Abstract
Chronic stress is considered to be a major risk factor in the development of psychopathological syndromes in humans. Cognitive impairments and long-term potentiation (LTP) impairments are increasingly recognized as major components of depression, anxiety disorders and other stress-related chronic psychological illnesses. It seems timely to systematically study the potentially underlying neurobiological mechanisms of altered cognitive and synaptic plasticity in the course of chronic stress. In the present study, a rat model of chronic unpredictable stress (CUS) induced a cognitive impairment in spatial memory in the Morris water maze (MWM) test and a hippocampal LTP impairment. CUS also induced hippocampal microglial activation and attenuated phosphorylation of glutamate receptor 1 (GluR1 or GluA1). Moreover, chronic treatment with the selective microglial activation blocker, minocycline (120 mg/kg per day), beginning 3 d before CUS treatment and continuing through the behavioral testing period, prevented the CUS-induced impairments of spatial memory and LTP induction. Additional studies showed that minocycline-induced inhibition of microglia activation was associated with increased phosphorylation of GluR1. These results suggest that hippocampal microglial activation modulates the level of GluR1 phosphorylation and might play a causal role in CUS-induced cognitive and LTP disturbances.
Introduction
Chronic stress is considered a key risk factor for the development of a variety of human ailments (Ehlert et al., Citation2001; Lopez et al., Citation1999). Lack of adaptation to stress can lead to the development of pathological syndromes, such as depression, anxiety and cognition impairment (Shah et al., Citation1998; Sheline et al., Citation1996). Stress affects cognition in a number of ways. For example, stress induces changes in glutamate neurotransmission in the hippocampus, a region critical for the formation of spatial memories (Eichenbaum, Citation2000; Nadel & MacDonald, Citation1980), thereby influencing some aspects of cognitive processing. Recent studies have shed light on the mechanisms by which stress affects hippocampal neuronal glutamate transmission, including effects on glutamate release, glutamate clearance and metabolism and glutamate receptors (McEwen & Sapolsky, Citation1995).
The glutamate receptor 1 (GluR1) is a subtype of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor. Long-term potentiation (LTP) in the hippocampus, an experimental model of synaptic plasticity, requires the GluR1 subunit. It is widely believed that synaptic plasticity provides the neural mechanism that underlies learning and memory in the mammalian brain (Sanderson et al., Citation2008).
Recent data suggest that stress induces inflammatory responses in various brain regions (Barnum et al., Citation2008; Blandino et al., Citation2006, Citation2009; Carty et al., Citation2008; Goshen et al., Citation2008; Goshen & Yirmiya, Citation2009; Grippo et al., Citation2005; O'Connor et al., Citation2003), including hippocampus (Diz-Chaves et al., Citation2012). Proinflammatory cytokines administered to experimental animals induce cognitive deficits (Dantzer et al., Citation2008; Dunn & Swiergiel, Citation2005; Godbout et al., Citation2008; Miller et al., Citation2009). Research on acute inflammation induced by lipopolysaccharide (LPS; 10 mg) revealed that GluR1 expression was prominent after LPS injection, and then decreased gradually 1 d after LPS injection. However, chronic stress effects on GluR1 expression have not been reported.
Microglial cells are important resident immunoreactive cells in the central nervous system (CNS) (van Rossum & Hanisch, Citation2004; Vilhardt, Citation2005) and are producers of proinflammatory factors. Published evidence suggests that minor changes in the microenvironment can cause the activation of microglia with subsequent release of a host of endogenous factors, such as nitric oxide (NO), tumor necrosis factor-alpha (TNF-α) and interleukin 1β (IL-1β).
Recent reports have also shown that stress plays important roles in microglial activation. For instance, repeated restraint stresses induce proliferation of microglial cells in the mouse brain (Nair & Bonneau, Citation2006). Furthermore, repeated immobilization stresses induce morphological microglial activation in the hippocampus and striatum in mice (Kwon et al., Citation2008). These observations clearly indicate that exposure of animals to certain types of stress could lead to microglial activation in the brain (Sugama, Citation2009). Studies also show that chronic unpredictable stress (CUS) may induce microglial activation (Bian et al., Citation2012), and the latter has been associated with learning and memory impairment, regulated by release of microglial-borne interleukin (IL)-1 (Tanaka et al., Citation2011). Inhibition of microglial activation can effectively rescue the learning and memory deficits in a murine model of human immunodeficiency virus (HIV) type-1 encephalitis (Keblesh et al., Citation2009).
Given the above, we posited that microglial activation may play an important role in stress-induced cognition deficits. Chronic stress may cause microglial activation followed by microglial-induced changes in glutamate receptor activity, eventually leading to LTP impairments and cognition dysfunction. To test this hypothesis and clarify its underlying mechanisms, we investigated the effect of CUS on microglial activation and cytokine release, hippocampal LTP levels, as well as hippocampal GluR1 phosphorylation in adult Sprague–Dawley rats (SD-rats). The role of microglial activation in CUS-induced cognitive deficits and LTP impairment was also confirmed by inhibiting microglial activation with minocycline, a microglial-specific inhibitor (Arvin et al., Citation2002; Tomas-Camardiel et al., Citation2004; Yrjanheikki et al., Citation1999).
