Abstract
In healthy, non-challenged individuals, the secretion of cortisol typically follows a diurnal profile characterized by a peak in the period following waking (cortisol awakening response) and a gradual decline throughout the day. In addition, cortisol secretion is increased in response to acutely stressful stimuli, particularly stressors involving social evaluation. The current study is the first to assess the impact of an anticipated acute laboratory stressor upon the typical diurnal pattern of HPA activation and relationship to acute cortisol secretion. A sample of 23 healthy young adults provided salivary cortisol samples at four time points (immediately upon awakening, 30-min post-awakening, 1200 h and before bed) on 2 consecutive days. On the second day, participants attended the laboratory and undertook an anticipated acute socially evaluative stressor immediately following provision of their 1200 h saliva sample. Heart rate, blood pressure and mood were recorded immediately before and after the stressor and at 10 and 20 min post-stressor along with additional salivary cortisol samples. Typical patterns of cortisol secretion were observed on both days and exposure to the laboratory stressor was associated with the expected increases in cortisol, heart rate, blood pressure and negative mood. However, significant differences in diurnal cortisol secretion were observed between the two days with greater secretion, in particular, during the period following awakening, evident on the day of the anticipated laboratory stressor. Furthermore, secretion of cortisol during the period following awakening was positively related to secretion during the acute reactivity periods. This is the first study to integrate a laboratory stressor into a typical day and assess its impact on indices of diurnal cortisol secretion in an ambulatory setting. The current findings support the notion that the cortisol awakening response is associated with anticipation of the upcoming day and the subsequent demands required of the individual.
Introduction
Cortisol, secreted by the hypothalamic–pituitary–adrenal (HPA) axis follows a marked diurnal profile characterized by a rapid increase in the 30–45 min following awakening (the cortisol awakening response – CAR) and a diurnal decline to a nadir around midnight (Saxbe, Citation2008). Aspects of this profile are associated with a range of psychosocial factors. Higher levels of perceived and accumulated psychosocial stress (Abercrombie et al., Citation2004; Bauer, Citation2005; Lovell et al., Citation2011) are associated with a flattening of the diurnal decline, characterized by diurnal hypersecretion and higher levels of evening cortisol. Similarly, higher levels of evening cortisol are observed in individuals with greater trait anxiety (Van den Bergh et al., 2008) and more symptoms of depression (Van den Bergh & Van Calster, 2009). Reactivity of cortisol to acute challenges is also predictive of diurnal cortisol secretion (Kidd et al., 2014) demonstrating that the ability to mount an appropriate response to an acute challenge relates to basal functioning of the HPA axis.
The CAR is a related but distinct aspect of the diurnal cortisol profile (Wilhelm et al., Citation2007). Blunted CARs, characterized by reduced responses following awakening, have been observed in chronic fatigue syndrome (Roberts et al., Citation2004), post-traumatic stress disorder (Rohleder et al., Citation2004), burnout (de Vente et al., Citation2003), exhaustion (Mommersteeg et al., Citation2006) and depression (Stetler & Miller, Citation2005). Conversely, increased CARs predict first onset of anxiety disorders (Adam et al., Citation2014) and have been observed in individuals experiencing proximal stress such as work overload and worry (Schlotz et al., Citation2004; Schulz et al., Citation1998) and job stress (Steptoe et al., Citation2004).
The CAR is determined more by situational than trait-like factors (Hellhammer et al., Citation2007) and as such reflects proximal circumstances. It has recently been posited that the CAR is an adaptive response to maximize day-to-day functioning (Clow et al., Citation2010) and plays a crucial role in preparing for forthcoming demands (Fries et al., Citation2009). In support, increased CARs have been observed in circumstances that require increased demand. Newly qualified doctors demonstrated greater CARs at the beginning of a clinical placement, characterized by a lack of control, compared to the end of a placement (Brant et al., Citation2010). Increased CARs have also been observed on workdays, characterized by feelings of stress, compared to less stressful weekend days, in civil servants (Kunz-Ebrecht et al., Citation2004), in seafarers during onshore training (Liberzon et al., Citation2008) and in teachers following an observed demonstration lesson compared to a regular working day (Wolfram et al., Citation2013). Increased CARs have also been observed on the day of temporary stressors. Increased cortisol has been observed on mornings of dancing; motorcycling and tennis competitions compared to control days (Filaire et al., Citation2007, Citation2009; Rohleder et al., Citation2007) and levels remained elevated across the day of competition. Levels of cortisol during the CAR period have also been associated with affect. In a longitudinal study, indices of the CAR were positively associated with self-reported anticipation of the forthcoming day and state tension and stress recorded 45 min following awakening (Stalder et al., Citation2010).
