Abstract
Research in to short-term cardio-respiratory changes in animals in reaction to a psychological stressor typically describes increases in rate of oxygen consumption () and heart rate. Consequently, the broad consensus is that they represent a fundamental stressor response generalizable across adult species. However, movement levels can also change in the presence of a stressor, yet studies have not accounted for this possible confound on heart rate. Thus the direct effects of psychological stressors on the cardio-respiratory system are not resolved. We used an innovative experimental design employing accelerometers attached to king penguins (Aptenodytes patagonicus) to measure and thus account for movement levels in a sedentary yet free-to-move animal model during a repeated measures stress experiment. As with previous studies on other species, incubating king penguins (N = 6) exhibited significant increases in both
and heart rate when exposed to the stressor. However, movement levels, while still low, also increased in response to the stressor. Once this was accounted for by comparing periods of time during the control and stress conditions when movement levels were similar as recorded by the accelerometers, only
significantly increased; there was no change in heart rate. These findings offer evidence that changing movement levels have an important effect on the measured stress response and that the cardio-respiratory response per se to a psychological stressor (i.e. the response as a result of physiological changes directly attributable to the stressor) is an increase in
without an increase in heart rate.
Introduction
External conditions or events that upset the homeostasis of the body typically trigger a stress response through neural and/or hormonal pathways. These stressors can be broadly categorised as either physical stressors, such as changes in temperature, or psychological stressors, which stress the body indirectly, such as the nearby presence of an aggressive conspecific or predator. Short-term cardio-respiratory responses to psychological stressors have been investigated over several decades, predominantly in humans, rats and dogs. Typically, both across studied species and type of psychological stressor, the cardio-respiratory stress response is described by an increase in both rate of oxygen consumption () and heart rate, with heart rate increasing more than expected given the increase in
(Blix et al., Citation1974; Boerth et al., Citation1969; Carroll et al., Citation1986; Langer et al., Citation1979). Based on these studies, the key short-term cardio-respiratory stress response is widely considered to be increased heart rate, and this is stated as fact in many university-level physiology textbooks (Campbell, Citation1996; Hill et al., Citation2008; Sherwood, Citation2013; Stanfield et al., Citation2011).
Indeed, heart rate is the most typical measure used to assess the response to a short-term psychological stressor (e.g. fear of receiving a shock) in humans and other animals. Changes in heart rate occur almost immediately, are relatively easy to record and typically represent a more sensitive response than outward signs of a stressed state such as changes in behavior (Nimon et al., Citation1995; von Borell et al., Citation2007), epitomised by the preparation for “fight or flight” where heart rate increases (Cannon, Citation1929) or, by contrast in “freezing” where bradycardia has been measured in juveniles (Buwalda et al., Citation1992; Jacobsen, Citation1979). Thus, the measurement of heart rate has been applied to a wide range of animals to evaluate stressed state, including various farmed animal breeds (von Borell et al., Citation2007), wandering albatrosses Diomedea exulans (Weimerskirch et al., Citation2002), giant petrels Macronectes halli (de Villiers et al., Citation2006), koalas Phascolarctos cinereus (Ropert-Coudert et al., Citation2009) and several penguin species: Adelie Pygoscelis adeliae (Culik & Wilson, Citation1991), gentoo Pygoscelis papua (Nimon et al., Citation1995) and king A. patagonicus (Viblanc et al., Citation2012).
However, despite the aforementioned studies on the stress response, there is reason to question what the direct physiological effects of a psychological stressor are on the cardio-respiratory system. Small movements such as mastication can cause relatively large increases in heart rate (Major, Citation1998) and none of the above studies investigating the cardiac or cardio-respiratory response to a stressor accounted for possible changes in movement levels (as either increases or decreases). Yet in the majority of these studies, movement was an inevitable element of the stressor response (e.g. piloting a plane during landing or taking off (Blix et al., Citation1974) or avoiding an electrical shock (Langer et al., Citation1979)). Thus measures of and heart rate in these studies represent not only the short-term cardio-respiratory response to stress “per se” (i.e. cardio-respiratory responses directly attributable to the stressor, for example to support increases in hormone production, release and action and consequent metabolic responses such as up-regulation of the cardio-respiratory system; Brener, Citation1987; Moberg & Mench, Citation2000), but also the response to changes in movement levels as a reaction to the stressor. In turn, in cases where movement increases in response to a stressor, even if this is only slight, elevations in
and heart rate could be due to a stress response per se and/or a change in behavior (von Borell et al., Citation2007).
