (Russell et al., Citation2012). Therefore, it is a useful long-term biomarker for detection of chronic stress in humans. How exactly cortisol enters the hair is not yet fully understood. It is assumed that a passive diffusion into the medulla of the hair shaft stems primarily from the bloodstream (Pragst & Balikova, Citation2006).
Military training was thought to be a real-life stressor (Bernton et al., Citation1995; Hellhammer et al., Citation1997). Even though the Swiss Armed Forces is a compulsory military service, where participation in war is not the first training objective, basic military training (BMT) may be experienced as especially stressful. In support of the stressful character, studies found a significant increase in salivary cortisol concentration during the first weeks of military training (Bernton et al., Citation1995; Hellhammer et al., Citation1997). This might be caused by a combination of high physical and mental demands during their training. Despite the fact that the military service is compulsory, the physical demands imposed on Swiss recruits are comparable to the demands on armed forces of other nations or professional athletes (Wyss et al., Citation2012). In a recent study, a positive correlation between training volume and HCC was found (Skoluda et al., Citation2012).
While the effect of stress on the HPA axis is unquestioned, findings show inconsistent associations between ratings of perceived stress and acute cortisol secretion measured with previous methods (Campbell & Ehlert, Citation2012). Hair cortisol has been investigated in terms of different real-life stressors and subjective stress perception. Kalra et al. (Citation2007) were the first to examine the relationship between HCC and self-reported stress. They found a significant positive correlation between HCC and self-reported stress levels. Van Uum et al. (Citation2008) found higher levels of perceived stress and HCC in patients with severe chronic pain as compared to healthy controls, while the correlation between both measures was not significant. Several other studies examining different stressors (e.g. unemployment) were also unable to find a significant relationship between HCC and perceived stress ratings (e.g. Dettenborn et al., Citation2010; Stalder et al., Citation2010). Karlen et al. (Citation2011) found that only the occurrence of serious life events predicted increased HCC in healthy students. Taken together, no clear relationship between perceived stress and HCC exists.
Since little is known about the characteristics of hair cortisol, a significant research interest focuses on the factors influencing hair cortisol concentrations (HCC). In this regard, natural hair color (Dettenborn et al., Citation2012), daily use of hair products (Manenschijn et al., Citation2011a) and smoking (Skoluda et al., Citation2012) do not affect HCC. Meanwhile, inconsistent findings were found with regard to the influence of age, sex, weight-related parameters and hair washing (Dettenborn et al., Citation2012; Stalder & Kirschbaum, Citation2012; Stalder et al., Citation2012). In addition, Russell et al. (Citation2014) recently demonstrated that human sweat contains cortisol, which likely contributes to hair cortisol content. Furthermore, the authors showed that when human hair samples are exposed to a sweat-like solution with physiological concentrations of sweat cortisol, hair cortisol concentrations were significantly elevated after an hour of exposure. Interestingly, these concentrations could not be reduced by regular methods of hair washing with isopropanol. Therefore, previous studies examining HCC in different populations (e.g. Ugandan individuals, from Steudte et al., Citation2011) or under different physical conditions (e.g. endurance athletes, from Skoluda et al., Citation2012) have to be considered with caution.
In this study, we expected (a) a positive effect of BMT on HCC and perceived stress, and (b) in line with previous findings, no relationship between perceived stress and HCC. In addition, we examined potential covariates mentioned in the literature (e.g. hair color, sociodemographic variables) as well as sweat-related candidate covariates (meteorological data).
Materials and methods
Participants and procedure
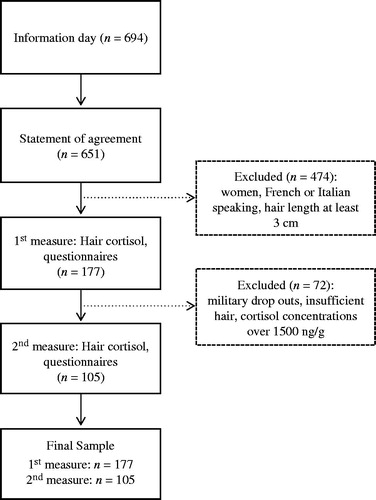
This study was part of a larger project on mental and physical health during military service and received ethical approval from the Ethics Committee of the Canton of Aargau, Switzerland. Subjects were recruited during the first week of basic training in the Swiss Armed Forces Infantry school in 2011 (overall: n = 694). Switzerland has a compulsory military service (Annen et al., Citation2010) and every young man who, after a recruitment process, is rated to be mentally and physically healthy, is required to complete military service. Typically, these recruits represent a sample of healthy young men. After the researchers described the study, volunteer recruits signed an informed consent (n = 651; ). Inclusion criteria were defined as male sex and German speaking. Exclusion criteria were: female sex (n = 1), Italian or French speaking (n = 81) and recruits with a hair length shorter than 3 cm (n = 392). One hundred and seventy-seven men met the inclusion criteria.