To empirically assess the effects of an anticipated challenge on diurnal indices of cortisol, it is necessary to develop protocols that allow for the manipulation of forthcoming demand. The current study assessed the effects of anticipated challenge in relation to diurnal cortisol secretion and assessed the association between acute cortisol reactivity and basal functioning. It was predicted that anticipation of a challenging stressor would differentiate between the 2 days in terms of diurnal cortisol secretion and that acute cortisol reactivity would be representative of basal secretion.
Methods
Participants
Healthy young participants were recruited from an undergraduate population and interested participants were screened on the basis of the following exclusion criteria: self-reported current or previous anxiety or stress-related disorder, hypertension, pregnancy, current medication apart from over-the-counter analgesia and the contraceptive pill. A total of 27 participants were recruited; however, N = 4 failed to provide saliva samples. A final sample of 23 participants (female = 17, male = 6, Mage = 20.21 years, SD = 4.23) provided complete data and were included in analyses.
Materials and apparatus
Perceived stress was measured using the 10 item perceived stress scale (PSS; Cohen et al., Citation1983), which, using responses ranging from “never” to “very often” provides a measure of perceived stress in the preceding month. Brief psychological distress was measured using the short form profile of mood states (POMS-SF; Shacham, Citation1983), which comprises 37 items with response ranging from “not at all” to “extremely”. Scores from the POMS-SF are used to derive a total score for “mood disturbance”, as well as subscores for the domains of “tension”, “depressed”, “anger”, “vigor”, “fatigue” and “concentration”. Paper diaries were used to record information regarding the provision of saliva samples including waking time, timing of samples as well as self-reports of the prior nights’ sleep and menstrual cycle stage. Heart rate and blood pressure measurements were taken using an upper arm inflatable cuff (Omron M2, Omron Worldwide, UK)
Procedure
All procedures were approved by the institutional ethics review board. Participants attended a baseline session to provide written consent and to complete the PSS and the POMS-SF. Participants were informed that the study would involve testing over two consecutive days: day one would involve the provision of saliva samples in their own homes and day two would involve an additional testing session in the laboratory.
All participants were given training regarding the appropriate collection and storage of saliva samples including a demonstration of how to provide saliva using salivettes (Sarstedt Ltd., Nümbrecht, Germany). In addition, the importance of the timing of samples and abstinence from behaviors known to affect the concentrations of cortisol in saliva were emphasized. Specifically, participants were asked to refrain from consumption of food, caffeinated or alcoholic beverages, nicotine, brushing of teeth, the use of mouthwashes or antacids and exercise for 1 h prior to provision of each sample (Kudielka et al., Citation2003).
Details of the day two testing session were then provided; specifically participants were informed that they would be required to attend the laboratory in the afternoon to take part in a stress task that would involve the completion of challenging speech and mental arithmetic tasks whilst being socially evaluated by a panel. Participants were provided with labeled salivettes and written instructions regarding the saliva collection protocol and the testing days were agreed between the researcher and the participant.
