Abstract
Ethnic minority groups across the world face a complex set of adverse social and psychological challenges linked to their minority status, often involving racial discrimination. Racial discrimination is increasingly recognized as an important contributing factor to health disparities among non-dominant ethnic minorities. A growing body of literature has recognized these health disparities and has investigated the relationship between racial discrimination and poor health outcomes. Chronically elevated cortisol levels and a dysregulated hypothalamic–pituitary–adrenal (HPA) axis appear to mediate effects of racial discrimination on allostatic load and disease. Racial discrimination seems to converge on the anterior cingulate cortex (ACC) and may impair the function of the prefrontal cortex (PFC), hence showing substantial similarities to chronic social stress. This review provides a summary of recent literature on hormonal and neural effects of racial discrimination and a synthesis of potential neurobiological pathways by which discrimination affects mental health.
Introduction
Racial discrimination affects minority groups across the world and has a negative impact on both physical and mental health, thus creating and perpetuating substantial health inequalities. The terms “racism” and “racial discrimination” overlap in meaning and are often used interchangeably in the literature. Defining these terms is necessary to provide a solid base for a discussion about their impact on health. Both terms refer to an unequal distribution of power in societies and inter-individual relations due to a notion of phenomenological, ancestral or cultural difference. While there is clearly no foundation for a biological concept of “race” (Association of American Physical Anthropologists, Citation1996, Heinz et al., Citation2012; Livingstone & Dobzhansky, Citation1962), “race” functions as a social construct and is often used to disadvantage certain groups of society. Most research reviewed in this article investigated racial discrimination in the context of inter-individual racism, including the subjective notion of being disadvantaged or socially excluded due to racism or prejudice. As racism is often implicit in its nature and pervasive in society, it may not always be perceived and reported, resulting in underreporting (Krieger et al., Citation2005). Therefore, relying on self-reported discrimination might only reveal a small portion on the actual effect of racism on the individual.
Discrimination and health
Health inequalities affect minority groups across the world. Reports have shown that ethnic minority groups suffer from lower life expectancy, lower access to health services and a higher burden of disease (Australian Institute of Health and Welfare, Citation2006; Kung et al., Citation2008). A growing body of literature has identified strong associations between discrimination and adverse health outcomes, most notably non-communicable diseases. These associations appear to be consistent across different countries and populations. Systematic reviews revealed an overall negative effect of racial discrimination on self-reported physical health (Brondolo et al., Citation2011; Pascoe & Richman, Citation2009; Williams & Mohammed, Citation2009; Williams et al., Citation2003,) and general well-being (Schmitt et al., Citation2014), which was stronger in adults than in adolescents (Priest et al., Citation2013), indicating an increasing detrimental effect over the lifespan. Experiencing racial discrimination seems to be associated with obesity (Hunte & Williams, Citation2009) and some studies found effects on blood pressure (Brondolo et al., Citation2003; Dolezsar et al., Citation2014; Din-Dzietham et al., Citation2004), cardiovascular disease (Chae et al., Citation2010, Citation2012; Lewis et al., Citation2014), glycaeted haemoglobin (Piette et al., Citation2006), coronary artery calcification (Lewis et al., Citation2006) and oxidative stress (Szanton et al., Citation2012). Chronic cardiovascular, inflammatory and metabolic risk factors have been found to be elevated in African Americans and Hispanics in the USA even after controlling for health behaviour such as smoking, physical exercise or dietary variables (Crimmins et al., Citation2007).
Mental health impact of discrimination
The negative impact of discrimination on mental health is evident but the mechanisms are still poorly understood. High rates of mental health disorders, notably schizophrenia, mood and anxiety disorders and psychological distress, have been found in various ethnic minority groups across the world. Based on these findings, a body of literature emerged over the last 15 years examining the relationship between ethnic minority status and mental health outcomes. A number of meta-analyses have identified strong associations between racial discrimination and poor mental health outcomes in adults (Paradies, Citation2006; Pascoe & Richman, Citation2009; Williams & Mohammed, Citation2009; Williams et al., Citation2003) and adolescents (Priest et al., Citation2013). Besides the well documented associations with psychosis, racial discrimination is associated with mood and anxiety disorders (Gee et al., Citation2007). While a large proportion of the research was carried out in the USA and examined health outcomes in African Americans, similar results have been established for Asian Americans (Gee et al., Citation2007; Lee & Ahn, Citation2011), Indigenous Australians (Larson et al., Citation2007) and ethnic minority groups in the UK (Chakraborty et al., Citation2010).
In the case of schizophrenia, the notion that the increased risk persists into the second generation among migrants gave rise to the hypothesis that post-migratory social environmental risk factors may be responsible rather than risk factors related to the experience of migration itself (Cantor-Graae & Selten, Citation2005; Selten et al., Citation2007). Indeed, racial discrimination was found to contribute to unequal rates of schizophrenia in former migrants in the UK (Karlsen et al., Citation2005) and Netherlands (Veling et al., Citation2007). More recently, reports suggested that racial discrimination is also associated with sub-threshold psychotic symptoms (Anglin et al., Citation2014; Saleem et al., Citation2014). While most studies in this area rely on correlational data, the impact of racism on psychotic symptoms was also confirmed in longitudinal designs (Janssen et al., Citation2003). However, it should be noted that not all studies investigating racism as a risk for psychosis found an association between the two (Veling et al., Citation2008).
Taken together, the notion that the elevated risk for adverse health outcomes is related to minority status and discrimination rather than ethnicity per se and that this risk is higher in the post-migratory environment compared to the country of origin strongly suggests a significant environmental contribution to these health inequalities. Additionally, it has been argued that different prevalence of mental disorders in different ethnicities are better explained by different age profiles in the studied population (Choi et al., Citation2006) and an early hypothesis that people at risk for psychosis are more likely to migrate has been dismissed (Selten et al., Citation2002). In light of these findings it seems highly unlikely that a substantial proportion of health inequalities can be attributed to ethnicity related traits.
