Abstract
The stress hormone copeptin, which is co-secreted with arginine vasopressin, increases in seriously ill patients and can predict outcome in several organic diseases. Information about the influence of psychological stress on copeptin levels is lacking, but is important for interpretation of copeptin levels in the clinical setting. The aim of this study was to evaluate the influence of psychological stress on copeptin levels. We measured copeptin levels in 25 healthy medical students before and after a written examination. The primary endpoint was change in copeptin levels from immediately prior to examination compared with after the examination. Median copeptin levels prior to the examination were significantly higher than those after its conclusion. Similar results were found for serum cortisol and salivary cortisol. Serum cortisol prior to examination was significantly higher in students with a superior examination result, compared to those with a lower score. In conclusion, psychological stress leads to a subtle increase in copeptin level and might therefore be taken into account as a confounding factor in disorders with small diagnostic copeptin range. Higher cortisol levels, but not copeptin, correlated with a better academic performance in this cohort of students.
Introduction
Copeptin is a peptide derived from the protein precursor of arginine vasopressin (AVP) and is secreted in equimolar ratio to AVP, and thus released in physical stress (Katan et al., Citation2008b; Morgenthaler et al., Citation2006). The clinical use of AVP is limited as it is very unstable at room temperature, is attached to platelets and has a short half-life. Consequently, it is difficult to measure. In contrast, copeptin is stable at room temperature for at least seven days and can be measured easily by a sandwich immunoassay (Morgenthaler et al., Citation2006). Copeptin is a useful prognostic biomarker in several diseases such as acute coronary syndrome, stroke, transient ischemic attacks, pneumonia, acute and chronic heart failure, chronic obstructive pulmonary disease and traumatic brain injury (Dong et al., Citation2011; Katan et al., Citation2011; Katan & Christ-Crain, Citation2010; Stolz et al., Citation2007; Urwyler et al., Citation2010). It is hypothesized that the close and reproducible relation of copeptin levels to the degree of activation of the stress axis is the basis of its unique usefulness as a biomarker (Katan & Christ-Crain, Citation2010). However, to our knowledge, there are no data on copeptin levels in association with psychological stress.
Numerous other stress hormones have been studied in the context of psychological stress. The Trier Social Stress Test, a standard laboratory protocol for experimental induction of psychological stress, is associated with the largest HPA axis stress responses relative to other laboratory stressors (Dickerson & Kemeny, Citation2004). Psychological stress also increases cortisol during ambulatory settings, e.g. during a theoretical driving license examination (Dugue et al., Citation2001), one hour prior to an oral academic examination (Lacey et al., Citation2000) and in Spanish medical graduates taking an examination to apply for their medical specialty training position (Gonzalez-Cabrera et al., Citation2014). To our knowledge, only preliminary findings from a very small study are available about a possible correlation between salivary cortisol levels and examination success (Ng et al., Citation2003).
The goal of this study was to determine whether a psychological stressor (examination stress) leads to changes in circulating copeptin. Correlations between copeptin and cortisol, and between performance and hormone measures were included as secondary endpoints.
Participants and methods
Participants
We included 25 medical students from fourth year course at Basel University, who participated in the written examination. Participants were recruited at Basel University medical school by word-of-mouth propaganda and e-mail. Exclusion criteria were BMI >30 kg/m2, evidence of acute disease, history of severe chronic illness including psychiatric disease, reported or assumed pregnancy and smoking.
The local ethical committee approved this study; written informed consent was obtained from all voluntary medical students. This study was pre-registered on clinicaltrials.gov (NCT02051647) and was conducted according to the current version of the Declaration of Helsinki, the ICH-GCP as well as all national legal and regulatory requirements.
Description of the examination
The written examination consists of 120 multiple-choice questions (maximal duration: 4 h) at the end of the semester. Students have to pass the examination to be eligible to continue the next study year. Our study was conducted during the first written examination of fourth medical course in January 2014.