Experimental procedures
Materials
Anti-GluR1 (Wang et al., Citation2012), anti-phospho (P)-GluR1 (Ser845) (Kelly et al., Citation2014) and anti-P-GluR1 (Ser831) (Barria et al., Citation1997) were purchased from Millipore (Billerica, MA). Polyclonal mouse anti-OX42 (CD11b) antibody (a microglial marker) (Grossmann et al., Citation2002) was purchased from Chemicon (Hampshire, UK). Fluorescent secondary antibodies were purchased from Vector Laboratories (Burlingame, CA). TNF-α and IL-1β enzyme-linked immunosorbent (ELISA) kits were purchased from eBioscience (San Diego, CA). Minocycline was bought from Sigma (Sigma-Aldrich, St Louis, MO). All other reagents were purchased from Sigma (Sigma-Aldrich, St Louis, MO). The inverted microscope and the fluorescence microscope were bought from Olympus (Tokyo, Japan). The Patch clamp system was bought from Axon (Axon Instruments, Union City, CA). The enzyme-linked immunosorbent spectrophotometer was obtained from Shimadzu (Nakagyo-ku, Kyoto, Japan).
Experimental animals
Male SD rats (250–300 g) were obtained from the Animal Experimental Center of the Fourth Military Medical University. Animal husbandry and experimental protocols were carried out in strict accordance with the international standards for animal care guidelines (Guide for the Care and Use of Laboratory Animals) and were approved by the institutional animal care and use committee of the Fourth Military Medical University (Permit Number: 12001). Efforts were made to minimize animal pain, suffering or discomfort, and to minimize the number of rats used.
Chronic unpredictable stress procedure
Rats were randomly divided into four groups: a control group; a stress group; a control plus minocycline group; and a stress plus minocycline group. Rats assigned to the CUS group were subjected to the following stressors (one stressor a day), and reflect modifications of published procedures (Bondi et al., Citation2008; Gouirand & Matuszewich, Citation2005; Ossowska et al., Citation2004): food and water deprivation overnight (12 h); 24 h reversal of the day–night cycle; 12 h cage tilt 45 degrees (overnight); individual housing (24 h); 2 h restraint (6 × 21.6 cm Plexiglas commercial restrainer; Harvard Apparatus, Inc., Holliston, MA); 2 h 4 °C cold exposure; and 15 min forced swimming (placing the rat in a cylindrical tank, 60 cm height × 30 cm diameter). Stressors were applied randomly and each stressor was repeated 3 times during the 3-week stress procedure. Control group and stress group rats received 5% sucrose solution by oral gavage. Control plus minocycline group and stress plus minocycline group rats treated with minocycline (Zhao et al., Citation2009) (120 mg/kg per day in 5% sucrose; Sigma-Aldrich, St Louis, MO) by oral gavage.
Morris water maze test
Morris water maze (MWM) studies were performed the day after the 3-week stress period to assess spatial learning. The MWM consisted of a circular water tank (120 cm diameter, 50 cm height) that was partially filled with water (25 °C, dyed white with edible pigment). The pool was divided into four equal quadrants labeled 1-2-3-4 in the middle position of each pool wall. A white escape platform (10 cm in diameter) was hidden 2 cm below the surface of the water in a fixed location in one of the four quadrants (defined as the target quadrant) of the pool. The platform remained in the same place during the entire experiment. The maze was surrounded with a curtain and located in a quiet test room, surrounded by fixed visual cues (e.g. the label on each quadrant’s pool wall, rack, etc), which were visible from within the pool and could be used by the rats for spatial orientation. The movement of the animals was recorded by a TV camera located over the center of the pool and was connected to a personal computer. Before the training started, rats were allowed to swim freely in the pool for 60 s without the platform and were then put on the platform for 30 s in order to let them be familiar with the experimental condition. Rats were given four trials (once from each starting position, Figure S2) per session for 7 d, with each trial having a ceiling of 120 s and a trial interval of approximately 30 s. After climbing onto the platform, the animal remained there for 30 s before the commencement of the next trial. The recording was automatically terminated as escape latency when the animal found the target. The time required to reach the platform is defined as the escape latency. When a tested rat could not escape to the platform within 120 s, it was placed on the platform and allowed to remain there for the same amount of time and their escape latency was recorded as 120 s. The mean latency of finding the invisible platform was measured for individual animals on each day. The day after the acquisition phase, a probe test was conducted by removing the platform. Rats were allowed to swim freely in the pool for 60 s. The time spent in the target quadrant, which had previously contained the hidden platform, was recorded. The result was presented as the percent of time spent in the target quadrant.
Long-term potentiation recording in hippocampal slices
Slice preparation
Coronal brain slices (300 μm) from SD rat containing the hippocampus were prepared according to standard methods (Wei et al., Citation2001). Slices were transferred to a submerged recovery chamber containing oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) (124 mM NaCl, 4.4 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 25 mM NaHCO3, 1 mM NaH2PO4, and 10 mM glucose) at room temperature for at least 1 h.