During the evaluation of hair color, 15 participants were excluded because of data quality (bad picture quality and poor light conditions for defining hair color). Therefore, the relationship between hair color and HCC was studied for 163 participants. For all other calculations, the entire sample size was used (n = 177).
Subjects were asked to provide a hair sample and complete a set of questionnaires twice, once during the first week and once during the 11th week of military service (). The psychobiological examinations were performed during an ordinary day of military service. BMT in the Swiss Armed Forces requires recruits mainly to participate in physically demanding tasks (e.g. endurance running, indoor sports, power circuit, sensomotoric balance training and games) and theoretical training.
Between the first and second examinations, 34 recruits quit the service because of physical or psychological issues. An additional 37 participants were excluded because their hair was too short for hair cortisol analysis, and data from one recruit was excluded from the second examination due to a HCC higher than 1500 ng/g, possibly indicating Cushing’s syndrome or glucocorticoid contamination (Thomson et al,. Citation2010). This resulted in a total sample size of 105 recruits completing the second examination.
Sociodemographic, anthropometric and perceived stress measures
We completed assessment of sociodemographic and anthropometric characteristics (age, education and smoking status), and the measurement of anthropometrical data (height and weight) using a portable stadiometer (model 213; Seca, Hamburg, Germany) and a calibrated scale (Seca model 861). In addition, the German version of the Perceived Stress Questionnaire (PSQ; Fliege et al., Citation2005; Levenstein et al., Citation1993) was used to assess perceived stress during the last month. The PSQ consists of 20 items such as “you have too many things to do”, “you feel lonely or isolated” and “you find yourself in situations of conflict”. Participants indicated how often the items applied to them, based on a four-point Likert-scale ranging from “almost never” to “usually”. The PSQ consists of a main scale (total stress experience) and four subscales (tension, worries, joy and demands).
Hair collection and analysis
Hair samples were collected from the posterior vertex region at the back of the head. The samples were cut by clean scissors as close as possible to the scalp, and then stored in an envelope at room temperature before shipping to the laboratory (The Ivey Chair in Molecular Toxicology, University of Western Ontario, Canada). HCC were determined from the 2 cm hair segment closest to the scalp. Based on a hair growth rate of 1 cm per month (Wennig, Citation2000), these segments are assumed to reflect hair growth over the two months prior to the sampling points.
Each hair segment grouping weighed 10–15 mg. Hair samples were washed twice with isopropanol. Each wash consisted of a 3-min immersion in 3 ml of isopropanol, while rotating at 0.28 g at room temperature. Hair samples were then analyzed using a previously published method (Sauvé et al., Citation2007). Each hair segment was immersed in 1 ml of methanol and minced with surgical scissors until it became granular in appearance. The samples were then incubated at 50 °C for 16 h, while rotating at 0.28 g. The methanol solution was subsequently evaporated on a hot plate (50 °C) under a steam of nitrogen gas. The residue was reconstituted with 250 µl of phosphate buffered saline (pH 8.0) and run on a salivary cortisol immunoassay (ALPCO Diagnostics, Salem, NH). The cortisol concentration was then corrected to the mass of the hair analyzed and reported in nanograms of cortisol per gram of hair. The intra- and inter-assay coefficients of variation were 6.1 and 10.7%, respectively.
Natural hair color
Participants' natural hair color was rated by two independent judges from pictures taken under standardized light conditions at the beginning of their military service (n = 163). Ratings followed predefined color categories (red/blonde, light brown, brown and black). Inconclusive ratings were categorized by two additional judges, while a final rating was made after consultation of all four judges.