On two consecutive typical days, participants collected saliva by chewing on the cotton roll of a salivette for 1–2 min at four time points: immediately upon awakening, 30 min post-awakening, at 1200 h and immediately before bed. On day one, all samples were provided in participants’ homes. On day two, participants provided their awakening, 30 min post-awakening and pre-bed samples at home and their 1200 h sample was provided during a testing session in the laboratory. Samples collected in homes were refrigerated by participants until they were returned to the researcher. All samples were then frozen (−20 °C) and subsequently assayed in house using the enzyme-linked immunosorbent assay method (Salimetrics-Europe, Cambridge UK, intra and inter assay coefficients <10%). To maximize adherence to the saliva collection protocol and as a means of assessing the timing of samples, participants were instructed to record the precise time at which they provided each of their saliva samples using a paper diary (Lovell et al., 2011).
On the test day, participants attended the laboratory prior to the provision of their 1200 h saliva sample, completed the PSS and were reminded that they were to take part in a stress task involving challenging tasks whilst being socially evaluated. A stress protocol based on the Trier Social Stress Test (Kirschbaum et al., Citation1993) was then administered. The chair of a three person panel instructed participants that they would have 10 min to prepare for a mock job interview. Following a 10-min preparation period, participants presented to the panel and were asked to explain why they were the best candidate for the chosen job for a period of 5 min; if speech faltered, they were prompted by the chair to continue. No other verbal interaction occurred. At the end of a 5-min period, the participant was stopped and informed that they would be assessed for their mental arithmetic abilities. Participants were instructed to subtract aloud from 1017 in multiples of 13 for 5 min; if an incorrect response was given the Chair informed them that their response was incorrect and they must begin the task again. The POMS-SF was completed and heart rate and blood pressure recorded immediately before and after the stressor and 10 and 20 min following stressor cessation. Saliva samples were obtained immediately before (12:00), and 10 and 20 min following the stressor. Following provision of the final samples, participants were debriefed and remunerated £10.
Data analysis
The efficacy of the stressor was assessed using a series of one-way ANOVAs with four sampling points (pre-stress, immediately post-stress, +10 min, +20 min) for POMS-SF items, heart rate, systolic blood pressure and diastolic blood pressure, and three sampling points (pre-stress, +10 min and +20 min) for salivary cortisol. Post-hoc analyses were conducted to assess post-stress changes (immediately post-stress, +10 min and +20 min) from pre-stress and those comparisons that remained significant following Bonferroni corrections for multiple comparisons are reported. Differences in perceived stress and POMS-SF items between the baseline and test day were assessed using paired samples t-tests.
The diurnal secretion of cortisol was assessed using two-way repeated measures ANOVAs with day (day 1, test day) and time (awakening, +30, noon, bed) and individual time points were compared across days using paired samples t-tests. Total cortisol secretion was assessed by area under the curve with respect to ground (AUCG). AUCG was calculated for each participant on each day using the cortisol level (nmol/l) at each sampling point and the time (min) between each sample (Pruessner et al., 2003) for diurnal secretion (awakening, awakening +30, noon and bed) and for total cortisol secretion during the CAR period (awakening and awakening +30). In addition, area under the curve with respect to increase (AUCI) from waking (Pruessner et al., 2003) and mean increase (awakening +30 values minus values at awakening) were also calculated during the CAR period.
Diurnal/CAR AUC was not calculated for participants who did not provide sufficient information regarding the timing of their saliva samples (n = 2). AUCG was also calculated on the test day (pre stress, 10 and 20 min post-stress) to assess cortisol secretion in response to acute stress. Differences between day 1 and test day were compared using paired samples t-tests and relationships between diurnal/CAR indices and acute cortisol reactivity were assessed using Pearson correlations.
results
Given the unequal number of males (n = 6) and females (n = 15) in the final sample, potential sex differences in cortisol indices were assessed. Males demonstrated significantly greater levels of cortisol at 1200 on Day 1 [t(5.52) = 2.79, p = 0.034] but no other significant differences were observed.
Stressor manipulation
Psychological, cardiovascular and cortisol measures of acute reactivity and recovery are presented in .
Table 1. Mean (SE) psychological, cardiovascular and cortisol measures of reactivity and recovery.