Pathways from discrimination to disease
Several pathways by which discrimination impacts on health have been described. One of the most accepted concepts of discrimination and its adverse effects on health characterizes discrimination as a psychosocial stressor. Within the framework of this concept, the experience of discrimination and social exclusion due to racism provokes a stress response in the individual, which then has detrimental effects on health. When an experience of discrimination is – consciously or not – perceived as stressful, this may generate a series of negative emotional responses and physiological arousal. Physiological arousal involves activation of the hypothalamic–pituitary–adrenal (HPA) axis and subsequently the release of cortisol. Cortisol is the main systemic hormonal endpoint of the stress response and has numerous effects on the body and the brain. These effects include metabolic changes and effects on the immune system as well as behavioural alterations, such as mood changes and cognitive impairment (Juster et al., Citation2010; Radley et al., Citation2011). Simultaneously, stress activates the sympathetic-adrenal-medullary axis (SAM), leading to changes in cardiovascular function with increased heart rate, blood pressure and vasoconstriction. Indeed, racial discrimination leads to increased chronic stress (Sellers et al., Citation2003) and chronic stress has been shown to partially mediate the effects of racial discrimination on depression (Paradies & Cunningham, Citation2012). Cognitive appraisal, the personal interpretation of a situation regarding significance for the individual, has been shown to mediate the effects of discrimination on the stress response (King, Citation2005). This finding may explain why racial discrimination is perceived as stressful. Conceptualising racial discrimination within the stress and coping paradigm provides a promising theoretical framework for the investigation of negative health consequences of racial discrimination. However, it seems most appropriate where racial discrimination is a symptom of institutional or individual racism and where racial discrimination creates a situation that is consciously perceived and interpreted as racism by the individual.
While the association between discrimination and adverse health outcomes is evident and several models of causality exist, the neural mechanisms have long been unclear (Morgan et al., Citation2010; Veling, Citation2013; Veling et al., Citation2008). Most of the previous research assessed the effects of racial discrimination in cross-sectional designs. In contrast, longitudinal studies are rare. However, within the last years, an increasing number of publications emerged testing hypotheses regarding the experience of discrimination and social rejection in experimental designs. These studies aim to investigate the biological effects of racism on the body and brain using neuroimaging and endocrinological techniques and provide novel insight into the effects of racial discrimination. The present review aims to shed light on the effects of discrimination on the brain and the potential consequences of these effects on mental health.
Activation of the biological stress response
Cortisol and the HPA axis
The hormonal consequences of experiencing or merely imagining racial discrimination have long been known (see Harrell et al. (Citation2003) for a review) (). Racial discrimination in a laboratory setting evokes a peripheral stress response, characterized by an increase in blood pressure and serum/salivary cortisol (Armstead et al., Citation1989; McNeilly et al., Citation1995; Sawyer et al., Citation2012). These findings illustrate the acute activating effects of experiencing discrimination on the biological stress axis. However, they do not take the cumulative toll of discrimination into account and leave the question open whether recurrent experiences of racial discrimination lead to an altered stress response. More recently, research has investigated the hormonal stress response in populations affected by frequent racial discrimination. Three studies have examined diurnal cortisol rhythms in African Americans, Hispanics and white Americans and found flatter cortisol slopes and higher evening cortisol among non-white groups (Cohen et al., Citation2006b; DeSantis et al., Citation2007; Martin et al., Citation2012). These results were partially mediated by low socioeconomic status but not influenced by psychological factors and suggest that ethnic differences in cortisol levels may be a result of chronic stress caused by environmental factors such as discrimination. The mediating role of low socioeconomic status here is not surprising as low socioeconomic status can be the consequence of institutional racism and may add up to the effects of discrimination on health. Another study investigated perceived racism in native Hawaiians and found a similar relationship (Kaholokula et al., Citation2012). Individuals who reported higher perceived discrimination had lower overall cortisol levels. These findings strongly suggest that perceptions of discrimination are associated with altered circadian cortisol levels. However, another study assessed American born adolescents of Mexican descent for self-reported racial discrimination and their cortisol levels (Zeiders et al., Citation2012) and found an opposite relationship. Higher perceived discrimination was associated with higher overall cortisol levels (higher area under the curve, AUC) and in contrast to previous studies steeper but not flatter awakening response. It has to be noted though that this study used only two saliva samples on one day, one at varying times in the morning and one 12 h later, but no sample representing the cortisol peak after waking up was recorded. Therefore, no conclusions can be drawn regarding the awakening response, as this requires at least two consecutive samples in the morning (wake up and 30 min later) to capture the morning peak. Another study did not find associations between cortisol and self-reported discrimination in pre-adolescents (Martin et al., Citation2012).
Table 1. Summary of the effects of racial discrimination on the activation of the HPA axis.
While changes in the HPA axis activity are well known under the influence of chronic stress, the direction of the change (heightened or attenuated) appears to depend on numerous factors (Miller et al., Citation2007). This again may limit the interpretation of the findings. In conclusion, studies investigating the effects of racial discrimination on the HPA axis revealed inconsistent results. While altered HPA-axis activity has been observed across different studies, the direction of this effect seems to depend on various factors and cannot always be fully explained. Altered HPS axis activity is a potential pathway by which racial discrimination affects overall health, as flat cortisol slopes are associated with cardiovascular mortality (Kumari et al., Citation2011).
Heart rate variability
Besides the HPA axis, the autonomic nervous system appears to underlie chronic changes resulting in altered balance between the sympathetic nervous system and the parasympathetic nervous system. Heart rate variability (HRV) quantifies the changes in beat-to-beat intervals caused by the balance between sympathetic (SNS) and parasympathetic nervous system (PNS). The influence of the PNS on HRV can be measured in the high frequency band (HF-HRV) and low HF-HRV has been identified as a negative prognostic factor for a number of diseases including myocardial infarction, heart failure and diabetic neuropathy (La Rovere et al., Citation2003; Ponikowski et al., Citation1997). More recently, low HF-HRV has been found in patients with post-traumatic stress disorder (PTSD) (Minassian et al., Citation2014; Moon et al., Citation2013), schizophrenia (Moon et al., Citation2013), depression, anxiety (Åhs et al., Citation2009; Gorman & Sloan, Citation2000), high levels of chronic stress and daily worry (Brosschot et al., Citation2007) and some reports suggest to consider low HF-HRV as an index for chronic stress. HRV appears to decrease with age and therefore may be a sign of aging. However, this seems not to be the case in African Americans, who show low HRV even at a young age (Choi et al., Citation2006). One possible interpretation of this finding is that low HRV may represent premature aging, perhaps due to the effect of chronic stress and increased allostatic load over time (see below). To date, only one study has investigated changes in HF-HRV in relation to racial discrimination in a sample of white and African American women with diabetes (Wagner et al., Citation2013). This study found a significant association between perceived discrimination and low HF-HRV at baseline and in response to a laboratory psychosocial stress task and no correlation between ethnic group status and HF-HRV. In other words, lifetime history of perceived discrimination but not skin colour explained the association. The central nervous top-down control of HF-HRV, which mainly represents the activity of the PNS, has been localized predominantly in the medial prefrontal cortex (mPFC, BA9/10), the subgenual anterior cingulate cortex (subACC, BA25) and the left sub-lenticular extended amygdala/ventral striatum (SLEA), a region comprising parts of the amygdala and ventral striatum (Thayer et al., Citation2012; Thayer & Lane, Citation2000, Citation2009). Disruption of the inhibitory effects of the PNS on the heart may ultimately be a pathway by which chronic stress and worry due to discrimination affect health.