Thus the direct effects of psychological stressors on the cardio-respiratory system are not clear. The present study addresses this important gap in our understanding of the stress response by measuring and heart rate in a free-to-move animal model, while employing a novel protocol to account for differences in movement levels. Our aim was to determine the major effects of psychological stress per se on the cardio-respiratory system of incubating king penguins (A. patagonicus). This model was chosen because during incubation these birds are sedentary and exhibit a limited range of behaviors, and lack motivation to flee in stressful situations, such as the presence of a predator. We instrumented wild incubating birds with a heart rate logger and a miniature accelerometer to record heart rate and body motion, respectively. The birds were then introduced into a respirometer, allowing measurement of their
, and received a dummy egg to incubate. Once habituated to the respirometer, they were exposed to a psychological stressor. Acceleration is a highly sensitive measurement to determine animal posture and movement levels (Fourati et al., Citation2009; Wilson et al., Citation2006; Yoda et al., Citation2001), and thus recording acceleration during trials enabled us to account for even slight, peripheral movements of the birds without inhibiting their natural responses to the stressor. We hypothesised that while king penguins would exhibit the typical cardio-respiratory response to a stressor under standard experimental analysis, having accounted for slight body movements during the presence of a stressor, the cardio-respiratory response per se would be different, described by at least an attenuation of the increase in heart rate.
Methods
Animals
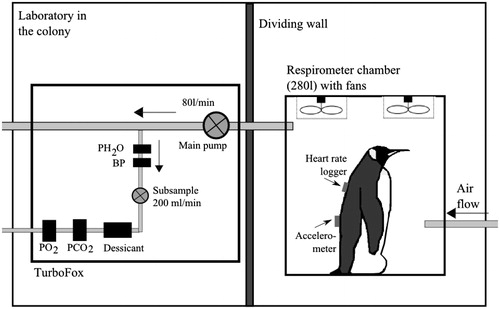
Six king penguins (11–13 kg) of unknown sex and within the first 20 days of incubation (both individuals of a breeding pair take turns to incubate the egg over multiple days at a time) were selected and colored with “pig marker” spray (Porcimark©, Langeskov, Denmark). The birds were late breeders, nesting in a peripheral area of the colony (Barrat, Citation1976), and thus were unlikely to reproduce successfully that year. Incubating birds used for the study were hooded and equipped with two data loggers while in the colony. We used a modified Polar heart rate logger (models RS400, RS800 or RS800lite, Polar Electro Oy, Kempele, Finland) to record heart rate (Groscolas et al., Citation2010) and a triaxial accelerometer (Macrologger FCMP Medina, R. Laesser, IPHC, Strasbourg, France; dimensions: 85 × 35 × 18 mm, 80 g) to record body movement. Loggers were taped to the bird’s back (central, midline) using Tesa tape (Beiersdorf AG, Hamburg, Germany). Gold-plated safety pins were used as heart rate electrodes and were placed dorsally (upper and lower back), ∼0.5 cm underneath the skin (Groscolas et al., Citation2010). The egg of the bird was then replaced by a plaster dummy egg and the real egg was placed in an incubator at 37.5°C and 60% relative humidity. Maintaining the incubating posture, with the dummy egg held against the brood patch, the bird was moved to the adjacent laboratory and placed into the respirometer chamber. The hood was removed and the bird left alone overnight for at least 10 h, during which the respirometry system was running (i.e. air was pulled through the chamber, at a rate of 80 l/min). Stress experiments were conducted the following day. Upon completion, the egg was returned to the bird and it was placed back at its original location in the colony. Each bird was observed for the following three days to ensure that egg desertion did not occur.