Self-report stress and mood
There was a significant effect on feelings of tension [F(3,20) = 6.4, p = 0.003, η2 = 0.49]; anger [F(3,20) = 4.66, p = 0.013, η2 = 0.41]; concentration [F(3,20) = 4.01, p = 0.022, η2 = 0.38] and total mood disturbance [F(3,20) = 3.44, p = 0.037, η2 = 0.34]; however, post-stress values were not significantly different from pre-stress values following correction for multiple comparisons. The stressor led to significant reductions in feelings of vigor [F(3,20) = 3.97, p = 0.023, η2 = 0.37]; levels immediately (p = 0.018); 10 min (p = 0.009) and 20 min (p = 0.006) post-stress were significantly lower than pre-stress. In contrast, levels of fatigue reduced [F(3,20) = 6.46, p = 0.003, η2 = 0.49] with significant reductions from pre-stress to 10 min (p = 0.018) and 20 min (p = 0.003) post-stress. There were no significant effects on feelings of depression [F(3,20) = 0.75, p = 0.54, η2 = 0.10].
Cortisol reactivity
The stressor paradigm induced changes in cortisol which approached significance [F(2, 21) = 3.44, p = 0.051, η2 = 0.25]. Bonferroni adjusted post-hoc comparisons revealed that following initial increases from pre to post-stress, cortisol significantly reduced from 10 to 20 min post-stress (p = 0.04).
Cardiovascular reactivity
The stressor had a significant effect on levels of SBP [F(3, 20) = 28.80, p < 0.001, η2 = 0.81]; levels immediately post-stress were greater than pre-stress (p < 0.001). The stressor exerted a similar effect on DBP [F(3, 20) = 11.57, p < 0.001, η2 = 0.63], with significantly greater DBP immediately post-stress, (p < 0.001); 10 min (p < 0.001) and 20 min (p = 0.009) post-stress relative to pre-stress. No significant changes were observed in HR.
Basal stress
Measures of psychological distress (baseline and test day) and cortisol indices (day 1 and test day) are presented in .
Table 2. Mean (SE) psychological and cortisol measures at baseline, day 1 and test day.
Self-report stress and mood
Levels of perceived stress [t(22) = 3.46, p = 0.002], tension [t(22) = 2.34, p = 0.025] and concentration [t(22) = 2.78, p = 0.01] were significantly greater on the test day relative to baseline. There was also a trend towards greater levels of anxiety on the stress day compared to the baseline [t(22) = 1.81, p = 0.08]. There were no significant differences between the baseline and stress days for depression, anger, vigor, fatigue or total mood disturbance.
Cortisol indices
There were no significant differences in self-reported time of awakening between day one and test day (p = 0.35). There was a significant main effect of time on diurnal cortisol [F(3, 20) = 37.22, p < 0.001, η2 = 0.86]; t-tests revealed significant differences between all time-points (p < 0.001; p = 0.039) representing the typical diurnal profile of cortisol characterized by a peak from awakening to 30 min post-awakening and a subsequent decline from the + 30 min sample to the afternoon and pre-bed samples. There was also a main effect of day [F(1, 20) = 14.03, p = 0.001, η2 = 0.41] representing significantly greater levels of cortisol secretion on the test day relative to day one. This was supported by greater secretion of cortisol as indexed by AUCG on the test day compared to day 1 [t(20) − 2.39; p = 0.027). Furthermore, CAR indices of CAR AUCG [t(20) −3.26 p = 0.004), CAR mean output [t(20) −3.26, p = 0.004) and peak levels [t(20) −2.99, p = 0.007) were greater on the test day compared with day 1 as were the mean increase during the CAR period [t(20) −1.77, p = 0.09) and the CAR AUCI [t(20) −1.77, p = 0.09) although not significantly so. Diurnal cortisol profiles on day one and the test day are presented in .
Figure 1. Diurnal cortisol profiles on day 1 and test day (SE). **p < 0.01 (CAR peak, CAR AUCG); *p < 0.05 (Diurnal AUCG).