Meteorological data
Meteorological data were collected to examine the potential effects of air temperature and humidity on HCC. A dense network of weather sensors in Switzerland (MeteoSwiss) made it possible to define weather conditions for the 2 months prior to the first hair sampling for each participant individually, according to the individual domicile. During the second measurements, the participants were separated into two larger military units. Therefore, for the second hair sampling, meteorological conditions were the same for all participants within a unit. Mean values of air temperatures (°C) and humidity (%) were based on measurements taken 2 m above ground, as assessed by MeteoSwiss.
Statistical analyses
Statistical analyses were performed using SPSS for Macintosh, version 19.0 (SPSS Inc., Chicago, IL). Outliers more than three standard deviations above the mean were excluded (n = 2; Field, Citation2009). In order to detect variables related to HCC, bivariate correlations after Pearson were computed. Subsequently, independent variables were stepwise integrated (ordered by the highest significant value) in a hierarchical regression analysis, after which the dependent variable (HCC) was integrated. Explained variance in regression models is reflected by R2 (the variance of the whole regression model) and adjusted R2 (considering the generalization of the model). In this study, hierarchical regression analyses were calculated only for the first examination due to violation of normal distribution for temperature and humidity (since recruits were stationed in two different companies during the second examination, as previously described).
In addition, partial correlations were used to assess the influence of variables on HCC, when controlling for the different significant variables. For comparing the examination values, Student’s t-tests were computed. Data are presented as mean ± standard deviation (SD). All analyses were two-tailed, with a level of significance of p < 0.05.
Results
Subject characteristics
In the first collection, the sample consisted of 177 male recruits, with an average age of 20.14 years (SD = 1.11) and a mean body mass index (BMI) of 23.55 kg/m2 (SD = 3.11). Of the initial sample, 36.0% were smokers. Level of education completed ranged from 29.1% lower secondary school, 35.2% upper secondary school and 35.8% academic high school (general qualification for university entrance). The sample consisted mostly of brown-haired individuals (45.7%), followed by light brown- (24.1%) and black-haired (24.1%) and blond- and red-haired (6.2%) subjects. Before the second examination, 34 recruits quit the service due to physical or psychological issues; they exhibited higher PSQ-scores in the first examination, but no difference in the HCC.
Effect of basic military training on perceived stress and HCC
Subjectively perceived stress (PSQ-total) increased from the first to the second examination [t(113) = −6.708, p < 0.001]. With the exception of worries [t(113) = 0.362, p = 0.718], all subscales altered significantly, with a decrease in joy [t(113) = 9.221, p < 0.001] and an increase in tension [t(113) = −6.529, p < 0.001) and demands [t(113) = −4.723, p < 0.001]. In contrast, no significant difference was found in the concentration of hair cortisol between the first and the second examination [t(96) = −1.06, p = 0.292]. Pearson's correlations further revealed no significant association between total perceived stress during the last month (PSQ) and HCC (1st examination: r = −0.061, p = 0.431; 2nd examination: r = 0.003, p = 0.980). Results were the same even when considering significant covariates in partial correlations (all p > 0.05).
Correlations between HCC and possible covariates
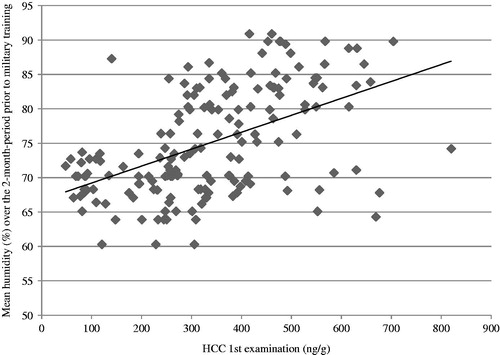
shows the results of bivariate correlations analyses involving HCC. Hair cortisol was found to be unrelated to age (1st examination: r = 0.057, p = 0.425; 2nd examination: r = 0.078, p = 0.431), BMI (1st examination: r = 0.134, p = 0.079; 2nd examination: r = 0.138, p = 0.162), smoking status (1st examination: r = 0.134, p = 0.079; 2nd examination: r = 0.113, p = 0.249), and natural hair color (1st examination: r = −0.087, p = 0.275; 2nd examination: r = −0.147; p = 0.151). In contrast, there were significant correlations between education and HCC (1st examination: r = −0.265, p = 0.001; 2nd examination: r = −0.314, p = 0.002). Similar significant correlations were found between temperature (1st examination: r = −0.452, p < 0.001; 2nd examination: r = −0.533, p < 0.001) and humidity (1st examination: r = 0.509, p < 0.001; 2nd examination: r = 0.533, p < 0.001) and HCC. Temperature was negatively correlated with humidity (r = −0.671, p < 0.001).