Whole-cell recordings
Experiments were performed in a recording chamber on the stage of an Axioskop 2 FS microscope with infrared differential interference contrast (DIC) optics for visualizing whole-cell patch-clamp recordings. Excitatory postsynaptic currents (EPSCs) were recorded from pyramidal neurons in the CA1 region with an Axon 200B amplifier (Axon Instruments, Union City, CA), and stimulations were delivered with a bipolar tungsten-stimulating electrode, which was placed on Schaffer collateral-commissural fibers in the CA3 stratum radiatum. AMPA receptor-mediated EPSCs were induced by repetitive stimulations at 0.02 Hz and neurons were voltage clamped at −70 mV. After obtaining stable EPSCs for at least 10 min, LTP was induced by 80 pulses at 2 Hz paired with postsynaptic depolarization at +30 mV (referred to as pairing training). The recording pipettes (3–5 MΩ) were filled with solution containing (mM) 145 K-gluconate, 5 NaCl, 1 MgCl2, 0.2 EGTA, 10 HEPES, 2 Mg-ATP, and 0.1 Na3-GTP (adjusted to pH 7.2 with KOH). Picrotoxin (100 µM) was always present to block gamma-aminobutyric acid (GABA)-A receptor-mediated inhibitory synaptic currents. Access resistance was 15–30 MΩ and monitored throughout the experiment. Data were discarded if access resistance changed more than 15% during an experiment. Results are expressed as means ± SEM.
Immunocytochemistry
Hippocampal microglial cells were detected with anti-OX42 antibody. Briefly, slices form cryopreserved tissues were followed by blocking for 1 h with phosphate buffered saline (PBS) containing 0.4% Triton X-100, 2% bovine serum albumin (BSA) and 3% normal goat serum. After blocking, slices were incubated with primary antibody overnight at 4 °C. Slices were then washed with PBS and incubated for 1 h with the secondary antibodies (anti-mouse-Rhodamine, Jackson ImmunoResearch, West Grove, PA) at room temperature and next rinsed with PBS buffer. Cells were examined and recorded blindly under an Olympus BX51 fluorescent microscope equipped with DP-BSW software (Olympus, Tokyo, Japan). The number of activated microglia was determined by counting the number of OX42-immunoreactive cells in DG zone of hippocampus (Burguillos et al., Citation2011). Briefly, each image results from one group were divided equally into 16 lattices, and then only calculate the activated cell number (based on morphological characteristics of the cell) (Figure S3). The total number microglial cells were detected and calculated by Image pro plus (IPP) software (only OX42 and Hoechst double-labeling cells were calculated).
Quantitation of secreted TNF-α and IL-1β
Rat brain hippocampal homogenizations
The hippocampus from each hemisphere was separately dissected out and homogenized using a Dounce homogenizer in ice-cold lysis buffer containing HEPES 25 mM, pH 7.4, 3-[(3-cholamidopropyl) dimethyl-ammonio]1-propanesulfonate 0.1%, MgCl2 5 mM, EDTA 1.3 mM, EGTA 1 mM, 10 μg/ml pepstatin, aprotinin, and leupeptin, and 1 mM PMSF. The homogenates were centrifuged (15 min at 50,000 rpm) and stored at −80 °C.
TNF-α and IL-1β detection
TNF-α and IL-1β in the hippocampus were detected by ELISA. Briefly, the 96-well culture plates were coated with coating buffer at 4 °C overnight, the plates were rinsed five times with a wash buffer, and the plates were blocked with 1 × assay diluent and incubated for 1 h at room temperature. The standard preparation was diluted with Assay Diluent and 100 μl of the standard preparation was added to the wells, at the same time, 100 μl of the sample was added to the wells, and the plate was covered and incubated for 2 h at room temperature. Next, the plate was rinsed and 100 μl of the detecting antibody was added to the wells and incubated for 1 h at room temperature. A total of 100 μl of Avidin-HRP was added to each well and incubated for 30 min at room temperature, the plate was rinsed seven times, 100 μl of base solution was added to each well and incubated for 15 min at room temperature. Next, 50 μl of stop solution was added to each well and the plate was read at 450 nm. The cytokine content was expressed at picogram cytokine per gram tissues.
Western blot analysis
Protein samples from the hippocampus of the different groups were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Non-specific binding sites were blocked by immersing the membranes in 5% BSA in PBS at room temperature, followed by incubation with primary antibodies. Subsequently, the membranes were incubated with appropriate secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). All secondary antibodies were horseradish peroxidase conjugated. ECL Western Blotting Substrate (Pierce Biotechnology, Rockford, IL) was used to detect the immunoreactive signals with an ECL-based Fluorchem® FC2 image system (Alpha Innotech, San Leandro, CA). Primary antibodies included GluR1 antibody (Millipore, USA), P-GluR1 (Ser845) and P-GluR1 (Ser831) antibody (Millipore, Billerica, MA). All the Western blot analyses were performed in triplicate. FluorChem FC2 software was used to analyze the gray value of the protein expression in each group.