The study was conducted during February and March 2011 within the wild king penguin colony at “Baie du Marin” on Possession Island, Crozet Archipelago (46°25′S; 51°52′E). All procedures used in the present study were approved by the Ethical Committee of the Institut Polaires Français-Paul Emile Victor (IPEV). The requirements of the United Kingdom (Scientific Procedures) Act 1986, amended 2012, were followed.
Respirometry protocol
Open-circuit respirometry was used to measure oxygen consumption rates () of penguins standing inside a respirometer chamber built with Plexiglass (0.8 m long × 0.5 m wide × 0.7 m high; 280 l) and equipped with a number of fans to facilitate rapid mixing of expired gas. During a trial, the primary flow control unit (FK-100) of a “TurboFOX-RM” integrated field respirometry system (Sable Systems International, Las Vegas, NV) pulled air through the chamber at a rate of 80 l/min (automatically corrected to standard temperature and pressure (STP), 273 K and 101.3 kPa). A subsample (200 ml/min) was pulled into the analyzer unit of the system, where it passed through a humidity meter (RH-300), scrubbing columns to remove water vapor (Drierite, Xenia, OH), a CO2-analyzer, and a fuel-cell oxygen-analyzer. Oxygen and CO2 concentrations within the chamber, main flow rate through the chamber, humidity of the gas sample and barometric pressure were recorded every second onto a laptop computer using ExpeData (Sable Systems International, Las Vegas, NV). All connections between the various components of the respirometry system were made using gas-impermeable Bev-a-Line or Tygon tubing. The O2-analyzer was calibrated before each trial using ambient air scrubbed free of water vapor and CO2 (set to 20.95% O2; the zero point was fixed and not subjected to drift). The CO2-analyzer was calibrated daily using 99.995% pure N2 and 1.00% CO2 (Air Liquide, Paris, France). The humidity meter was calibrated weekly according to recommendations of the manufacturer. Before a trial, the entire system was tested for leaks by infusing pure N2 gas. Time constant of the respirometer chamber was calculated to be 3.5 min. To account for analyzer drift, the system was frequently baselined (every 30 min) by switching the air inflow into the analyzer unit from the animal respirometer chamber to ambient air. The respirometry system was set-up inside a well-ventilated laboratory, adjacent to the penguin colony. To reduce any visual disturbance from the experimenter, the respirometer chamber was placed behind an opaque plastic curtain ().
Respirometry calculations
Respirometry data were analyzed using Expedata. During analysis, analyzer drift and lag time of the respirometry system were corrected. Main flow rate was corrected to STP dry (STPD) using equation 8.6 in Lighton (2008). To improve the response time of our system, we used a modified version of the “instantaneous” correction, first applied by Woakes & Butler (1983), as described by Halsey et al. (Citation2009). In brief, the volumes of O2 uptake (, ml) and CO2 output (
, ml) between any two points in time (t1 and t2, min) were calculated as:
where
and
are the excurrent O2 fractions at t1 and t2, V is chamber volume (ml), and
is the flow rate through the chamber (ml min−1). Rates of O2 uptake (
, ml min−1) were then calculated as
Measuring body movement
Body movement was calculated as the vector sum of dynamic body acceleration in three dimensions (VeDBA; Qasem et al., Citation2012). We used a low-pass filter to separate dynamic acceleration from static acceleration in the raw acceleration signal recorded by the accelerometer, where the vector sum of the static body acceleration in the three axes equals 1 g (Fourati et al., Citation2009). When an accelerometer is attached to a central, fixed point on an animal’s body, dynamic acceleration represents the element of recorded acceleration due to movement of the animal’s center of mass as a consequence of movement of any part of the body (Gleiss et al., Citation2010; Halsey et al., Citation2009; Wilson et al., Citation2006). Thus, it provides a highly sensitive measure of amount of animal movement. The acceleration data analyses were performed using custom-written computer programs (Yves Handrich) adapted from Fourati et al. (Citation2009), using Matlab 6.0 (The MathWorks, Natick, MA).