Table 1. Bivariate correlations between hair cortisol concentration (HCC; 1st and 2nd examination) and sociodemographic, anthropometric and meteorological data.
Partial correlations confirmed the significant correlation between education and HCC, even when temperature and humidity were controlled (1st examination: r = −0.168, p = 0.038; 2nd examination: r = −0.260, p = 0.011). When level of education was controlled, temperature and humidity remained significantly related to HCC (p < 0.001 for both). shows the association between HCC (from the 1st examination) and the mean humidity of the 2 months prior to the hair sample collection. The explained variance in the regression model of air temperature was R2 = 0.204 (p < 0.001) and for air humidity R2 = 0.259 (p < 0.001).
Hierarchical regression analyses were calculated with HCC (1st examination) as the dependent variable. Based on the analysis of correlation, we entered education, temperature and humidity as independent variables. Humidity as a predictor explained 26.8% (β = 0.339, p < 0.001, R2 = 0.268), temperature as a predictor explained 3.1% (β = −0.215, p = 0.021, adj. R2 = 0.031) and education as a predictor explained 2.0% (β = −0.145, p = 0.031, adj. R2 = 0.020). The whole model within these three predictors explained 31.8% (=R2) of HCC.
Discussion
The findings of this study reveal that in contrast to increasing subjective ratings of perceived stress during BMT, HCC appeared unaffected. Similarly, self-reported stress was not correlated to HCC. In addition, a significant influence of air temperature and humidity on HCC was found, and an association between HCC and education level was detected.
Military training was perceived as stressful, as indicated by self-ratings in this study. Similarly, previous studies confirm an increase in mental load during military training (Bernton et al., Citation1995). In contrast, this study did not show any verifiably concomitant increase in HCC during BMT. A few studies examined changes of saliva cortisol related to military training and found an increase in concentration during the first few weeks of military school. Thereafter, a habituation of the HPA is reported (Bernton et al., Citation1995; Hellhammer et al., Citation1997). This could indicate a normalization of the HCC until the 11th week when the second sample was collected.
Our findings of unchanged HCC are supported by Karlen et al. (Citation2011), who found that only the experience of serious life events in the past 3 months significantly increased levels of hair cortisol. Military training was indeed perceived as stressful, but not as a serious life event. Therefore, it can be interpreted that military training is not consistently stressful enough to alter HCC, with possible adaptation during training. A further point of criticism could be the restricted period of measurement, which perhaps was too short to cause measurable effects on HCC.
Previous studies have revealed that excessive sport could lead to an increase in HCC (Gerber et al., Citation2013). Even though BMT includes significant physical demands, we found no increase in HCC. One possible explanation might be the presence or absence of other psychological or physiological resources and demands (e.g. the group cohesiveness, social support and discontinuation of stressful conditions in daily life). In addition, the role of sweat in affecting HCC is not well understood and needs further examination. Furthermore, one must consider that BMT constitutes a considerable change in daily life, and therefore stress prior to and during the first week of BMT (e.g. due to anticipatory stress, separation stress and changes in sleeping habits) might represent not an unaffected baseline level but a stress measurement itself. This might have prevented a possible further increase of HCC.
We did not find a relationship between HCC and stress perception. This is in line with most studies (Dettenborn et al., Citation2010; Dowlati et al., Citation2010; Kramer et al., Citation2009; Stalder et al., Citation2010). An explanation could be that psychological stress responses occur in a rapid fashion with dynamic changes, especially during prolonged stress situations, while HPA-axis responses may be less dynamic (Dettenborn et al., Citation2010). In line with these findings, Campbell & Ehlert (Citation2012) found that emotional perception during a laboratory stressor (TSST) is unrelated to physiological parameters, supporting the hypothesis that these are two independent dimensions.