Design and procedure
Students were seen at the day of the written examination immediately prior to the examination, and straight afterwards. The examination started at 1 p.m. Students were allowed to eat breakfast as usual and drink a maximum of 300 ml for breakfast until at latest 9 a.m. After 9 a.m., they were not allowed to drink anymore. For lunch, they were allowed to eat normally, but without drinking. Students were not allowed to drink alcohol during the 24 h prior to blood sampling nor to participate in sports within 8 h of sampling.
Immediately prior to and after the examination, clinical parameters were measured, blood and salivary samples were taken to assess serum and salivary cortisol and copeptin levels, respectively, and students were asked to indicate their current individual stress level on the adapted distress thermometer, defined as: 0 absolutely not stressed and 10 extremely stressed (Mehnert et al., Citation2006). During the examination, students were allowed to eat and drink as much as they liked. To assess examination outcome, students were asked to e-mail their examination mark to the study center.
Blood sampling and salivary cortisol
At each time point, two tubes of blood were collected. One sample of 7.5 ml in a Monovette® EDTA KE and one sample of 7.5 ml in a Monovette® Serum Gel Z/7.5 ml to assay cortisol. Copeptin was assessed with a chemiluminescence sandwich immunoassay (BRAHMS CT-proAVP KRYPTOR, from BRAHMS GmbH, Hennigsdorf, Germany). This assay has a lower detection limit of 0.4 pmol/l, intra-assay coefficient of variation of <3–15%, inter- and an inter-assay coefficient of variation of <5–17%. Cortisol was measured with an electrochemilumineszenzimmunoassay (Elecsys Cortisol Test; Roche Diagnostics GmbH, Mannheim, Germany) with an intra- assay coefficient of variation of 1.0–1.7%, inter and inter- assay coefficient of variation of 1.4–2.8%.
Salivary cortisol was collected by chewing for at least 30 s on a cylindrical cotton swab (Salivette® Cortisol (Art.– Nr. 51.1534.500) from Sarstedt AG & Co., Nümbrecht, Germany). Routine laboratory measurements of serum sodium, glucose, urea and uric acid were performed in the central laboratory of the hospital. Serum osmolality was calculated according to the formula (2 × serum sodium + glucose + urea).
Endpoints
The primary endpoint of this study was the change of copeptin levels from immediately prior to the written examination as compared to after the examination. Secondary endpoints were correlation between stress hormones and exam success and correlation of copeptin to cortisol as the classical stress marker.
Statistics
Discrete variables were expressed as counts (percentage) and continuous variables as medians (interquartile range), unless stated otherwise. To test our primary hypothesis, a two-group comparison was performed by Wilcoxon–MWU test. To test for gender differences, the same analysis was repeated separately in male and female students. To investigate how well hormonal levels are able to predict examination results, we compared students with high performance (score: ≥84 points (median)) with students who had a lower performance (<84 points) using the Mann–Whitney U test. We also used linear regression models to study associations of cortisol and copeptin and their change (delta) at the two time points. Hormone levels were log-transformed before entering into the models due to non-normal distribution. To check for differential responses in regard to HPA activation and also score results, we repeated the analysis in each of the subgroups (i.e. high vs low HPA activation based on median baseline cortisol levels (=560 nmol/l) and high vs low exam score results based on median exam points = 84), and also used interaction terms to test for effect modification. Univariate regression models including different potential confounders including age, gender, BMI and baseline heart rate, sodium and osmolality levels were calculated.
All testing were two-tailed, and p values less than 0.05 were considered as statistically significant. All data were calculated using STATA 12.1 and GraphPad Prism®, version 4.00, for Windows (GraphPad Software, San Diego, CA).
Results
Of the 25 voluntary medical students (median age 23 years, median BMI: 21.9 kg/m2), 15 (60%) were female. No student had a history of psychiatric disease, in two students, somatic diseases (vitiligo and migraine) were reported, and 9 of 15 female students (60%) were taking a contraceptive pill. Apart from this, no medication intake was reported. Hydration status assessed prior to examination was euvolemic in all participants.