Statistical analysis
The effects of CUS and minocycline on the MWM performance were assessed using a repeated measures two-way analysis of variance (ANOVA) with trials as the repeated measure (within subject factor) and CUS and minocycline as independent factors (between subject factors). When interactions between the independent factors or between an independent factor and a repeated factor were detected, Bonferroni post-hoc tests were performed in order to identify specific differences. All electrophysiological data were analyzed by one-way ANOVA followed by LSD t-test comparing the baseline responses to the stimulation to analyzing the within group effects. Effects of group on magnitude of LTP were assessed by ANOVA, followed by LSD t-test to identify specific group differences. For cell activation assessment, statistical significance (p < 0.05) was determined using one-way ANOVA. For Western blots, each experiment was repeated three times, and the value of three samples averaged was compared among groups.
Results
Effect of CUS on spatial memory and hipopocampal long-term potentiation in rats
We investigated the effects of CUS on spatial learning and memory in rats using the MWM (Con: n = 9, CUS: n = 9). Repeated measures ANOVA demonstrated an interaction between time and group (F(6, 96) = 20.058, p < 0.01), in which the CUS group’s mean latency was significantly prolonged. Isolated effects of time (F(6, 96) = 280.469, p < 0.01) and group (F(1, 16) = 139.834, p < 0.01) were also observed ().
Figure 1. CUS caused impairment of spatial learning and memory in the Morris water maze. (A) CUS exposure rats expend a longer time to escape onto hidden platform than control rats (*p < 0.01 compared to control). (B) CUS induced memory retrieval impairment as indicated by less time to search for in the target quadrant than control rats (Con, 37.99 ± 5.27%; CUS, 22.02 ± 2.9%. *p < 0.05, CUS versus Con). (C) There was no significant difference among the CUS groups and control group for the swim speed. (Con: n = 9, CUS: n = 9. p > 0.05).
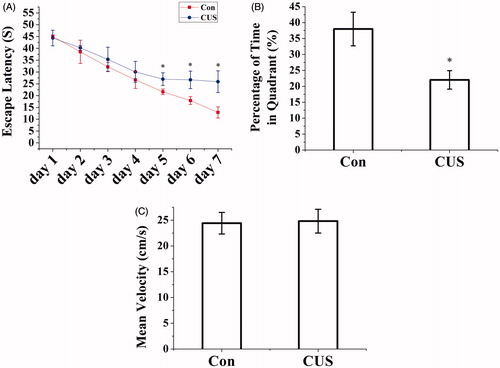
On the probe trial, with the platform removed, CUS impaired memory retrieval as indicated by a reduced amount of time spent in the target quadrant (i.e. the quadrant where the hidden platform was placed during the training session) (F(1, 16) = 373.168, p < 0.05, CUS versus Con, ). No significant differences in swimming speed were noted among the four groups (F(1, 16) = 0.188, p > 0.05, ) (Figure S1).
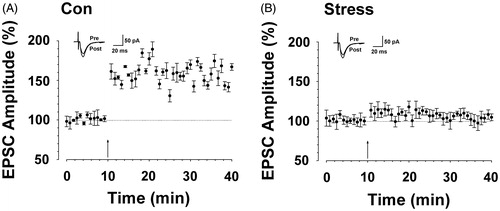
LTP represents an important mechanism underlying hipopocampal learning and memory (Bird & Burgess, Citation2008; Poucet et al., Citation2010; Rolls, Citation2010). Thus, we investigated the effect of CUS on hippocampal LTP. We used the traditional LTP induction paradigm to trigger LTP in hippocampal slices (Zhao et al., Citation2005). The results showed that pairing training induced a significant LTP of synaptic responses in slices from control rats (F(1, 47) = 247.055, p < 0.01 compared with baseline responses, ). In contrast, synaptic potentiation was attenuated in slices from CUS rats (F(1, 47) = 1.123, p > 0.05 compared with baseline, F(1, 71) = 520.810, p < 0.05 versus control group, ).
Figure 2. CUS caused impairment of hippocampal long-term potentiation. (A) LTP was induced in hippocampal pyramidal neurons in control rats (167.88 ± 2.34% of baseline, n = 6 slices/6 rats, t-test; p < 0.01 compared with baseline). (B) LTP was attenuated in hippocampal pyramidal neurons in CUS-treated rats (104.22 ± 3.38%, n = 6 slices/6 rats, t-test; p < 0.05 versus Con). Pairing training is indicated by an arrow. The dashed line indicates the mean basal synaptic responses.
Effect of CUS on hipopocampal microglial activation and proinflammatory factors expression
To investigate whether CUS induces microglial activation, we assessed the number of activated microglia in hippocampal slices. Results showed that microglia of control rats were mostly in the resting state (), while microglia in the CUS-exposed rats appeared activated status (), suggesting that CUS-induced microglial activation (F(1, 39) = 310.898, p < 0.05 versus control, ).