No relationship was found between HCC and smoking status, as is the case with most studies. For example, Skoluda et al. (Citation2012) examined amateur endurance athletes compared to controls, and Dettenborn et al. (Citation2012) investigated participants covering a wide range of ages. In both studies, no relationship between HCC and smoking were found, despite the fact that smokers are known to have a dysregulation of the HPA axis and cigarette smoking induces an increase in plasma cortisol levels (Xue et al., Citation2010). This discrepancy might be due to the young age of the subjects, who have had fewer years to sustain the physical damages of smoking.
Our findings revealed no significant association between BMI and HCC, which reflects the inconsistency found in literature. Pereg et al. (Citation2011) and Manenschijn et al. (Citation2011b) found a positive correlation between HCC and BMI, but an additional study by Manenschijn et al. (Citation2011a) was unable to confirm this relationship, while presenting significant association between waist to hip ratio and HCC. In a recent study, Manenschijn et al. (Citation2013) again found no significant relationship between HCC and BMI. A further reason for the missing correlation could be the restricted BMI range in this study as compared to the broader ranges described by others (Manenschijn et al., Citation2013).
We did not find an influence of age on HCC. This contradicts the findings of a study by Dettenborn et al. (Citation2012). However, they examined a large age range (1–91 years) and discovered a curvilinear relationship between HCC and age. According to these authors, children and elderly people have higher HCC than middle-aged subjects. In contrast, other studies did not find a significant correlation between age and HCC (Dettenborn et al., Citation2010, Citation2012; Manenschijn et al., Citation2011a; Raul et al., Citation2004). Reasons for this inconsistency could be attributed to small sample sizes or a limited age range, as our sample’s characteristics showed.
Melanin, the pigment responsible for hair color, is thought to influence the incorporation of cortisol in hair. In a study of 360 participants, Dettenborn et al. (Citation2012) found an insignificant trend influence of hair color on the HCC (p = 0.081), wherein darker hair showed higher HCC. Other studies were neither unable to confirm this association (Kirschbaum et al., Citation2009; Manenschijn et al., Citation2011a; Sauvé et al. Citation2007); nor did our results.
A negative relationship between education level and HCC was found in this study. The higher the level of education, the lower the HCC. No previous study has found this direct relationship, although Vaghri et al. (Citation2013) found that lower maternal and paternal education was associated with higher hair cortisol levels in preschool children (n = 339). Together these findings suggest a possible influence of socioeconomic status on HCC, with poorer people displaying higher levels of stress (Vaghri et al., Citation2013).
Our results have identified relative humidity as the strongest predictor of HCC (26.8% explained variance). The higher the relative humidity, the higher the HCC. Evaporation of sweat strongly depends on relative air humidity. In a setting of high relative humidity sweat evaporates more slowly, and therefore remains longer on the scalp than under less humid conditions (Lim et al., Citation2008). Since sweat contains cortisol (Russell et al., Citation2014), it can be assumed that hair has more time to absorb cortisol in cases of high humidity.
HCC was further negatively correlated with air temperature. Lower temperature exposure during the previous two months was related to higher HCC. Since air temperature is inversely related to humidity, the negative association with air temperature might be based on the positive association of HCC with humidity. Alternatively, it is possible that high humidity and low temperature during intense army maneuvers may affect stress levels, as measured by HCC. On the other hand, influences of seasonal variations in HPA axis were observed previously. Some studies revealed a reduction of urinary cortisol (Hansen et al., Citation2001), plasma cortisol (Walker et al., Citation1997) and saliva cortisol levels (Persson et al., Citation2008) during summer as well as an increase in winter. One possible explanation for the lower summer cortisol concentrations could be a daylight-dependent mechanism that mediates the metabolism of cortisol (Hansen et al., Citation2001).
Several limitations of this study should be addressed. Even though an overall increase in perceived stress was found in this study, BMT might not be perceived as stressful by every subject. Other standardized real-life stressors (e.g. school examinations) perhaps would have elicited a more homogenous stress response and therefore constituted a better option to examine the effects of chronic stress. Then, BMT allowed us to measure people with different educational levels and from all over the country, who were exposed to a broader variety of meteorological conditions.