Copeptin levels immediately prior to written examination were higher compared with after its conclusion (median (IQR) 3.1 pmol/l (2.4–4.3) vs 2.3 pmol/l (1.7–2.8), p < 0.001). These results were confirmed also in a multivariate model adjusted for osmolality, sodium, age, gender, heart rate and BMI. Similarly, serum cortisol (561 nmol/l (386–651) vs 297 nmol/l (235–327), p < 0.001) and salivary cortisol levels (12.5 nmol/l (8.2–17.1) vs 6.9 nmol/l (5.7–8.1), p < 0.001) were elevated prior to examination in comparison to its conclusion. There was no gender difference. Immediately prior to examination, students had a higher heart rate (79/min (74–96) vs 71/min (62–76), p < 0.001) and reported a subjective higher stress level on the adapted distress thermometer (5 (4–6) vs 3 (2–3), p < 0.001) ().
Table 1. Characteristics of medical students before and after the written examination.
There was no association between copeptin and cortisol concentrations prior to examination in the overall population (linear regression coefficient – 0.12, 95% CI: −0.50 to 0.24). Results were similar in subjects with activated HPA axis compared with subjects without HPA activation (p interaction = 0.60). Similarly, there was no significant association between copeptin and cortisol concentrations prior to examination in subjects with high examination scores (linear regression coefficient 0.23, 95% CI: −0.48 to 0.56). In the subgroups of subjects with low scores, we found a significant negative association between copeptin and cortisol concentrations prior to examination (linear regression coefficient −0.57, 95% CI: −1.1 to −0.01). However, there was no evidence for a significant interaction (p interaction = 0.09). After the examination, there was again no association of copeptin and cortisol concentrations in the overall population (linear regression coefficient – 0.20, 95% CI: −0.65 to 0.25). Furthermore, we found no evidence for effect modification by HPA activation (p interaction = 0.39) and examination results (p interaction = 0.84). Moreover, there was no association between Δ-copeptin levels and Δ-serum cortisol levels (linear regression coefficient −13.15, 95% CI: −69.00 to 42.70) in the overall sample population. No differences were found in the subgroups of students with low- and high-score results (p interaction = 0.33) and in students with and without HPA axis activation (p interaction = 0.15).
Subjective stress levels did not correlate with cortisol (linear regression coefficient 0.03, 95% CI: −0.01 to 0.08), copeptin (linear regression coefficient 0.01, 95% CI: −0.07 to 0.45) or salivary cortisol levels (linear regression coefficient −0.03, 95% CI: −0.08 to 0.26).
Students scored with a median score of 84 points (IQR: 79–90) (maximal points 119) and a median grade of 5 (IQR: 4.5–5.5). Serum cortisol levels (p = 0.03) were higher in students with a good result ().
Figure 1. Cortisol as outcome predictor for examination result. Comparison of cortisol levels in students who had high performance (≥84 points of 119) with students who had poor performance (<84 points) using the Mann–Whitney U-test. Error bars show mean and SD.
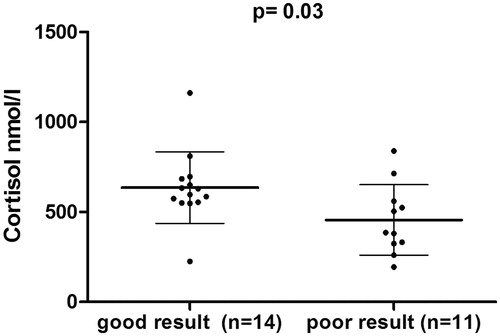
Logistic regression analysis found a significant association between cortisol levels prior to the examination (OR of log transformed cortisol: 399, 95% CI: 1.01 to >1000, p < 0.05) and good test results. No associations between good test performance and copeptin, salivary cortisol or subjective stress level (as indicated on the adapted distress thermometer) were observed (data not shown).