Allostatic load
The chronic over-activation of the HPA axis found in persons reporting racial discrimination likely contributes to chronic wear and tear on the body, termed allostatic overload (McEwen, Citation1998, Citation2007). Allostasis describes the adaption of the body to changing demands over time. Chronically increased allostasis, on the other hand, may lead to pathophysiology and is termed “allostatic load” or “allostatic overload”. Chronic exposure to stressors such as racial discrimination contributes to this maladaptation and leads to changes mediated by glucocorticoids and pro-inflammatory cytokines. These changes are known to have long-term effects on the brain, particularly areas expressing a high number of glucocorticoid receptors, such as prefrontal cortex, hippocampus and amygdala (Blix et al., Citation2013; Dannlowski et al., Citation2012). Furthermore, allostatic load leads to metabolic changes (McEwen, Citation2007). The concept of allostasis might therefore be a final common pathway for different stressors and may link racial discrimination to poor health outcomes (Harrell et al., Citation2011).
Insights from neuroimaging studies
Neuroimaging techniques including the use of functional magnetic resonance imaging (fMRI) allow the study of brain activity and functional connectivity between brain regions in vivo (). This provides an insight into the areas of the brain that are active during certain tasks or states and allows for interpretation of the evoked responses. While early neuroimaging work focused on individual brain regions, research into large-scale brain networks has developed over the last years. This research is based on the finding that sets of individual regions activate in a coordinated manner during certain tasks and at rest.
Table 2. Summary of functional brain imaging studies on the effects of discrimination and social exclusion.
Anterior cingulate cortex
Social adversity evokes a number of responses in the human brain. These responses involve evolutionary old and conserved brain regions in the midbrain regulating basic survival related functions as well as higher order control regions in the cortex. The anterior cingulate cortex (ACC; BA24, 32, 33) is a cortical area that integrates social cues and knowledge related to complex social experiences such as social exclusion. It is connected to the limbic system and closely involved in emotional reactivity (Bush et al., Citation2000). This region of the brain has been found to be affected by early life stress (Cohen et al., Citation2006a; Dannlowski et al., Citation2012; Korgaonkar et al., Citation2013). Furthermore, perceived social stress is associated with altered ACC activity (Wang et al., Citation2005). Recent research investigating neural correlates of social exclusion found that the dorsal ACC (dACC, BA32) is activated by the experience of social exclusion (Eisenberger & Lieberman, Citation2004; Wesselmann et al., Citation2013). Most of the experimental studies investigating the neural correlates of social exclusion used the paradigm of a ball tossing game (cyberball). Participants are asked to play a video game in which they have to play with two computer-controlled players while believing that these two players are other human participants (Eisenberger et al., Citation2003). During the course of the game the participant is then either included by the computer players or excluded from the game by not receiving any ball contacts. An early study has found an increased activity of the dACC during social exclusion and a positive correlation between self-reported distress due to exclusion and dACC activity (Eisenberger et al., Citation2003). Other studies investigated distinct patterns of social exclusion. The inclusion of peers after the subject has previously been excluded (creating differences between subjects) for instance led to activity in the subgenual ACC (subACC, BA25) and dACC, while being excluded when everyone else in the experiment was excluded resulted in different patterns of brain activity and no activity in brain regions linked to distress and social pain (Masten et al., Citation2013).
Rejection sensitivity, the individual’s importance of being accepted and fears and believes about the likelihood of being accepted, could be an important variable in the context of racial discrimination. Higher rejection sensitivity seems to be associated with greater activity in the subACC and ventral–lateral prefrontal cortex (VLPFC; BA47), a region involved in distress control (Masten et al., Citation2009, Citation2013). Furthermore, higher perceived stress during social exclusion was shown to be associated with higher dACC and subACC activity (Eisenberger et al., Citation2003, Masten et al., Citation2009, Citation2011). One study used the same paradigm and investigated the attribution of social exclusion to racial discrimination. When subjects believed that the experienced negative social treatment was due to racial discrimination, lower dACC and higher rostralACC (rACC; BA24, 32) activity was found. This finding led to the conclusion that attributing negative social treatment to racial bias may be seen as a coping mechanism (blame for the negative treatment is projected externally, i.e. due to bias of others), as the pattern of brain activity suggests lower emotional pain but higher emotion- and pain-regulation (Masten et al., Citation2011). This is in line with another study that found lower rates of depression in individuals who felt amusement or sorrow for a racist person (Paradies & Cunningham, Citation2012).
While results of these studies show the neural correlates in the event of social exclusion due to racial discrimination, it has to be considered that in most cases racial discrimination is a long lasting condition and likely takes its toll over time, hence creating a cumulative effect of adversity. The long term influences of racial discrimination on neural social stress processing have been studied in members of ethnic minority groups reporting various levels of racial discrimination (Akdeniz et al., Citation2014). This study found a significant correlation between perceived discrimination and activity of the pACC, a region known to have a high density of glucocorticoid receptors, suggesting altered stress processing in individuals affected by racial discrimination. Moreover, chronic stress was associated with stronger functional connectivity between pACC and dACC and mediated the effects of racial discrimination. Another study found a negative correlation between perceived discrimination due to stigma and concentrations of N-acetylaspartate (NAA) in the ACC in patients with heroin addiction (Frischknecht et al., Citation2013). NAA can be seen as a metabolite representing energy metabolism and is mainly found in the brain. Reduced levels of NAA suggest decreased metabolism or cell loss. While in this study influences of drug withdrawal or medication could not be ruled out, the findings suggest altered neuroplasticity in the ACC of a highly stigmatized group depending on self-reported discrimination.