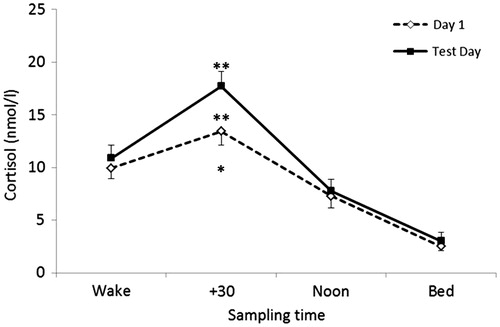
Significant relationships were observed between indices of basal function and acute cortisol reactivity. Greater secretion of cortisol across the diurnal period was related to greater pre-stress levels of cortisol (r = 0.91) and higher individual peak response (r = 0.59) and greater secretion of cortisol (r = 0.72) across the stress period. Levels of cortisol upon awakening were higher in those that demonstrated the greatest levels of cortisol immediately pre-stress (r = 0.57) and the greatest secretion of cortisol during the acute stress period (r = 0.44). Finally, greater secretion of cortisol during the CAR period was related to greater pre-stress levels (r = 0.59), greater individual peak response (r = 0.57) and greater cortisol secretion (r = 0.61) during the stressor period. Correlation coefficients are reported in .
Table 3. Correlation coefficients for basal and acute reactivity indices.
discussion
Using a two-day testing protocol this is the first study to assess the effects of an anticipated laboratory stressor on indices of diurnal cortisol secretion. Typical patterns of cortisol secretion, characterized by an increase in the 30 min following awakening and a diurnal decline towards a nadir before bedtime, were observed on both days; however, the second day was characterized by greater diurnal secretion of cortisol, in particular during the CAR period. The two sampling days were the same with the exception of participation in a laboratory stressor on the test day and as such, the greater secretion of cortisol can be attributed to this atypical but anticipated challenging event. Specifically, the greater secretion of cortisol on the test day is in the main, driven by greater secretion of cortisol during the CAR period. This supports previous observations of an anticipatory effect such that cortisol levels increase when faced with novel challenging procedures (Lovallo et al., 2010) and identifies the influence of state factors on the CAR (Hellhammer et al., Citation2007).
The current design has enabled us to directly compare the CAR on a typical day (on which trait factors alone impacted upon the CAR) with a day in which both trait and state factors influenced the CAR. Given that the participants in our study had been explicitly told that the test day would involve exposure to a laboratory stressor involving social evaluation and cognitive challenge, we suggest that our data reflect diurnal cortisol variation in response to anticipation of forthcoming demands. Specifically, the greater levels following awakening observed on the test day reflect the proposed adaptive nature of the CAR (Clow et al., Citation2010) and suggest that the CAR may play a role in preparation for forthcoming daily challenges. Further research investigating the influence of stress anticipation on the CAR, and the role of the CAR in preparing the individual for forthcoming demands is therefore warranted.
While this is the first study to report that the CAR is modulated by anticipation of a manipulated laboratory stressor, similar increases have been associated with periods of increased demand (Brant et al., Citation2010; Kunz-Ebrecht et al., Citation2004; Liberzon et al., Citation2008) and single anticipated challenges (Filaire et al., Citation2007, Citation2009; Rohleder et al., Citation2007). Furthermore, higher levels of cortisol in the CAR period have been associated with increased reports of state tension, stress and anticipation of forthcoming tension (Stalder et al., Citation2010). In support, self-reported levels of perceived stress, tension and anxiety in the current study were also greater on the morning of the anticipated stressor. These findings are concomitant with the notion that the CAR serves to maximize day to day functioning (Clow et al., Citation2010) and plays a role in the preparation of forthcoming challenges (Fries et al., Citation2009). The current study adds further support to this notion by replicating the observations from naturalistic studies through the explicit manipulation of forthcoming demand.