Stress experiment
In the control (i.e. unstressed) condition, the birds were exposed to a consistent environment; the bird could not see the experimenter, there was no movement within the laboratory and the noise levels were continually low. The day after the bird was placed in the respirometer chamber, the stress condition was invoked; the bird was exposed to a continuous stressor over a period of 15 min. , heart rate and VeDBA were continuously recorded. The stressor was simultaneously visual and auditory. The visual element was provided by the movement of the researcher (ASTW), while the auditory element consisted of noise of about 70 dB at source generated by striking together the broad sides of two flat pieces of metal (each with a surface area of 10 × 20 cm and with a handle on the rear face) at a maximum rate of four times per minute. The researcher stood approximately 2 m from the respirometer chamber when the stressor was applied. During these stressing periods, birds were incubating and, hence, did not move away from the dummy egg.
Data processing and statistical analysis
Mean values of , heart rate and VeDBA during the control condition (i.e. before birds were exposed to the stressor) were calculated at 5 min periods, when mean
was lowest. To account the effects of circadian rhythms on
(Halsey et al., Citation2008), only data recorded during daylight hours (07:00 h to 21:00 h) were considered; stress experiments were conducted between 08:00 h and 14:00 h. For the stress condition, mean values of
, heart rate and VeDBA were calculated over the 15-min period, during which the stressor was applied. To ensure that birds were in physiological equilibrium, pilot tests were undertaken prior to data collection to ascertain the time needed for the three parameters to stabilise. VeDBA and heart rate stabilised almost instantaneously while
stabilised after ca. 1 min. Consequently, the first minute of measurement of
during each 15-min stress period was discarded from the analysis. A preliminary analysis showed that movement levels of birds during the stressed condition were low but nonetheless higher than during the control condition. To account for this difference in movement level, we scanned the data recorded during the control condition (daytime only) and selected 15-min periods for which movement levels (i.e. mean VeDBA) were similar to those during the stressed condition. Mean values for
and heart rate were also calculated for these periods; the R software package “CaTool” was used to calculate running means (R Core Team, Citation2012). Q–Q plots indicated that the data were normally distributed. Paired t-tests were used to test for differences in mean values of
, heart rate and VeDBA between the control and stress condition. Tests were run for both datasets (i.e. when differences in movement level were accounted for and when not; N = same 6 birds for each analysis). Results were considered to be statistically significant, thus providing evidence for an effect, when p < 0.05. All statistical analyses and graphs were produced using R (R Core Team, Citation2012).
Results
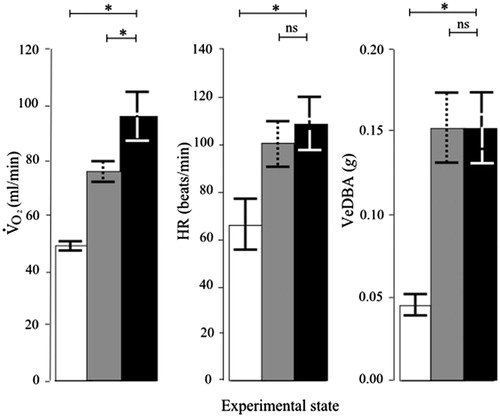
In the control (i.e. unstressed) condition, the birds (N = 6) exhibited little if any movement, which when present was observed to comprise solely comfort behaviors (Viblanc et al., Citation2011). In contrast, in the presence of the stressor, in the same birds, low-level vigilance behavior and displacement behavior, such as scratching and feather preening, were observed on occasion. During the control condition, mean heart rate was 66 beats min−1, which is similar to the heart rate of incubating and brooding king penguins in the wild during periods of rest (Groscolas et al., Citation2010; Viblanc et al., Citation2011). Comparison between the control and stressed conditions, without accounting for movement levels, showed a significant increase in both mean and heart rate during the stressed condition (mean ± SEM: for
97 ± 21%, t5 = 4.98, p = 0.004 and for heart rate 80 ± 21%, t5 = 6.00, p = 0.002; ). Mean levels of movement were significantly higher during the stressed condition than during the control condition (mean ± SEM: + 286 ± 78%, t5 = 5.13, p = 0.004; ). Although this represents a large percentage increase compared to the control condition, the level of movement exhibited during the presence of the stressor was still small (mean VeDBA: 0.05 v. 0.15 g, while mean VeDBA during walking is around 2.8 g; Willener, unpublished). When accounting for differences in movement levels between the two conditions by comparing periods in the stressed condition with periods in the control condition where movement levels were similar, results for
and heart rate in response to the stressor changed. While
remained significantly higher in the stressed condition when compared with the control condition (+26 ± 9%, t5 = 2.78, p = 0.040; ), this was not the case for heart rate (+8 ± 5%, t5 = 1.47, p = 0.20).