Apart from mental health disorders, the experience of racial discrimination seems to be associated with bodily pain, as has been found in a cohort of African American veterans (Burgess et al., Citation2009) and in an epidemiological study (Edwards, Citation2008). These findings remained significant even after controlling for potential mediating factors such as socioeconomic status and health related variables. In the context of social rejection paradigms it is noteworthy that the emotional pain during exclusion creates similar patterns of brain activity as physical pain (Dewall et al., Citation2010; Eisenberger & Lieberman, Citation2004; Eisenberger et al., Citation2003) and it has been argued that high sensitivity to social rejection may concur with higher sensitivity to physical pain (Eisenberger & Lieberman, Citation2004). This shared sensitivity may explain the relationship between social rejection through discrimination and the perception of pain. Additionally, regions involved in chronic pain such as the dACC and rACC, the MPFC, the precuneus and the putamen are also central parts of the salience network and the role of the salience network in chronic pain is a matter of ongoing discussion (Borsook et al., Citation2013).
Prefrontal cortex
The prefrontal cortex is another area of interest in the context of racial discrimination. There is some consensus that the ventral prefrontal cortex (vPFC) is involved in the top-down regulation of emotions and stress during social rejection (Eisenberger et al., Citation2003; Onoda et al., Citation2009). In the cyberball paradigm, the right vental prefrontal cortex (rvPFC) is activated during exclusion together with the dACC/subACC and AI (Eisenberger et al., Citation2003; Masten et al., Citation2009, Citation2011). Greater dACC and lower rvPFC activity in turn were observed in individuals reporting higher levels of perceived stress during the experiment (Eisenberger et al., Citation2003; Masten et al., Citation2009, Citation2011). The rvPFC has been shown to be involved in the stress response and it shows sustained activity after the psychological stress task ended (Wang et al., Citation2005). Chronic psychosocial stress further disrupts connectivity in the PFC (Arnsten, Citation2011; Liston et al., Citation2009) and between the ACC and the PFC (Jovanovic et al., Citation2011). Moreover, psychosocial stress is associated with decreased grey matter volumes in the ACC and dorsolateral prefrontal cortex (DLPFC; lateral part of BA9, 46) (Blix et al., Citation2013). These stress-induced changes appear to be partially mediated by glucocorticoid (GC) receptors as artificially elevated levels of GCs result in similar changes as can be observed in patients reporting chronic stress (Lucassen et al., Citation2014). One study also found that enduring daily psychosocial stress seems to be associated with a reduction of 5-HT(1A) receptor binding in the ACC and functional disintegration of ACC/mPFC (Jovanovic et al., Citation2011).
The salience network
The salience network (SN) has been proposed as a large-scale neurocognitive system that integrates sensory information and shifts attention towards potential threats (Corbetta et al., Citation2008; Seeley et al., Citation2007). It consists of the amygdala, the dACC and hypothalamus, the medial prefrontal cortex (MPFC), the anterior insula (AI), striatum, the pulvinar, the thalamus and inferotemporal and temproparietal regions (Corbetta et al., Citation2008) and the posterior parietal cortex and the precuneus have also been implicated in SN functions (Arcizet et al., Citation2011; Cavanna & Trimble, Citation2006). On one hand, brain regions activated by social exclusion and increased stress processing in individuals reporting racial discrimination are core parts of the salience network. Discriminatory events may well be seen as salient stimuli in individuals who are exposed to them over a long period of time. Altered patterns of activation and connectivity in the SN may impair the brain’s ability to integrate and evaluate stimuli. This may lead to heightened sensitivity to racial and ethnic issues and the fact that merely anticipating racial discrimination leads to a stronger stress response compared to a stressful situation without the involvement of racial biases further supports this hypothesis (Sawyer et al., Citation2012). Rejection sensitivity in this context could be interpreted as an increased salience of social exclusion due to prior experience. On the other hand, the stress associated with experiences of racial discrimination appears to have effects on the salience of external stimuli. Acute social stress leads to increased activity in the amygdala and increased functional connectivity with the dACC, anterior insula and the locus coeruleus, and prolonged activity remains in the aftermath of stress (van Marle et al., Citation2010). Interestingly, activity of the HPA axis predicted connectivity between the subACC and the SN in one study, strongly suggesting that heightened subACC activity as it is found in individuals reporting experiences of racial discrimination results in over-activation of the HPA axis and a maladaptive stress response (Thomason et al., Citation2011). Furthermore, stress leads to higher sensitivity and lower specificity for potential stimuli, thereby increasing vigilance and as a consequence non-harmful stimuli may evoke a stress response (van Marle et al., Citation2009). This activity seems to be sensitive to noradrenaline but not to dopamine and can be decreased by the administration of the beta-adrenergic blocker propranolol (Hermans et al., Citation2011). In conclusion, there seems to be an up-regulation of the SN during and perhaps after stress (Hermans et al., Citation2014). From a psychological viewpoint, an individual sensitive to racial discrimination may be on guard for signs of discrimination and the emerging chronic vigilance likely results in chronic stress. Chronic vigilance is often accompanied by worry and worry has been shown to have negative effects on health (Brosschot et al., Citation2006). Salience dysregulation also occurs in a number of illnesses, most notably Parkinson’s disease (Nagy et al., Citation2012), schizophrenia (Roiser et al., Citation2009, Citation2013), addiction (Ma et al., Citation2010) and perhaps chronic pain (Borsook et al., Citation2013). The higher rates of chronic pain found in individuals reporting discrimination may also be a result of heightened vigilance and salience dysregulation (Borsook et al., Citation2013; Burgess et al., Citation2009).