Figure 3. CUS induced activation of microglia and expression of TNF-α and IL-1β. Microglia activation was detected by immunocytochemistry (OX42) (hippocampal DG zone). (A–C) control group; (D–F) CUS group. (G) The results were quantified (Con: 3.5 ± 0.53%; CUS: 21.3 ± 1.3%, n = 20). Results (A–F) are expressed as the mean ± SD of relative increase of the activated microglia cells in random fields (n = 20). *p < 0.05 versus control groups. Scale bar indicates 50 μm. TNF-α (Con: 41.42 ± 7.35 pg/g; CUS: 88.40 ± 19.36 pg/g, n = 6) (H) and IL-1β (Con: 738.32 ± 77.38 pg/g; CUS: 1411.25 ± 145.30 pg/g, n = 6) (I) were determined by ELISA (n = 6). *p < 0.05 versus control groups.
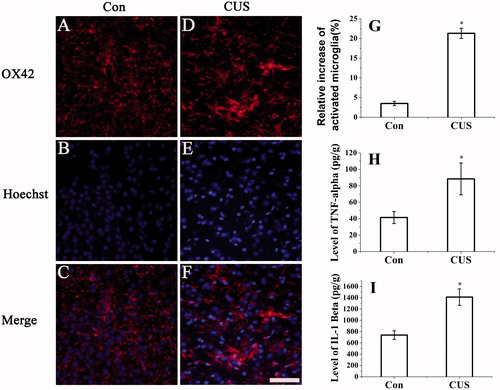
Activation of microglia has been reported to secrete numerous proinflammatory factors (Frigo et al., Citation2005; Liu et al., Citation2009). To examine whether CUS exposure can induce expression of proinflammatory factors, we measured the levels of TNF-α and IL-1β using ELISA. As shown in , rats in the CUS group expressed a significantly higher levels of TNF-α (F(1, 11) = 32.547, p < 0.05 versus control, ) and IL-1β (F(1, 11) = 212.107, p < 0.05 versus control, ) than rats in the control group.
Minocycline treatment alleviates CUS-induced hipopocampal microglia activation and expression of proinflammatory cytokines
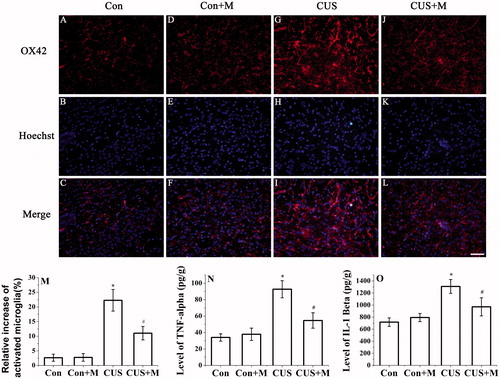
Minocycline, a tetracycline antibiotic, has anti-inflammatory properties and is used experimentally in the treatment of several CNS disorders. Minocycline has been shown to inhibit microglial activation and protect the CNS from inflammatory conditions (Arvin et al., Citation2002; Tomas-Camardiel et al., Citation2004; Yrjanheikki et al., Citation1999). To investigate whether activated microglia causes cognitive dysfunction, rats were treated with minocycline by oral gavage during CUS exposure. The results showed that minocycline treatment alleviated CUS-induced microglial activation (F(3, 79) = 412.399, p < 0.05, CUS + Minocycline versus CUS) (). Minocycline treatment also decreased CUS-induced expression of TNF-α (F(3, 23) = 110.927, p < 0.05, CUS + Minocycline versus CUS) () and IL-1β (F(3, 23) = 31.678, p < 0.05, CUS + Minocycline versus CUS) (). There were no significant differences in microglial activation between control and minocycline-treated rats (F(1, 39) = 0.081, p > 0.05, Con versus Con + Minocycline, ). In addition, pro-inflammatory mediator expression did not differ between the two groups (TNF-α: F(1, 11) = 2.750, p > 0.05, Con versus Con + Minocycline; IL-1β: (F(1, 11) = 2.628, p > 0.05, Con versus Con + Minocycline, ).
Figure 4. Minocycline treatment inhibited the activation of microglia and CUS-induced secretion of TNF-α and IL-1β. Microglia activation was detected by OX42 antibody (hippocampal DG zone). (M) The results were quantified (Con: 2.63 ± 1.15%; Con + Minocycline: 2.74 ± 1.25%; CUS: 22.27 ± 3.68%; CUS + Minocycline: 11.02 ± 2.28%, n = 20). Results (A–L) are expressed as the mean ± SD of relative increase of the activated microglia cells in random fields (n = 20). *p < 0.05 versus control groups. #p < 0.05 versus CUS groups. TNF-α (Con: 33.96 ± 4.63 pg/g; Con + Minocycline: 37.85 ± 7.49 pg/g; CUS: 92.62 ± 10.42 pg/g; CUS + Minocycline: 54.61 ± 9.38 pg/g, n = 6) (N) and IL-1β (Con: 717.05 ± 70.35 pg/g; Con + Minocycline: 793.63 ± 68.36 pg/g; CUS: 1308.74 ± 115.32 pg/g; CUS + Minocycline: 970.76 ± 150.25 pg/g, n = 6) (O) were determined by ELISA (n = 6). *p < 0.05 versus control groups. #p < 0.05 versus CUS groups.