There were 34 dropouts of recruits who were unable to complete the basic training due to mental issues or somatic reasons. These participants rated themselves to be significantly more stressed during the first examination as compared to the other recruits, but did not reveal altered HCC at baseline. It might be possible that these dropouts, due to an increased stress perception, would have had higher levels of hair cortisol during the second data collection, and their attrition left behind only the more resilient subjects for the second examination. It would have been interesting to collect a hair sample immediately after the recruits dropped out, to investigate a potential change of HCC compared to the first collection.
Most importantly, levels of perceived stress and HCC should have been measured not only during BMT but also before. This would allow a more accurate measurement of stress alterations, while controlling, at least for the period before BMT, for excessive physical demands.
The second measurement point perhaps was too late to capture the effects of chronic stress induced by BMT. It is possible that HCC measurements could have correlated with training if measured after 5 weeks of military training due to possibility of HPA habituation.
As hair samples were exclusively taken from young healthy men, our observations cannot be generalized to women or other age groups. Our results should be confirmed in more heterogeneous populations. Previous research indicates higher HCC in men than in women (Dettenborn et al., Citation2012; Manenschijn et al., Citation2013; Skoluda et al., Citation2012). Examining female HCC should verify whether temperature and humidity have the same effect as in men.
Recently, several original reports on various variables affecting HCC have been published, with somewhat contradictory findings. The innovative aspect of our study is the investigation of meteorological measures in relation to HCC. This study is the first to examine temperature and humidity in relation to hair cortisol and the first to research HCC in the context of BMT. Furthermore, this is the first study to directly examine the association between individual education level and HCC, revealing a significant relationship. Future investigations using hair cortisol should incorporate humidity and the effect of sweating as well as seasonal influences. Hair cortisol should be assessed longitudinally under different temperature and humidity conditions. We assume that most of the previous studies took place in a single city, so the authors could try to explore temperature and humidity data retrospectively, to validate our findings in their samples.
Conclusion
This study confirms a missing relationship between BMT and HCC, while revealing new factors influencing HCC. BMT induced an increase in perceived stress, but did not affect HCC. This could be interpreted as a missing influence of mainly physical stress (e.g. exercise) on HCC. Similarly, self-reported stress was not related to HCC. On the other hand, we found a significant negative influence of air temperature and a significant positive influence of air humidity on HCC. In addition, a negative association between HCC and education level was revealed. Future studies should control for meteorological data and educational status when interested in the examination of HCC.
Declaration of interest
This study was supported by a financial grant from the Swiss Federal Department of Defence, Civil Protection and Sport (DDPS). The authors alone are responsible for the content and writing of the paper. All authors declare that there is no conflict of interest.
Acknowledgements
The authors would like to thank the whole project team for supporting and contributing to the accomplishment of the project. A special thank you goes to the recruits of the Infantry school of Aarau, Switzerland.
References
- Annen H, Seiler S, Jonas K. (2010). Military psychology in Switzerland: a short story with a long history. Swiss J Psychol 69(2):75–82
- Bernton E, Hoover D, Galloway R, Popp K. (1995). Adaptation to chronic stress in military trainees: adrenal androgens, testosterone, glucocorticoids, IGF-1, and immune function. Ann NY Acad Sci 774:217–31
- Campbell J, Ehlert U. (2012). Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 37:1111–34
- Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. (2010). Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology 35(9):1404–9
- Dettenborn L, Tietze A, Kirschbaum C, Stalder T. (2012). The assessment of cortisol in human hair: associations with sociodemographic variables and potential confounders. Stress 15(6):578–88
- Dowlati Y, Herrmann N, Swardfager W, Thomson S, Oh PI, Van Uum S, Koren G, Lanctôt KL. (2010). Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsychiatr Dis Treat 6:393–400
- Field A. (2009). Discovering statistics using SPSS. London: Sage Publications