Figure 2. Cardio-respiratory stress responses of incubating king penguins. Values are means ± SEM. : rate of oxygen consumption; HR: heart rate; VeDBA: vectorial sum of dynamic body acceleration (indicating levels of body movement). Data are for the same penguins, each in the chamber throughout (repeated measures design; N = 6). Open bars: before stressor; gray bars: before stressor but with similar movement levels to stressed penguins; black bars: during stressor exposure. Asterisks indicate p < 0.05 and ns indicates p > 0.05, paired t-tests.
Discussion
Standard analysis of the present data indicated an increase in both and heart rate in response to the stressor, as found in previous studies of adult animals. However, the typical response measured in studies of psychological stress includes behavioral adjustments associated with “fight or flight”, and this was reflected in the current data by the presence of low-level vigilance and displacement behavior such as scratching. Such movement levels in king penguins in the wild are associated with increases in heart rate of around 30 beats min−1 (Viblanc et al., Citation2011), which is comparable to the increases recorded in the present study in the presence of the stressor (). When experimentally accounting for movement levels, the observed cardio-respiratory stress response per se was represented by an increase in
only, with no evidence for a systematic change in heart rate. This increase, reflecting increased metabolic rate, probably resulted at least in part from an increase in physiological function such as hormone up-regulation, brain stimulation (Brener, Citation1987; Moberg & Mench, Citation2000) and possibly also increased striated muscle tone. It may also represent an increase in heat production to compensate for heat loss during the first moments of the stress response due to short-term peripheral vasodilation. These results provide evidence that the change in heart rate during exposure to a stressor reported by previous studies is associated with changes in body movement, rather than stress per se (and this inference includes observations of reduced heart rate during freezing behavior in juvenile animals; Buwalda et al., Citation1992; Jacobsen, Citation1979). The present results therefore contradict the widely held belief that a fundamental cardio-respiratory response to a psychological stressor is principally an increase in heart rate.
Of course, an increase in by the tissues ultimately requires an increase in rate of oxygen delivery. According to Fick’s convection equation for the cardiovascular system (Fick, Citation1870), since
of the birds increased in the presence of the stressor while their heart rate did not when comparing periods of time during which equal levels of movement occurred, either heart stroke volume, the quantity of oxygen per unit volume of blood or oxygen extraction by the body tissues must have increased. Van Zanten et al. (Citation2004) described increased hemo-concentrations in humans in response to a stressor, which would result in a greater transport of oxygen per unit volume of blood and thus an increase in oxygen extraction. Penguins have a relatively large heart mass compared with other birds (Drabek, Citation1989), which enables them to transport more oxygen per heart beat due to a greater stroke volume, and this might attenuate an increase in heart rate in response to increased oxygen demand. Evidence supporting this possibility is provided by studies on humans in which those with relatively larger heart masses (due to regular physical training) exhibited smaller changes in heart rate to a stressor (an avoidance task; Vandoornen & Degeus, Citation1989).
Indeed, the possibility should be considered that the stress response per se of king penguins is atypical, perhaps due to their large heart mass. Alternatively, they have few terrestrial predators and thus may not have evolved a strong “fight or flight” response to predator presence. Furthermore, it is reasonable to question how unstressed the birds could be while present in a laboratory environment even after many hours to habituate (although there is lack of evidence that after the long-term rest overnight they were stressed). However, the data in the present study show that when the small movement levels of incubating king penguins exhibited during exposure to the stressor are not accounted for the cardio-respiratory stress response is similar to other adult species (a considerable increase in both heart rate and ). Thus, the most likely explanation for the novel findings of the current study is not founded on unusual physiology of the subject species but on the experimental protocol employed enabling measurement of the stress response per se.