CUS inhibits hipopocampal GluR1 phosphorylation and minocycline reverses this effect
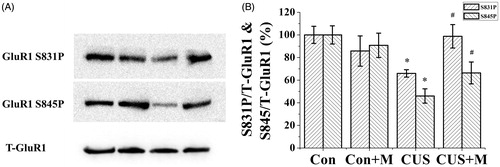
Glutamate receptors are synaptically localized, and they function in neural communication, memory formation and learning. GluR1 phosphorylation enhances the conductivity of AMPA receptor-mediated single channel conductance. Our results showed that after CUS exposure, the phosphorylation of GluR1 (Ser845, Ser831) significantly decreased compared with the control group (Ser845: F(1, 11) = 8.787, p < 0.05, Con versus CUS; Ser831: F(1, 11) = 10.129, p < 0.05, Con versus CUS, ). To confirm that this decrease was caused by the activation of microglia, we treated the CUS group with minocycline as previously described. Minocycline treatment alleviated the CUS-induced decline in GluR1 phosphorylation (Ser845, Ser831) (Ser845: (F(1, 11) = 98.868, p < 0.05, CUS versus CUS+Minocycline; Ser831: (F(1, 11) = 44.649, p < 0.05, CUS versus CUS+Minocycline, ). No significant difference was noted between the control and control plus minocycline groups (Ser845: (F(1, 11) = 8.787, p > 0.05, Con versus Con+Minocycline. Ser831: (F(1, 11) = 2.146, p > 0.05, Con versus Con+Minocycline, ). These results indicate that the activation of microglia and the subsequent expression of proinflammatory factors from these cells likely caused the decrease in GluR1 (Ser845, Ser831) phosphorylation.
Figure 5. Minocycline treatment restored hipopocampal GluR1 phosphorylation in CUS-exposed rats. Stress significantly inhibit GluR1 phosphorylation compared to controls (Ser845: Con, 100.00 ± 8.04% of total GluR1; CUS, 46.00 ± 6.36% of total GluR1, n = 6. Ser831: Con, 100.00 ± 7.61% of total GluR1; CUS, 65.96 ± 3.25% of total GluR1, n = 6) (*p < 0.05). Minocycline treatment reverses the inhibition of CUS on hipopocampal GluR1 phosphorylation (Ser845: CUS + Minocycline, 66.40 ± 9.70% of total GluR1, n = 6. Ser831: CUS+Minocycline, 98.78 ± 10.35% of total GluR1, n = 6) (#p < 0.05). There is no significant difference between the control and control plus minocycline groups (Ser845: Con+Minocycline, 90.81 ± 10.78% of total GluR1, n = 6. Ser831: Con+Minocycline, 85.78 ± 13.37% of total GluR1, n = 6). *p < 0.05 versus control. #p < 0.05 versus CUS. Data are presented as mean ± SD.
Minocycline treatment alleviates CUS-induced impairments of spatial memory and hipopocampal long-term potentiation
The effects of minocycline treatment on spatial learning and memory were tested in the MWM. The results showed a significant effect of trial on latency (F(6, 192) = 375.141, p < 0.01, ) as revealed by two-way repeated measures (trials: day 1–7) ANOVA with CUS and minocycline as the independent factors. Additionally, a significant CUS × minocycline interaction (F(1, 32) = 31.696, p < 0.01, ) and main effects of CUS (F(1, 32) = 108.814, p < 0.01, ), minocycline (F(1, 32) = 20.160, p < 0.01, ) were also observed. As revealed by further analysis, among CUS rats the learning performance of the minocycline treated was improved relative to the non-minocycline-treated group (F(1, 16) = 33.374, p < 0.05, repeated measures (trials) one-way ANOVA with minocycline as independent factor), while no difference was observed between control and control plus minocycline groups (F(1, 16) = 1.395, p > 0.05).
Figure 6. Minocycline treatment alleviated spatial memory impairment induced by CUS. (A) CUS exposure rats require a longer time to escape onto hidden platform than control rats (*p < 0.05). Minocycline treatment (CUS + Minocycline) reduces the escape latency time than CUS group (#p < 0.05). (B) CUS induced memory retrieval impairment as indicated by less time to search for in the target quadrant (*p < 0.05 versus control groups). Minocycline treatment enhanced the search time in target quadrant than CUS group (*p < 0.05) (Con: 40.76 ± 11.05%; Con + Minocycline: 40.26 ± 7.83%; CUS: 21.58 ± 5.76%; CUS + Minocycline: 32.24 ± 8.37%). (C) There was no significant difference among each group for the swim speed (Con: n = 8, Con + Minocycline: n = 9, CUS: n = 9, CUS + Minocycline: n = 9. p > 0.05).
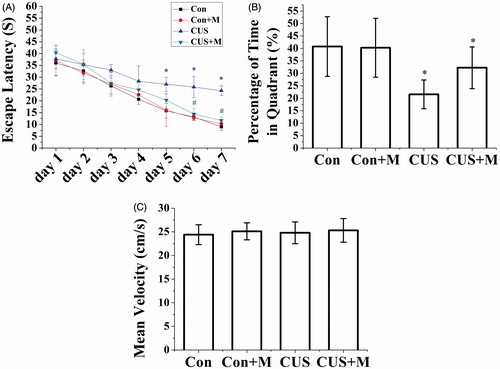
On the probe trial, with the platform removed, minocycline also alleviated the impairment of memory retrieval as indicated by greater percent of time which was spent in the target quadrant (F(1, 17) = 14.546, p < 0.05, CUS + Minocycline versus CUS, ). No significant differences in swimming speed were found between the four groups (F(3, 35) = 0.245, p > 0.05, ).