Linking stress hormones to brain networks
It has been recently pointed out that there is a lack of sufficient understanding of how discrimination leads to stress within the framework of the social stress model (Schwartz & Meyer, Citation2010). The association between minority group status and poor health outcomes (between group differences), as well as the association between stress and poor health outcomes (within group differences) had been shown but a lack of evidence on how racial minority status creates stress existed. Insights from neuroimaging and hormonal studies may contribute to filling this gap by highlighting the effects of racial discrimination on the brain and the stress response in vivo and the chronic changes resulting from discrimination over time.
The notion that a single event of racial discrimination is a form of acute social stress is well documented and indeed seems very intuitive. This finding alone, however, does not explain potential detrimental effects on health. It is the chronic exposure to discrimination that engages neural stress regulatory circuits and that leads to reorganizations in the brain. The ACC appears to be the key site on which discrimination impacts, resulting in hyperactivity in response to stress. Besides the ACC, the PFC also shows a different pattern of activity under the influence of long-term racial discrimination. Lower activity in the PFC associated with a stronger perceived stress likely represents an impaired top-down control on the ACC. Together with the over-activation of the HPA axis, this suggests that chronic influence of racial discrimination exacerbates the stress response. The SN may be involved in the heightened vigilance associated with racial discrimination. Social adversity in the form of discrimination activates key regions of the SN. While only speculative at the moment, salience dysregulation may be a result of chronic discrimination and may make the individual more susceptible for other stressors and perhaps even be a common pathway with mental disorders involving salience dysregulation, such as psychosis and schizophrenia ().
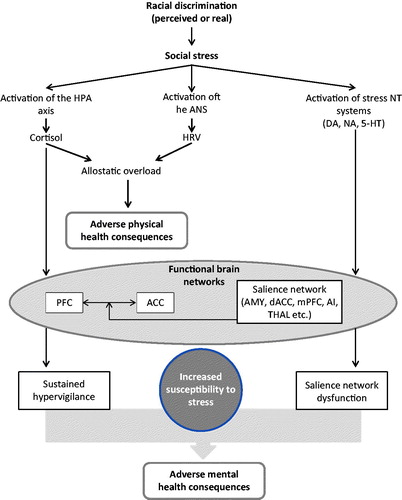
Figure 1. A working model of how racial discrimination may result in adverse mental health consequences. Briefly, racial discrimination is perceived as a form of social stress, leading to the activation of the HPA axis, the ANS and stress-related neurotransmitters in the brain. Sustained elevation of cortisol over time leads to allostatic overload, which, together with altered HRV, a measure of the abnormal activation of ANS, may contribute to adverse physical health consequences. Functional brain networks encompassing the PFC, ACC and the salience network receive hormonal inputs, cortisol, from the periphery and neurotransmitter innervation from lower, subcortical regions. Glucocorticoid-driven structural changes within these networks and altered network activity due to abnormal afferent neurotransmitter inputs may contribute in deleterious functions consequences, such as sustained hypervigilance and abnormal salience attribution, which, in turn, can result in increased susceptibility to everyday stressors, including discrimination. These together may contribute in the development of adverse mental health consequences associated with racial discrimination, including, anxiety disorders, depression and psychosis. See further details in the text. Abbreviations: 5-HT: 5-hydroxy-tryptamine, serotonin; ACC: anterior cingulate cortex; AI: anterior insula; AMY: amygdala; ANS: autonomic nervous system; DA: dopamine; dACC: dorsal anterior cingulate cortex; HPA: hypothalamic-pituitary-adrenal; mPRC: medial prefrontal cortex; NA: noradrenaline; NT: neurotransmitter; PFC: prefrontal cortex; THAL: thalamus.
The social defeat hypothesis may provide a useful framework for the influence of discrimination on schizophrenia (Selten & Cantor-Graae, Citation2005). This hypothesis posits that long-term exposure to social exclusion leads to sensitization of mesolimbic dopamine neurons, which is commonly found in patients with schizophrenia. Indeed, there is evidence that individuals with experiences of chronic social defeat react to stress with greater dopamine release (Novick et al., Citation2011; Pruessner et al., Citation2004; Soliman et al., Citation2008). Animal models of social defeat have yielded similar results (Tidey & Miczek, Citation1996) and showed that dopaminergic brain regions undergo neural adaptions in reaction to chronic stress (Fanous et al., Citation2010). Furthermore, animal studies have shown the functional connection between DA neurons and the HPA axis (Lemos et al., Citation2012), suggesting a tight interaction between chronic exposure to stress and dopaminergic neurons. Such social stress-induced activation of dopamine neurons that project to the ACC and PFC as well as to certain areas within the salience network may contribute to the abnormal activation pattern and connectivity.
A general limitation to the reviewed studies is the operationalization of the variable “racism” or “racial discrimination”. Self-report is most commonly used to quantify experiences of racial discrimination. This poses the question whether subjective impressions adequately represent objective events. However, the individual experience may have closer associations to the brains response than an event rated by an observer. Researchers have called for development of measures for racism independent of self-report (Williams & Mohammed, Citation2013).
Regarding the HPA axis, findings across different studies vary. This may be partially explainable by methodological differences and that the effect of racism on the brain and body likely depends on several individual factors. These complex interactions between social support, socioeconomic status, resilience and others may moderate the effect of racism and integrating them into research designs remains a challenge.
Although it has been widely acknowledged that stress, such as racial discrimination, activates a variety of hormonal systems, including the HPA axis, neurotransmitters such as dopamine, noradrenaline and serotonin, as well as pro-inflammatory cytokines (Juster et al., Citation2010; McEwen, Citation2007), cortisol plays a central role in linking stress to its long-term consequences (McEwen, Citation2007). Glucocorticoid receptors are centrally located in brain regions that belong to the salience circuit, including amygdala, hypothalamus and prefrontal cortex (McEwen, Citation2007) as well as in the cell body regions of the dopaminergic system, the ventral tegmental area (Butts & Phillips, Citation2013) and in the hippocampus (McEwen, Citation1997; McEwen & Magarinos, Citation1997). Chronic activation of these receptors can lead to abnormal long-term plasticity of neural systems that mediate emotions, attention, cognitive functions and hedonic homeostasis, which can lead to altered activity of the salience network and, ultimately, the emergence of psychopathology, such as anxiety, depression and psychosis () (Bizik et al., Citation2013; Maren et al., Citation2013; Misiak et al., Citation2014; Schwabe & Wolf, Citation2013).