In conclusion, the cause of the cardio-respiratory responses elicited by the presence of a psychological stressor should be reconsidered in light of the evidence presented here. Our results indicate that the apparently ubiquitous increase in heart rate in adult animals as a short-term response to a psychological stressor may be associated with increases in movement levels, and therefore not occur if movement levels are accounted for. In contrast, in our study increased significantly in response to a psychological stressor even after accounting for movement levels, attesting to as yet unclear physiological, and thus metabolic, up-regulation in response to a stressor per se. Aside from providing a revised understanding of the direct response of the cardio-respiratory system to a psychological stressor, our study calls into question the adequacy of heart rate as a general measure of short-term stress and highlights the need for further studies into the potentially important energetic costs associated with stress responses.
Acknowledgements
We thank the French Polar Institut (Institut Polaire Paul Emile Victor) for their financial and logistical support in realising this study. Marguerite Netchaieff gave invaluable help during data collection. Nicholas Decalmer built the respirometer chamber for the experiments. We would also like to thank Anne Robertson, René Groscolas, Charles-André Bost, Laëtitia Maréchal, Stuart Semple and Nils Arrigo for their advice during data collection, data analyses and manuscript writing.
Declaration of interest
The authors report no conflicts of interests. The Swiss Fund for Women at University (Association Suisse des femmes diplômées des universités), the Company of Biologists and the Society for Experimental Biology provided funding for this study.
References
- Barrat A. (1976). Quelques aspects de la biologie et de l'écologie du manchot royal (Aptenodytes patagonicus) des Iles Crozet. Comité National Français Recherches Antarctiques 40:9–52
- Blix AS, Stromme SB, Ursin H. (1974). Additional heart rate – an indicator of psychological activation. Aerosp Med 45(11):1219–22
- Boerth RC, Covell JW, Pool PE, Ross J. (1969). Increased myocardial oxygen consumption and contractile state associated with increased heart rate in dogs. Circ Res 24(5):725–34
- Brener J. (1987). Behavioral energetics – some effects of uncertainty on the mobilization and distribution of energy. Psychophysiology 24(5):499–512
- Buwalda B, Koolhaas J, Bohus B. (1992). Behavioral and cardiac responses to mild stress in young and aged rats: effects of amphetamine and vasopressin. Physiol Behav 51(2):211–16
- Campbell N. (1996). Biology. Series biology. California: The Benjamin, Cummings Publishing Company, Inc
- Cannon WB. (1929). Bodily changes in pain, hunger, fear and rage: an account of recent research into the function of emotional excitement. 2nd ed. New York: Appleton-Century-Crofts
- Carroll D, Turner JR, Prasad R. (1986). The effects of level of difficulty of mental arithmetic challenge on heart rate and oxygen consumption. Int J Psychophys 4:167–73
- Culik B, Wilson R. (1991). Penguins crowded out. Nature 351(6325):340
- de Villiers M, Bause M, Giese M, Fourie A. (2006). Hardly hard-hearted: heart rate responses of incubating northern giant petrels (Macronectes halli) to human disturbance on sub-Antarctic Marion Island. Pol Biol 29:717–20
- Drabek CM. (1989). Heart and ventricle weight of antarctic penguins. Can J Zool 67:2602–4
- Fick A. (1870). Uber die Messung des Blutquantums in den Herzventrikeln. Sitzungsberichte der Physiologisch - Medizinoschen Gesellschaft zu Wurzburg 2:16
- Fourati H, Manamanni N, Afilal L, Handrich Y. (2009). Sensors-based data fusion solution design for 3D motion estimation with application in bio-logging. Int J Sci Tech Auto Cont Comp Eng (IJ-STA) 3(2):1012–31