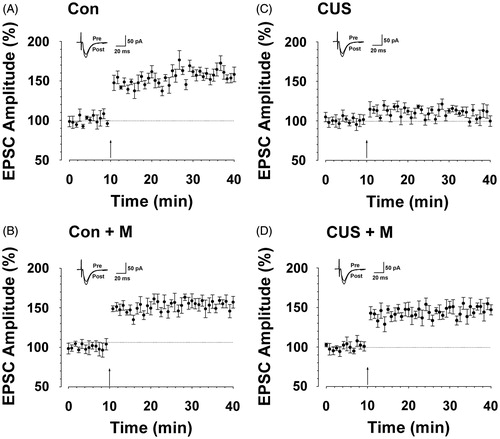
After confirming that minocycline alleviates CUS-induced spatial memory impairment, we determined if this effect was paralleled by rescued hippocampal LTP. The study on hippocampal LTP showed that minocycline administration (CUS + minocycline group) partly restored the induction of LTP (F(1, 47) = 483.762, p < 0.05 versus baseline, ) compared with the CUS group (F(1, 47) = 0.840, p > 0.05 compared with baseline, F(1, 71) = 521.124, p < 0.05 compared with CUS + minocycline group, ). The results also showed that there was no significant difference between control rats (F(1, 47) = 362.677, p < 0.05 compared with baseline, ) and minocycline-treated rats (F(1, 47) = 774.072, p < 0.05 compared with baseline, F(1, 71) = 1.205, p > 0.05 versus control group, ). Collectively, these data suggest that minocycline could partly prevent CUS-induced LTP impairment.
Figure 7. Minocycline treatment alleviated hipopocampal long-term potentiation impairment induced by CUS. (A) LTP was induced in hippocampal pyramidal neurons in control rats (164.14 ± 17.01%, n = 7 slices/7 rats, t-test; p < 0.05 compared with baseline). (B) LTP was induced in hippocampal pyramidal neurons in minocycline-treated rats (154.10 ± 13.08%, n = 8 slices/7 rats, t-test; p < 0.05 compared with baseline). (C) LTP was lost in hippocampal pyramidal neurons in CUS-treated rats (104.88 ± 8.99%, n = 9 slices/7 rats, t-test; p > 0.05 compared with baseline). (D) LTP was partly reversed in hippocampal pyramidal neurons in minocycline-treated rats during CUS exposure (149.84 ± 19.45%, n = 8 slices/seven rats, t-test; p < 0.05 compared with baseline). Pairing training is indicated by an arrow. The dashed line indicates the mean basal synaptic responses.
Discussion
This study evaluated the effects of CUS exposure on cognition and synaptic plasticity impairment and its underlying mechanisms. CUS exposure over a period of 21 d induced impairments of spatial memory and LTP in rats. Along with the cognition and synaptic plasticity impairment, rats subjected to CUS also displayed microglial activation and expression of proinflammatory factors, and reduced hippocampal GluR1 (Ser845, Ser831) phosphorylation. Further, the data suggest that the cognitive and synaptic plasticity impairments induced by CUS were prevented by the microglia-specific inhibitor, minocycline. Finally, the study shows that the inhibition of microglial activation and proinflammatory factors expression restores the CUS-induced inhibition of hippocampal GluR1 (Ser845, Ser831) phosphorylation.
The negative effect of CUS exposure on cognition and synaptic plasticity have been widely addressed (Alfarez et al., Citation2003; Alkadhi et al., Citation2011; Bondi et al., Citation2008; Zhang et al., Citation2011), but the underlying mechanisms have not been fully understood. Glutamate receptors appeared to be causative for the glutamate-mediated postsynaptic excitation of neural cells, and are important for neural communication, memory formation, and learning (De Leonibus et al., Citation2003; Gecz, Citation2010; Riedel et al., Citation1996). GluR1 is an ionotropic glutamate receptor, previous report showed that phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory (Lee et al., Citation2003). Our data demonstrated that CUS induced a significant inhibition of GluR1 (Ser845, Ser831) phosphorylation. Studies also demonstrated that GluR1 is essential for the induction of hippocampal LTP (Lisman & Raghavachari, Citation2006; Sanderson et al., Citation2008), and LTP is a long-lasting enhancement in signal transmission between two neurons that results from stimulating them synchronously (Cooke & Bliss, Citation2006). It is one of several phenomena underlying synaptic plasticity, reflecting the ability of chemical synapses to change their strength. As memories are thought to be encoded by modification of synaptic strength (Bliss & Collingridge, Citation1993), LTP is widely considered one of the major cellular mechanisms that underlie learning and memory (Bliss & Collingridge, Citation1993; Cooke & Bliss, Citation2006). Our research suggests that accompanied with the inhibition of GluR1 (Ser845, Ser831) phosphorylation, CUS also induced a significant inhibition of synaptic plasticity (LTP) as well as spatial memory.