Recent results reviewed here clearly establish the neurobiological stress response as a possible mechanism to mediate effects of racial discrimination on mental health. However, more research, longitudinal studies in particular that specifically focus on the effects of actual and perceived discrimination, is needed to identify the exact mechanisms and pathways through which psychopathology emerges in individual subjected to discrimination.
Declaration of interest
The authors declare that there is no conflict of interest.
References
- Åhs F, Sollers JJ III, Furmark T, Fredrikson M, Thayer JF. (2009). High-frequency heart rate variability and cortico-striatal activity in men and women with social phobia. NeuroImage 47:815–20
- Akdeniz C, Tost H, Streit F, Haddad L, Wust S, Schafer A, Schneider M, et al. (2014). Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry 71:672–80
- Anglin DM, Lighty Q, Greenspoon M, Ellman LM. (2014). Racial discrimination is associated with distressing subthreshold positive psychotic symptoms among US urban ethnic minority young adults. Soc Psychiatry Psychiatr Epidemiol 49:1545–55
- Arcizet F, Mirpour K, Bisley JW. (2011). A pure salience response in posterior parietal cortex. Cereb Cortex 21:2498–506
- Armstead CA, Lawler KA, Gorden G, Cross J, Gibbons J. (1989). Relationship of racial stressors to blood pressure responses and anger expression in black college students. Health Psychol 8:541–56
- Arnsten AF. (2011). Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci 29:215–23
- Association of American Physical Anthropologists. (1996). Statement on biological aspects of race. Am J Phys Anthropol 101:569–70
- Australian Institute of Health and Welfare. (2006). Australia's health. Canberra: AIHW
- Bizik G, Picard M, Nijjar R, Tourjman V, McEwen BS, Lupien SJ, Juster RP. (2013). Allostatic load as a tool for monitoring physiological dysregulations and comorbidities in patients with severe mental illnesses. Harv Rev Psychiatry 21:296–313
- Blix E, Perski A, Berglund H, Savic I. (2013). Long-term occupational stress is associated with regional reductions in brain tissue volumes. PLoS One 8:e64065
- Borsook D, Edwards R, Elman I, Becerra L, Levine J. (2013). Pain and analgesia: the value of salience circuits. Prog Neurobiol 104:93–105
- Brondolo E, Hausmann LR, Jhalani J, Pencille M, Atencio-Bacayon J, Kumar A, Kwok J, et al. (2011). Dimensions of perceived racism and self-reported health: examination of racial/ethnic differences and potential mediators. Ann Behav Med 42:14–28
- Brondolo E, Rieppi R, Kelly K, Gerin W. (2003). Perceived racism and blood pressure: a review of the literature and conceptual and methodological critique. Ann Behav Med 25:55–65
- Brosschot JF, Gerin W, Thayer JF. (2006). The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res 60:113–24
- Brosschot JF, van Dijk E, Thayer JF. (2007). Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. Int J Psychophysiol 63:39–47
- Burgess DJ, Grill J, Noorbaloochi S, Griffin JM, Ricards J, van Ryn M, Partin MR. (2009). The effect of perceived racial discrimination on bodily pain among older African American men. Pain Med 10:1341–52
- Bush G, Luu P, Posner MI. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–22
- Butts KA, Phillips AG. (2013). Glucocorticoid receptors in the prefrontal cortex regulate dopamine efflux to stress via descending glutamatergic feedback to the ventral tegmental area. Int J Neuropsychopharmacol 16:1799–807
- Cantor-Graae E, Selten JP. (2005). Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry 162:12–24
- Cavanna AE, Trimble MR. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–83
- Chae DH, Lincoln KD, Adler NE, Syme SL. (2010). Do experiences of racial discrimination predict cardiovascular disease among African American men? The moderating role of internalized negative racial group attitudes. Soc Sci Med 71:1182–8
- Chae DH, Nuru-Jeter AM, Lincoln KD, Jacob Arriola KR. (2012). Racial discrimination, mood disorders, and cardiovascular disease among black Americans. Ann Epidemiol 22:104–11
- Chakraborty AT, Mckenzie KJ, Hajat S, Stansfeld SA. (2010). Racism, mental illness and social support in the UK. Soc Psychiatry Psychiatr Epidemiol 45:1115–24
- Choi JB, Hong S, Nelesen R, Bardwell WA, Natarajan L, Schubert C, Dimsdale JE. (2006). Age and ethnicity differences in short-term heart-rate variability. Psychosom Med 68:421–6
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, et al. (2006a). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 59:975–82
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. (2006b). Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med 68:41–50
- Corbetta M, Patel G, Shulman GL. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–24
- Crimmins EM, Kim JK, Alley DE, Karlamangla A, Seeman T. (2007). Hispanic paradox in biological risk profiles. Am J Public Health 97:1305–10
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, et al. (2012). Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71:286–93
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. (2007). Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolescent Health 41:3–13
- Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, et al. (2010). Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci 21:931–7
- Din-Dzietham R, Nembhard WN, Collins R, Davis SK. (2004). Perceived stress following race-based discrimination at work is associated with hypertension in African–Americans. The metro Atlanta heart disease study, 1999–2001. Soc Sci Med 58:449–61
- Dolezsar CM, McGrath JJ, Herzig AJ, Miller SB. (2014). Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol 33:20–34
- Edwards RR. (2008). The association of perceived discrimination with low back pain. J Behav Med 31:379–89
- Eisenberger NI, Lieberman MD. (2004). Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci 8:294–300
- Eisenberger NI, Lieberman MD, Williams KD. (2003). Does rejection hurt? An FMRI study of social exclusion. Science 302:290–2
- Fanous S, Hammer RP Jr, Nikulina EM. (2010). Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience 167:598–607
- Frischknecht U, Hermann D, Heinrich M, Hoerst M, Weber-Fahr W, Vollstadt-Klein S, Kiefer F, et al. (2013). Experience of social discrimination correlates with neurometabolism: a pilot study in heroin addicts. Eur Arch Psychiatry Clin Neurosci 263:197–203