- Gleiss A, Wilson R, Shepard E. (2010). Making overall dynamic body acceleration work: on the theory of acceleration as a proxy for energy expenditure. Meth Ecol Evol 2(1):23–33
- Groscolas R, Viera V, Guerin N, Handrich Y, Cote S. (2010). Heart rate as a predictor of energy expenditure in undisturbed fasting and incubating penguins. J Exp Biol 213:153–60
- Halsey LG, Butler PJ, Fahlman A, Woakes AJ, Handrich Y. (2008). Behavioral and physiological significance of minimum resting metabolic rate in king penguins. Phys Biochem Zool 81(1):74–86
- Halsey LG, Portugal SJ, Smith JA, Murn C, Wilson RP. (2009). Recording raptor behavior on the wing via accelerometry. J Field Ornithol 80(2):171–7
- Hill RW, Wyse GA, Anderson M. (2008). Animal physiology. 2nd ed. Sunderland, Massachusetts: Sinauer Associates, Inc. 770 p
- Jacobsen NK. (1979). Alarm bradycardia in white-tailed deer fawns (Odocoileus virginianus). J Mammal 60(2):343–349
- Langer AW, Obrist PA, McCubbin JA. (1979). Hemodynamic and metabolic adjustments during exercise and shock avoidance in dogs. Am J Physiol 236(2):225–30
- Lighton J. (2008). Measuring metabolic rates. Oxford: Oxford University Press
- Major P. (1998). Subtle physical activity poses a challenge to the study of heart rate. Physiol Behav 63(3):381–4
- Moberg GP, Mench JA. (2000). The biology of animal stress: basic principles and implications for animal welfare. Cabi Pub
- Nimon AJ, Schroter RC, Stonehouse B. (1995). Heart rate of disturbed penguins. Nature 374:415
- Qasem L, Cardew A, Wilson A, Griffiths I, Halsey LG, Shepard ELC, Gleiss AC, Wilson R. (2012). Tri-axial dynamic acceleration as a proxy for animal energy expenditure; should we be summing values or calculating the vector? PLoS One 7(2):e31187
- R Core Team. (2012). R: a language and environment for statistical computing. R Foundation for Statistical Computing
- Ropert-Coudert Y, Brooks L, Yamamoto M, Kato A. (2009) ECG response of koalas to tourists proximity: a preliminary study. PLoS ONE 4(10):e7378
- Sherwood L. (2013). Introduction to human physiology. In: Series introduction to human physiology. Australia: Thomson Brooks Cole Publishing
- Stanfield CL, Germann WJ, Niles MJ, Cannon JG. (2011). Principles of human physiology. Boston, MA: Pearson, Benjamin Cummings
- Van Zanten J, Ring C, Burns VE, Edwards KM, Drayson M, Carroll D. (2004). Mental stress-induced hemoconcentration: sex differences and mechanisms. Psychophysiology 41(4):541–51
- Vandoornen LJP, Degeus EJC. (1989). Aerobic fitness and cardiovascular-response to stress. Psychophysiology 26(1):17–28
- Viblanc VA, Mathien A, Saraux C, Viera V, Groscolas R. (2011). It costs to be clean and fit: energetics of comfort behavior in breeding-fasting penguins. PLoS One 6(7):e21110
- Viblanc VA, Smith A, Gineste B, Groscolas R. (2012). Coping with continuous human disturbance in the wild: insights from penguin heart rate response to various stressors. BMC Ecol 12:10
- von Borell E, Langbein J, Despres G, Hansen S, Leterrier C, Marchant-Forde J, Marchant-Forde R, et al. (2007). Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals – a review. Physiol Behav 92(3):293–316
- Weimerskirch H, Shaffer SA, Mabille G, Martin J, Boutard O, Rouanet J-L. (2002). Heart rate and energy expenditure of incubating wandering albatrosses: basal levels, natural variation, and the effects of human disturbance. J Exp Biol 205:475–83
- Wilson RP, White CR, Quintana F, Halsey L, Liebsch N, Martin G, Butler PJ. (2006). Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J Anim Ecol 75(5):1081–90
- Woakes AJ, Butler PJ. (1983) Swimming and diving in tufted ducks, Aythya fuligula, with particular reference to heart rate and gas exchange. J Exp Biol 107:311–29
- Yoda K, Naito Y, Sato K, Takahashi A, Nishikawa J, Ropert-Coudert Y, Kurita M, Le Maho Y. (2001). A new technique for monitoring the behaviour of free-ranging adélie penguins. J Exp Biol 204:685–90