Discussion
This study has two main findings. First, examination stress leads to a small increase in copeptin levels, showing that psychological stress influences copeptin release. Second, serum cortisol levels measured prior to the examination are elevated as a consequence of the anticipation of the examination and predicted subsequent successful completion of the exams. It has already been shown in different stress models (Trier stress test, theoretical driving lesson, national medical examination and first jump of a parachutist) that cortisol is influenced by psychological stress (Dickerson & Kemeny, Citation2004; Dugue et al., Citation1993, Citation2001; Gonzalez-Cabrera et al., Citation2014). Our study demonstrates that psychological stress induced by a written examination not only leads to a rise of serum or salivary cortisol levels but also to a rise in copeptin levels. However, this rise is clearly less pronounced compared with physical stress in somatic diseases like in myocardial infarction or stroke (Katan et al., Citation2008a).
We found no correlation between serum or salivary cortisol levels and copeptin levels in this group of students overall. However, assuming that our students do not react in a homogenous way to examination stress, we showed in a subgroup analysis that students with a poor test result reacted with a different HPA-activation compared with those with a good test result. AVP is not only a stress hormone but also the major hormone involved in water homeostasis (Katan & Christ-Crain, Citation2010). One could postulate that copeptin levels, as surrogate marker of AVP levels, may have been decreased by the oral fluid intake (median amount = 700 ml) during the examination. However, copeptin remained an independent predictor for examination stress after adjusting for osmolality and sodium. Therefore, decreased sodium and osmolality can be excluded as confounding factor for inducing a lowering of copeptin levels after the examination. We also corrected for other confounding factors such as age and gender.
Students with a good examination result had higher serum cortisol levels prior to examination compared to those with a poor result, whereas salivary cortisol, copeptin levels and subjective stress level did not predict examination results. It is known that in sports, high cortisol levels are associated with better performance. For example, tennis players with higher salivary cortisol levels were shown to perform better than those with lower salivary cortisol (Lautenbach et al., Citation2014). However, in contrast to these findings, a small study showed that higher salivary cortisol tended to predict a worse examination outcome (Ng et al., Citation2003). One possible explanation for these discrepant findings might be the degree of cortisol elevation, as an inverted U-shape curve association has been described for glucocorticoid levels and cognitive performance, i.e. extremely high or extremely low cortisol levels are associated with cognitive impairment (Lupien et al., Citation2005). Another explanation might be that students in our study with higher cortisol levels immediately prior to examination also had higher cortisol levels during the consolidation period. High cortisol levels during consolidation period are known to improve memory and acquisition of new information (de Quervain et al., Citation2009). It is known that glucocorticoids have multiple and conflicting effects on memory function with positive effects on memory consolidation and activation of the glucocorticoid receptors seems to be essential for long-term storage of information (Lupien et al., Citation2005). However, stress and glucocorticoids can also impair retrieval of long-term memory. For example, healthy volunteers treated with 25 mg cortisone were significantly worse in recalling information relative to placebo controls (de Quervain et al., Citation1998).
Our study has several limitations. First, we did not measure cortisol and copeptin levels during the consolidation period. Second, we did not measure morning cortisol levels due to the fact that examinations took place in the afternoon. Third, there was no fully standardized eating and drinking protocol as students disagreed to change their habits during the examination. However, fluid intake between breakfast and beginning of the examination was reduced to 300 ml, assuring that prior fluid intake did not suppress copeptin levels.
Conclusion
In conclusion, psychological stress leads to a small increase in copeptin level, less pronounced than in somatic diseases. When using copeptin as a biomarker in clinical routine, psychological stress might only be taken into consideration in disorders with subtly abnormal levels such as polyuria/polydipsia syndrome. Moreover, we showed that stress preceding an examination has differential effects on HPA-axis activation in subgroups that report equal levels of subjective stress. Higher serum cortisol levels, but not copeptin, seem to predict a better examination performance.