The current study design also enabled the combination of a controlled acute event (the laboratory stressor) with otherwise typical activity in an ambulatory setting over a two-day period. This design provided the opportunity to assess basal functioning, as well as reactivity to and recovery from an acutely challenging event. During recruitment participants were instructed to select two consecutive, typical days for participation. As such, the diurnal profile obtained on day 1 provided an indication of each individual’s typical CAR and diurnal cortisol profile when acutely stressful events were not anticipated or experienced, whereas the cortisol samples collected on the test day enabled the influence of acute psychosocial stress exposure on the CAR, diurnal profile and cortisol reactivity to all be investigated. Comparing observations on the day of an acute stressor with a resting control day is a recommended approach to the assessment of individual differences in cortisol responding (Lovallo et al., 2010) and serves as a more appropriate reference point when assessing the effects of acute stress on cortisol responses (Wolfram et al., Citation2013). Furthermore, given the state-like influences on diurnal secretion, particularly during the CAR period, two-day protocols allow for the assessment of acute stress on the underlying diurnal cycle. In this instance, we have been able to observe the effects of anticipating an acutely stressful event on basal functioning of the HPA axis in a more controlled manner than afforded by an observation of a pre-existing event and with more flexibility and ecological validity than afforded by an entirely laboratory based protocol.
The current protocol also allowed for the investigation of potential relationships between acute cortisol reactivity and indices of basal HPA function. The association between acute and diurnal secretion supports recent observations from a large cross-sectional sample (Kidd et al., 2014) and reinforces the use of laboratory stressor techniques as valid analogues of everyday function. In addition, the current study observed the previously unreported association between cortisol secretion during the acute stressor and cortisol awakening periods suggesting that the CAR may play a priming role and influence subsequent function across the day (Clow et al., Citation2014).
The current study should however be considered in light of its limitations. First, the sample size is small; however, it was sufficient to detect meaningful differences in the predicted indices of diurnal secretion. Second, the reliability of diurnal cortisol measurement is reliant on good adherence to the sampling protocol, including the accuracy of the timing of samples. In line with recommendations (Adam & Kumari, Citation2009; Okun et al., Citation2010; Saxbe, Citation2008) steps were therefore taken to maximize protocol adherence. Participants were given verbal and written instructions and the importance of sample timing was emphasized. Individuals are generally accurate in the reporting of their own wake up times (DeSantis et al., 2012; Kraemer et al., Citation2006) and it is often easier for participants to integrate saliva sampling into more standardized morning routines (Golden et al., 2014). As such, participants were requested to accurately record the times at which they provided their samples in relation to waking and two participants were subsequently excluded on the basis of timing discrepancies and suspected non-adherence. The remaining participants reported good adherence to the sampling protocol. Although evidence suggests that the use of self-reported sample timings are effective in ensuring protocol adherence, and moreover are preferred by participants (Kraemer et al., Citation2006), other techniques, for example, Medical Event Monitoring (MEMS) caps (Smyth et al., 2013) and actigraphy (Clow et al., Citation2014) would provide additional markers of adherence with regards to sampling accuracy and sleep and awakening times, respectively.
Third, the current study used only two samples to index the CAR. Although the protocol was devised to reduce participant burden and ease adherence to protocol, a greater number of samples during the post-awakening period (e.g. 15, 45 and 60 min post-awakening) would provide more robust indices of the CAR (Stalder et al., Citation2009). These initial findings therefore warrant a more thorough assessment of the CAR in relation to manipulated forthcoming demand; however, protocols with increased sampling should be mindful of not over-burdening participants to minimize impact on recruitment or retention (Wetherell & Montgomery, 2014).
Finally, although ambulatory studies provide the opportunity for real life assessment, they lack the level of control afforded in laboratory studies. Participants were instructed to identify two typical consecutive days for study protocol and were notified of the second day stressor manipulation from the commencement of participation. Participants did not report any atypical events; however, in the absence of objective observation of participants across the study period, changes in the CAR cannot be solely attributed to the anticipation of the laboratory stressor. This naturalistic sampling also prevents the counterbalancing of conditions that would typically be employed in an experimental manipulation. That is, random allocation of participants to experience the stressor on either day 1 or day 2 would avoid potential systematic differences that may occur. Although we cannot rule out any such effect, there were no differences in sampling times or non-stressor procedures between the 2 days. Furthermore, the current design offers a good representation of how people typically function in relation to forthcoming events in the everyday life.