- Fliege H, Rose M, Arck P, Walter OB, Kocalevent RD, Weber C, Klapp BF. (2005). The perceived stress questionnaire (PSQ) reconsidered: validation and reference values from different clinical and healthy adult samples. Psychosom Med 67:78–88
- Gerber M, Jonsdottir IH, Kalak N, Elliot C, Pühse U, Holsboer-Trachsler E, Brand S. (2013). Objectively assessed physical activity is associated with increased hair cortisol content in young adults. Stress 16:539–99
- Hansen AM, Garde AH, Skovgaard LT, Christensen JM. (2001). Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clin Chim Acta 309:25–35
- Hellhammer DH, Buchtal J, Gutberlet I, Kirschbaum C. (1997). Social hierarchy and adrenocortical stress reactivity in men. Psychoneuroendocrinology 22:643–650
- Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. (2007). The relationship between stress and hair cortisol in healthy pregnant woman. Clin Invest Med 30:E103–7
- Karlen J, Ludvigsson J, Frostell A, Theodorsson E, Faresjö T. (2011). Cortisol in hair measured in young adults – a biomarker of major life stressors? BMC Clin Pathol 11(12):1–6
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. (2009). Hair as a retrospective calendar of cortisol production – increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34:32–7
- Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, Genest J, et al. (2009). Stress pathway to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol 169:1319–26
- Levenstein S, Prantera C, Varvo V, Scribaon ML, Berto E, Luzi C, Andreoli A. (1993). Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. J Psychosom Res 37(1):19–32
- Lim CL, Byrne C, Lee JKW. (2008). Human thermoregulation and measurement of body temperature in exercise and clinical settings. Ann Acad Med Singapore 37:347–53
- Manenschijn L, Koper JW, Lamberts SW, Van Rossum EFC. (2011a). Evaluation of method to measure long term cortisol levels. Steroids 76:1032–6
- Manenschijn L, Schaap L, Van Schoor NM, Van der Pas S, Peeters GMEE, Lips P, Koper JW, Van Rossum EFC. (2013). High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab 98:2078–83
- Manenschijn L, Van Kruysbergen RG, De Jong FH, Koper JW, Van Rossum EF. (2011b). Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab 96(11):E1862–5
- Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, Koren G. (2011). Hair cortisol and the risk for acute myocardial infarction in adult men. Stress 14:73–81
- Persson R, Garde AH, Hansen AM, Österberg K, Larsson B, Orbaek P, Karlson B. (2008). Seasonal variation in human salivary cortisol concentration. Chronobiol Int 25:923–37
- Pragst F, Balikova MA. (2006). State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 370:17–49
- Raul JS, Cirimele V, Ludes B, Kintz P. (2004). Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem 37:1105–11
- Russell E, Koren G, Rieder M, Van Uum S. (2012). Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37:589–601
- Russell E, Koren G, Rieder M, Van Uum S. (2014). The detection of cortisol in human sweat: implications for measurement of cortisol in hair. Ther Drug Monit 36:30–4
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum S. (2007). Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 30(5):E183–91
- Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. (2012). Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology 37:611–17
- Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L. (2010). Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol 85(3):357–60
- Stalder T, Kirschbaum C. (2012). Analysis of cortisol in hair – state of the art and future directions. Brain Behav Immun 26:1019–29
- Stalder T, Steudte S, Alexander N, Miller R, Gao W, Dettenborn L, Kirschbaum C. (2012). Cortisol in hair, body mass index and stress-related measures. Biol Psychol 90(3):218–23
- Steudte S, Kolassa I-T, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T. (2011). Increased cortisol concentration in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology 36:1193–200
- Thomson S, Koren G, Fraser L, Rieder M, Friedman TC, Van Uum S. (2010). Hair analysis provides a historical record of cortisol levels in Cushing’s syndrome. Exp Clin Endocrinol Diabetes 118(2):133–8
- Vaghri Z, Guhn M, Weinberg J, Grunau RE, Yu W, Hertzman C. (2013). Hair cortisol reflects socio-economic factors and hair zinc in preschoolers. Psychoneuroendocrinology 38(3):331–40
- Van Uum S, Sauvé B, Fraser LA, Morley-Forster TL, Koren G. (2008). Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress 11:483–8
- Walker BR, Best R, Noon JP, Watt GCM, Webb DJ. (1997). Seasonal variation in glucocorticoid activity in healthy men. J Clin Endocrionol Metab 82:4015–19
- Wennig R. (2000). Potential problems with the interpretation of hair analysis results. Forensic Sci Int 107:5–12
- Wyss T, Scheffler J, Mäder U. (2012). Ambulatory physical activity in Swiss army recruits. Int J Sports Med 33:716–22
- Xue Y, Morris M, Ni L, Guthrie SK, Zubieta JK, Gonzales K, McConnell DS, Domino EF. (2010). Venous plasma nicotine correlates of hormonal effects of tobacco smoking. Pharmacol Biochem Behav 95:209–15