Acknowledgements
We thank the staff of the laboratory, the staff of the Department of Endocrinology, Diabetology & Metabolism and our study nurses, especially Cemile Bathelt, Susanne Mueller and Silke Purschke, for their helpful support during this study. For the most helpful review of our manuscript, we thank Dr. Albert Shun, Head of the Liver and Renal Transplantation Unit at the Children’s Hospital, Westmead, Sydney, Australia.
Declaration of interest
This study was supported by a grant from the Swiss National Science Foundation (Swiss National Foundation Professorship), no. PP00P3-12346, to M. C-C. M. C-C. and P. S. have received speaking honoraria and research support from Thermo Scientific Biomarkers (formerly B·R·A·H·M·S AG), the copeptin assay developer and manufacturer. C. S. and S. A. U. have no disclosures.
ClincalTrials.gov number NCT02051647.
References
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. (2009). Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol 30:358–70
- de Quervain DJ, Roozendaal B, Mcgaugh JL. (1998). Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394:787–90
- Dickerson SS, Kemeny ME. (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130:355–91
- Dong XQ, Huang M, Yang SB, Yu WH, Zhang ZY. (2011). Copeptin is associated with mortality in patients with traumatic brain injury. J Trauma 71:1194–8
- Dugue B, Leppanen E, Grasbeck R. (2001). The driving license examination as a stress model: effects on blood picture, serum cortisol and the production of interleukins in man. Life Sci 68:1641–7
- Dugue B, Leppanen EA, Teppo AM, Fyhrquist F, Grasbeck R. (1993). Effects of psychological stress on plasma interleukins-1 beta and 6, C-reactive protein, tumour necrosis factor alpha, anti-diuretic hormone and serum cortisol. Scand J Clin Lab Invest 53:555–61
- Gonzalez-Cabrera J, Fernandez-Prada M, Iribar-Ibabe C, Peinado JM. (2014). Acute and chronic stress increase salivary cortisol: a study in the real-life setting of a national examination undertaken by medical graduates. Stress 17:149–56
- Katan M, Christ-Crain M. (2010). The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly 140:w13101
- Katan M, Morgenthaler N, Widmer I, Puder JJ, Konig C, Muller B, Christ-Crain M. (2008a). Copeptin, a stable peptide derived from the vasopressin precursor, correlates with the individual stress level. Neuro Endocrinol Lett 29:341–6
- Katan M, Muller B, Christ-Crain M. (2008b). Copeptin: a new and promising diagnostic and prognostic marker. Crit Care 12:117
- Katan M, Nigro N, Fluri F, Schuetz P, Morgenthaler NG, Jax F, Meckel S, et al. (2011). Stress hormones predict cerebrovascular re-events after transient ischemic attacks. Neurology 76:563–6
- Lacey K, Zaharia MD, Griffiths J, Ravindran AV, Merali Z, Anisman H. (2000). A prospective study of neuroendocrine and immune alterations associated with the stress of an oral academic examination among graduate students. Psychoneuroendocrinology 25:339–56
- Lautenbach F, Laborde S, Achtzehn S, Raab M. (2014). Preliminary evidence of salivary cortisol predicting performance in a controlled setting. Psychoneuroendocrinology 42:218–24
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. (2005). Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology 30:225–42
- Mehnert A, Lehmann C, Cao P, Koch U. (2006). [Assessment of psychosocial distress and resources in oncology – a literature review about screening measures and current developments]. Psychother Psychosom Med Psychol 56:462–79
- Morgenthaler NG, Struck J, Alonso C, Bergmann A. (2006). Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52:112–19
- Ng V, Koh D, Chia SE. (2003). Examination stress, salivary cortisol, and academic performance. Psychol Rep 93:1133–4
- Stolz D, Christ-Crain M, Morgenthaler NG, Leuppi J, Miedinger D, Bingisser R, Muller C, et al. (2007). Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest 131:1058–67
- Urwyler SA, Schuetz P, Fluri F, Morgenthaler NG, Zweifel C, Bergmann A, Bingisser R, et al. (2010). Prognostic value of copeptin: one-year outcome in patients with acute stroke. Stroke 41:1564–7