Conclusions
This study has observed for the first time, differences in cortisol secretion across the diurnal period in relation to a manipulated stressful event. Moreover, this difference was in the main, driven by changes during the period immediately following awakening. That increased levels of cortisol were evident during the CAR period on the day of a forthcoming challenging laboratory stressor provides empirical evidence for the notion proposed by Clow et al. (Citation2010) that the CAR is an adaptive mechanism that aids maximal day to day functioning. That is, an increased CAR serves as a preparatory mechanism that provides an individual with sufficient resources to cope with an anticipated demanding event, in this case an anticipated cognitively challenging stressor. Furthermore, secretion during the CAR period was concomitant with secretion during the acute stress phase, demonstrating that the CAR may influence subsequent functioning across the day (Clow et al., Citation2014).
Acknowledgements
The authors would like to thank Anthea Wilde for conducting cortisol assays.
Declaration of interest
The authors report no conflict of interest
References
- Abercrombie HC, Giese-Davis J, Sephton S, Epel S, Turner-Cobb JM, Spiegel D. (2004). Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology 29:1082–92
- Adam EK, Kumari M. (2009). Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34:1423–36
- Adam EK, Vrshek-Schallhorn S, Kendall AD, Minkea S, Zinbarg RE, Craske MG. (2014). Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up. Psychoneuroendocrinology 44:47–59
- Bauer ME. (2005). Stress, glucocorticoids and ageing of the immune system. Stress 8:69–83
- Brant H, Wetherell MA, Lightman SL, Crown AL, Vedhara K. (2010). An exploration into physiological and self-report measures of stress in pre-registration doctors at the beginning and end of a clinical rotation. Stress 13:155–62
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. (2010). The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev 35:97–103
- Clow A, Law R, Evans P, Vallence A-M, Hodyl NA, Goldsworthy MR, Rothwell JR, Ridding MC. (2014). Day differences in the cortisol awakening response predict day difference in synaptic plasticity in the brain. Stress 17(3):219–23
- Cohen S, Kamarck T, Mermelstein R. (1983). A global measure of perceived stress. J Health Soc Behav 24:385–96
- DeSantis AS, DiezRoux AV, Hajat A, Aiello AE, Golden SH, Jenny NS, Seeman TE, Shea S. (2012). Associations of salivary cortisol levels with inflammatory markers: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology 37:1009–18
- de Vente W, van Amsterdam JGC, Kamphuis JH, Emmelkamp PMG. (2003). Physiological differences between burnout patients and healthy controls: blood pressure, heart rate, and cortisol responses. Occup Environ Med 60:i54–61
- Filaire E, Alix D, Ferrand C, Verger M. (2009). Psychophysiological stress in tennis players during the first single match of a tournament. Psychoneuroendocrinology 34:150–7
- Filaire E, Filaire M, Le Scanff C. (2007). Salivary cortisol, heart rate and blood lactate during a qualifying trial and an official race in motorcycling competition. J Sports Med Phys Fitness 7:413–17
- Fries E, Dettenborn L, Kirschbaum C. (2009). The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 72:67–73
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. (2007). Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state and trait components. Psychoneuroendocrinology 32:80–6
- Kidd T, Caralho LA, Steptoe A. (2014). The relationship between cortisol responses to laboratory stress and cortisol profiles in daily life. Biol Psychol 99:34–40
- Kirschbaum C, Pirke KM, Hellhammer D. (1993). The trier social stress test – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28:76–81
- Kraemer HC, Giese-Davis J, Yutsis MBA, O'Hara R, Neri EBS, Gallagher-Thompson D, Taylor CB, Spiegel D. (2006). Design decisions in order to optimise reliability of daytime diurnal slopes in an older population. Am J Geriatr Psychiatry 14:325–33
- Kudielka BM, Broderick JE, Kirschbaum C. (2003). Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol profiles in noncompliant subjects. Psychosom Med 65:313–19