- Gee GC, Spencer M, Chen J, Yip T, Takeuchi DT. (2007). The association between self-reported racial discrimination and 12-month DSM-IV mental disorders among Asian Americans nationwide. Soc Sci Med 64:1984–96
- Gorman JM, Sloan RP. (2000). Heart rate variability in depressive and anxiety disorders. Am Heart J 140:S77–83
- Harrell CJP, Burford TI, Cage BN, Nelson TM, Shearon S, Thompson A, Green S. (2011). Multiple pathways linking racism to health outcomes. Du Bois Rev–Soc Sci Res Race 8:143–57
- Harrell JP, Hall S, Taliaferro J. (2003). Physiological responses to racism and discrimination: an assessment of the evidence. Am J Public Health 93:243–8
- Heinz A, Müller D, Kluge U. (2012). “Race”: Warum alte Begriffe keine neuen Perspektiven haben. J Neurol Neurochir Psychiatr 12:168–74
- Hermans EJ, Henckens MJ, Joels M, Fernandez G. (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37:304–14
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, et al. (2011). Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334:1151–3
- Hunte HE, Williams DR. (2009). The association between perceived discrimination and obesity in a population-based multiracial and multiethnic adult sample. Am J Public Health 99:1285–92
- Janssen I, Hanssen M, Bak M, Bijl RV, De Graaf R, Vollebergh W, Mckenzie K, van Os J. (2003). Discrimination and delusional ideation. Br J Psychiatry 182:71–6
- Jovanovic H, Perski A, Berglund H, Savic I. (2011). Chronic stress is linked to 5-HT1A receptor changes and functional disintegration of the limbic networks. NeuroImage 55:1178–88
- Juster RP, Mcewen BS, Lupien SJ. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 35:2–16
- Kaholokula JK, Grandinetti A, Keller S, Nacapoy AH, Kingi TK, Mau MK. (2012). Association between perceived racism and physiological stress indices in Native Hawaiians. J Behav Med 35:27–37
- Karlsen S, Nazroo JY, Mckenzie K, Bhui K, Weich S. (2005). Racism, psychosis and common mental disorder among ethnic minority groups in England. Psychol Med 35:1795–803
- King KR. (2005). Why is discrimination stressful? The mediating role of cognitive appraisal. Cultur Divers Ethnic Minor Psychol 11:202–2
- Korgaonkar MS, Antees C, Williams LM, Gatt JM, Bryant RA, Cohen R, Paul R, et al. (2013). Early exposure to traumatic stressors impairs emotional brain circuitry. PLoS One 8:e75524
- Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. (2005). Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 61:1576–96
- Kumari M, Shipley M, Stafford M, Kivimaki M. (2011). Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J Clin Endocrinol Metab 96:1478–85
- Kung HC, Hoyert DL, Xu J, Murphy SL. (2008). Deaths: final data for 2005. Natl Vital Stat Rep 56:1–120
- La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, et al. (2003). Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 107:565–70
- Larson A, Gillies M, Howard PJ, Coffin J. (2007). It's enough to make you sick: the impact of racism on the health of Aboriginal Australians. Aust N Z J Public Health 31:322–9
- Lee DL, Ahn S. (2011). Racial discrimination and Asian mental health: a meta-analysis. Couns Psychol 39:463–89
- Lemos JC, Wanat MJ, Smith JS, Reyes BA, Hollon NG, van Bockstaele EJ, Chavkin C, Phillips PE. (2012). Severe stress switches CRF action in the nucleus accumbens from appetitive to aversive. Nature 490:402–6
- Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, et al. (2006). Chronic exposure to everyday discrimination and coronary artery calcification in African–American women: the SWAN heart study. Psychosom Med 68:362–8
- Lewis TT, Williams DR, Tamene M, Clark CR. (2014). Self-reported experiences of discrimination and cardiovascular disease. Curr Cardiovasc Risk Rep 8:365
- Liston C, McEwen BS, Casey BJ. (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA 106:912–17
- Livingstone FB, Dobzhansky T. (1962). On the non-existence of human races. Curr Anthropol 3:279–81
- Lucassen PJ, Pruessner J, Sousa N, Almeida OF, van Dam AM, Rajkowska G, Swaab DF, Czeh B. (2014). Neuropathology of stress. Acta Neuropathol 127:109–35
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, et al. (2010). Addiction related alteration in resting-state brain connectivity. Neuroimage 49:738–44
- Maren S, Phan KL, Liberzon I. (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14:417–28
- Martin CG, Bruce J, Fisher PA. (2012). Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: the role of parental psychosocial risk and monitoring. Horm Behav 61:661–8
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. (2009). Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci 4:143–57
- Masten CL, Eisenberger NI, Pfeifer JH, Dapretto M. (2013). Neural responses to witnessing peer rejection after being socially excluded: fMRI as a window into adolescents' emotional processing. Dev Sci 16:743–59
- Masten CL, Telzer EH, Eisenberger NI. (2011). An fMRI investigation of attributing negative social treatment to racial discrimination. J Cognitive Neurosci 23:1042–51
- McEwen BS. (1997). Possible mechanisms for atrophy of the human hippocampus. Mol Psychiatry 2:255–62
- McEwen BS. (1998). Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci 840:33–44
- McEwen BS. (2007). Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904
- McEwen BS, Magarinos AM. (1997). Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci 821:271–84
- McNeilly MD, Robinson EL, Anderson NB, Pieper CF, Shah A, Toth PS, Martin P, et al. (1995). Effects of racist provocation and social support on cardiovascular reactivity in African American women. Int J Behav Med 2:321–38
- Miller GE, Chen E, Zhou ES. (2007). If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol Bull 133:25–45
- Minassian A, Geyer MA, Baker DG, Nievergelt CM, O'connor DT, Risbrough VB. (2014). Heart rate variability characteristics in a large group of active-duty marines and relationship to posttraumatic stress. Psychosom Med 76:292–301
- Misiak B, Frydecka D, Zawadzki M, Krefft M, Kiejna A. (2014). Refining and integrating schizophrenia pathophysiology – relevance of the allostatic load concept. Neurosci Biobehav Rev 45c:183–201
- Moon E, Lee SH, Kim DH, Hwang B. (2013). Comparative study of heart rate variability in patients with schizophrenia, bipolar disorder, post-traumatic stress disorder, or major depressive disorder. Clin Psychopharmacol Neurosci 11:137–43
- Morgan C, Charalambides M, Hutchinson G, Murray RM. (2010). Migration, ethnicity, and psychosis: toward a sociodevelopmental model. Schizophr Bull 36:655–64