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. (2004). Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology 29:516–28
- Liberzon J, Abelson JL, King A, Liberzon I. (2008). Naturalistic stress and cortisol response to awakening: adaptation to seafaring. Psychoneuroendocrinology 33:1023–6
- Lovallo WR, Farag NH, Vincent AS. (2010). Use of a resting control day in measuring the cortisol response to mental stress: Diurnal patterns, time of day, and gender effects. Psychoneuroedocrinology 35:1253–8
- Lovell B, Moss M, Wetherell MA. (2011). Perceived stress, common health complaints and diurnal patterns of cortisol secretion in young, otherwise healthy individuals. Horm Behav 60(3):301–5
- Mommersteeg PMC, Heijnen CJ, Verbraak MJPM, van Doornen LJP. (2006). Clinical burnout is not reflected in the cortisol awakening response, the day curve or the response to a low-dose dexamethasone suppression test. Psychoneuroendocrinology 31:216–25
- Okun ML, Krafty RT, Buysse DJ, Monk TJ, Reynolds CF, Begley A, Hall M. (2010). What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self report and PSG-assessed wake time. Psychoneuroendocrinology 35:460–8
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Roberts ADL, Wessely S, Chalder T, Papadopoulos A, Cleare AJ. (2004). Salivary cortisol response to awakening in chronic fatigue syndrome. Br J Psychiatry 184:136–41
- Rohleder N, Beulen ES, Chen E, Wolf JM, Kirschbaum C. (2007). Stress on the dance floor: the cortisol stress response to social-evaluative threat in competitive ballroom dancers. Pers Soc Psychol Bull 33:69–84
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. (2004). Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry 55:745–51
- Saxbe DE. (2008). A field (researcher's) guide to cortisol: tracking HPA axis functioning in common life. Health Psychol Rev 2:163–90
- Schlotz W, Hellhamme, J, Schulz P, Stone AA. (2004). Perceived work overload and chronic worrying predict weekend–weekday differences in the cortisol awakening response. Psychosom Med 66:207–14
- Schulz P, Kirschbaum C, Pruessner J, Hellhammer D. (1998). Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med 14:91–7
- Shacham S. (1983). A shortened version of the profile of mood states. J Person Assess 47:305–6
- Smyth N, Clow A, Thorn L, Hucklebridge F, Evans P. (2013). Delays of 5–15 min between awakening and the start of saliva sampling matter in assessment of the cortisol awakening response. Psychonueroendocrinology 38:1476–83
- Stalder T, Evans P, Hucklebridge F, Clow A. (2010). State associations with the cortisol awakening response in health females. Psychoneuroendocrinology 35:1245–52
- Stalder T, Hucklebridge F, Evans P, Clow A. (2009). Use of a single case study design to examine state variation in the cortisol awakening response: relationship with time of awakening. Psychoneuroendocrinology 34:607–14
- Steptoe A, Siegrist J, Kirschbaum C, Marmot M. (2004). Effort-reward imbalance, overcommitment, and measures of cortisol and blood pressure over the working day. Psychosom Med 66:323–9
- Stetler C, Miller G. (2011). Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73(2):114–26
- Stetler C, Miller GE. (2005). Blunted cortisol responses to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. J Abnormal Psychol 114(4):697–705
- Van den Bergh BRH, Van Calster B, Pinna Puissant S, Van Huffel S. (2008). Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: associations with diurnal cortisol profiles. Horm Behav 54:253–7
- Van den Bergh BRH, Van Calster B. (2009). Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children's Depression Inventory. Psychoneuroencocrinology 34:791–4
- Wetherell MA, Montgomery C. (2014). Basal functioning of the hypothalamic-pituitary-adrenal (HPA) axis and psychological distress in recreational ecstasy polydrug users. Psychopharmacology 231:1365–75
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. (2007). Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology 32:358–66
- Wolfram M, Belingrath S, Feurehahn N, Kudielka BM. (2013). Cortisol responses to naturalistic and laboratory stress in student teachers: comparison with a non-stress control day. Stress Health 29:143–9