- Nagy H, Levy-Gigi E, Somlai Z, Takats A, Bereczki D, Keri S. (2012). The effect of dopamine agonists on adaptive and aberrant salience in Parkinson's disease. Neuropsychopharmacology 37:950–8
- Novick AM, Forster GL, Tejani-Butt SM, Watt MJ. (2011). Adolescent social defeat alters markers of adult dopaminergic function. Brain Res Bull 86:123–8
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. (2009). Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Soc Neurosci 4:443–54
- Paradies Y. (2006). A systematic review of empirical research on self-reported racism and health. Int J Epidemiol 35:888–901
- Paradies YC, Cunningham J. (2012). The DRUID study: exploring mediating pathways between racism and depressive symptoms among indigenous Australians. Soc Psychiatry Psychiatr Epidemiol 47:165–73
- Pascoe EA, Richman LS. (2009). Perceived discrimination and health: a meta-analytic review. Psychol Bull 135:531–54
- Piette JD, Bibbins-Domingo K, Schillinger D. (2006). Health care discrimination, processes of care, and diabetes patients' health status. Patient Educ Couns 60:41–8
- Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, Adamopoulos S, Webb-Peploe K, et al. (1997). Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 79:1645–50
- Priest N, Paradies Y, Trenerry B, Truong M, Karlsen S, Kelly Y. (2013). A systematic review of studies examining the relationship between reported racism and health and wellbeing for children and young people. Soc Sci Med 95:115–27
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. (2004). Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci 24:2825–31
- Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP. (2011). Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes. Stress 14:481–97
- Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. (2013). Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull 39:1328–36
- Roiser JP, Stephan KE, Den Ouden HE, Barnes TR, Friston KJ, Joyce EM. (2009). Do patients with schizophrenia exhibit aberrant salience? Psychol Med 39:199–209
- Ratner KG, Halim ML, Amodio DM. (2013). Perceived stigmatization, ingroup pride, and immune and endocrine activity: evidence from a community sample of black and latina women. Soc Psychol Person 4:82–91
- Saleem MM, Stowkowy J, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, et al. (2014). Perceived discrimination in those at clinical high risk for psychosis. Early Interv Psychiatry 8:77–81
- Sawyer PJ, Major B, Casad BJ, Townsend SSM, Mendes WB. (2012). Discrimination and the stress response: psychological and physiological consequences of anticipating prejudice in interethnic interactions. Am J Public Health 102:1020–6
- Schmitt MT, Branscombe NR, Postmes T, Garcia A. (2014). The consequences of perceived discrimination for psychological well-being: a meta-analytic review. Psychol Bull 140:921–48
- Schwabe L, Wolf OT. (2013). Stress and multiple memory systems: from ‘thinking' to ‘doing'. Trends Cogn Sci 17:60–8
- Schwartz S, Meyer IH. (2010). Mental health disparities research: the impact of within and between group analyses on tests of social stress hypotheses. Soc Sci Med 70:1111–18
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–56
- Sellers RM, Caldwell CH, Schmeelk-Cone KH, Zimmerman MA. (2003). Racial identity, racial discrimination, perceived stress, and psychological distress among African American young adults. J Health Soc Behav 44:302–17
- Selten JP, Cantor-Graae E. (2005). Social defeat: risk factor for schizophrenia? Br J Psychiatry 187:101–2
- Selten JP, Cantor-Graae E, Kahn RS. (2007). Migration and schizophrenia. Curr Opin Psychiatry 20:111–15
- Selten JP, Cantor-Graae E, Slaets J, Kahn RS. (2002). Odegaard's selection hypothesis revisited: schizophrenia in Surinamese immigrants to The Netherlands. Am J Psychiatry 159:669–71
- Soliman A, O'driscoll GA, Pruessner J, Holahan AL, Boileau I, Gagnon D, Dagher A. (2008). Stress-induced dopamine release in humans at risk of psychosis: a [11C]raclopride PET study. Neuropsychopharmacology 33:2033–41
- Szanton SL, Rifkind JM, Mohanty JG, Miller ER III, Thorpe RJ, Nagababu E, Epel ES, et al. (2012). Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int J Behav Med 19:489–95
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ III, Wager TD. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 36:747–56
- Thayer JF, Lane RD. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 61:201–16
- Thayer JF, Lane RD. (2009). Claude Bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 33:81–8
- Thomason ME, Hamilton JP, Gotlib IH. (2011). Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents. J Child Psychol Psychiatry 52:1026–34
- Tidey JW, Miczek KA. (1996). Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 721:140–9
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. (2009). From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry 66:649–55
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. (2010). Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage 53:348–54
- Veling W. (2013). Ethnic minority position and risk for psychotic disorders. Curr Opin Psychiatry 26:166–71
- Veling W, Hoek HW, Mackenbach JP. (2008). Perceived discrimination and the risk of schizophrenia in ethnic minorities. Soc Psychiatry Psychiatr Epidemiol 43:953–9
- Veling W, Selten JP, Susser E, Laan W, Mackenbach JP, Hoek HW. (2007). Discrimination and the incidence of psychotic disorders among ethnic minorities in The Netherlands. Int J Epidemiol 36:761–8
- Wagner J, Lampert R, Tennen H, Feinn R. (2013). Exposure to discrimination and heart rate variability reactivity to acute stress among women with diabetes. Stress Health [Epub ahead of print]. doi: 10.1002/smi.2542
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. (2005). Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA 102:17804–9
- Wesselmann ED, Williams KD, Hales AH. (2013). Vicarious ostracism. Front Hum Neurosci 7:153
- Williams DR, Mohammed SA. (2009). Discrimination and racial disparities in health: evidence and needed research. J Behav Med 32:20–47
- Williams DR, Mohammed SA. (2013). Racism and health I: pathways and scientific evidence. Am Behav Sci 57:1152–73
- Williams DR, Neighbors HW, Jackson JS. (2003). Racial/ethnic discrimination and health: findings from community studies. Am J Public Health 93:200–8
- Zeiders KH, Doane LD, Roosa MW. (2012). Perceived discrimination and diurnal cortisol: examining relations among Mexican American adolescents. Horm Behav 61:541–8