Recent data suggest that stress induces inflammatory responses in various brain regions (Barnum et al., Citation2008; Blandino et al., Citation2006, Citation2009; Carty et al., Citation2008; Goshen et al., Citation2008; Goshen & Yirmiya, Citation2009; Grippo et al., Citation2005; O'Connor et al., Citation2003). Studies of human psychological stress show that psychological stress can enhance the expression of proinflammatory factors such as TNF-α, interleukin 6 (IL-6) and interferon γ (IFN-γ) (Maes et al., Citation1998). Previous studies also show that primary cultured hippocampal neurons treated with proinflammatory factors such as IL-1β can decrease neuronal surface expression and phosphorylation of postsynaptic neuronal GluR1 subunit (Lai et al., Citation2006). Further, studies find that the aged rats treated with TNF receptor 1 (TNFR1) signaling blockers-XPro1595 shows improved spatial memory and increased protein levels for the GluR1 type glutamate receptor in hippocampal CA1 neurons (Sama et al., Citation2012).
Microglial cells are the resident immune cell population of the mammalian CNS. They can be converted to a chronically activated state by a large number of different stimuli (Parkhurst & Gan, Citation2010). Activated microglial cells act as producers of proinflammatory factors such as IL-1β, TNF-α (Bianco et al., Citation2005; Frigo et al., Citation2005; Jiao et al., Citation2008). Studies of prenatal stress show that, stress can induce the morphological changes of hippocampal microglia, enhancing the mRNA levels of IL-1β, IL-10 and TNF-α in this region (Diz-Chaves et al., Citation2012). The role of microglia as a cellular effector in stress-induced CNS immune activation is further supported by recent findings showing that chronic restraint stress in mice induces brain microglia activation and proliferation (Nair & Bonneau, Citation2006) and restraint stress in rats induces microglia activation in the hippocampus (Frank et al., Citation2007). Recent studies also show that repeated restraint stress induces prelimbic cortex and infralimbic cortex microglia activation (Kopp et al., Citation2013) and impaired spatial working memory (Hinwood et al., Citation2012).
Activation of microglia and release of proinflammatory cytokines are not only critical for the development and progression of many neurodegenerative diseases (Perry et al., Citation2010), but also appear to be important for impairments in learning and memory (Tanaka et al., Citation2011). Studies show that the activation of microglia could release excitotoxic levels of glutamate, causing synaptic degeneration and neuronal death (Barger & Basile, Citation2001), and the inhibition of microglial activation can protect neurons from degeneration (Kohman et al., Citation2013) and rescue the learning and memory deficits (Keblesh et al., Citation2009). In addition, a study on the effect of microglia on cognition has demonstrated that under physiological state, the activation of microglial P2X7 receptors (P2X7R) induced by ATP increases the expression and phosphorylation of postsynaptic neuronal GluR1 subunit, enhancing the induction of LTP (Chu et al., Citation2010).
In this study, we showed that CUS exposure induced the activation of hippocampal microglia and enhanced the protein level of IL-1β and TNF-α in hippocampus. Accordingly, we speculated that CUS-induced microglia activation and the ensuing expression of proinflammatory cytokines may cause inhibition of GluR1 (Ser845, Ser831) phosphorylation, in turn, impairing spatial memory and synaptic plasticity.
Minocycline is a semi-synthetic tetracycline derivative and has been demonstrated as a specific inhibitor of microglia activation (Hinwood et al., Citation2012, Citation2013; Walker et al., Citation2013). Studies on mixed spinal cord cultures and pure microglia cultures both find that minocycline prevents excitotoxin-induced microglial proliferation and the increased release of NO metabolites and IL-1β. Further study shows that excitotoxins activated p38 mitogen-activated protein kinase (p38 MAPK) in microglia and minocycline can inhibit this activation (Tikka et al., Citation2001). Other studies indicate that minocycline treatment may inhibit the LPS-induced NF-κB upregulation in microglia (Kobayashi et al., Citation2013). Similarly, studies on restraint stress show that minocycline reduces the impact of stress on neuronal activation and working memory, as well as microglial activation (Hinwood et al., Citation2012; Walker et al., Citation2013), and further study shows that the minocycline treatment could largely abolished the microglial pro-ramifying effects of stress (Hinwood et al., Citation2013; Walker et al., Citation2013). In order to address the chronology of these events, we administrated the microglial activation-specific antagonists, minocycline, and established that after attenuated the microglial activation (and proinflammatory factors expression), the phosphorylation of GluR1 (Ser845, Ser831) was significantly enhanced. Our results also showed that the inhibition of microglial activation was associated with prevention of the impairments in spatial memory and LTP.
In summary, this study indicates that CUS-induced cognition and synaptic plasticity impairments are likely mediated by microglial activation and the expression of intracellular proinflammatory factors. Either alone or in combination, these proinflammatory factors attenuate hippocampal GluR1 (Ser845, Ser831) phosphorylation, leading to hippocampal LTP and cognition deficits. CUS-induced hippocampal microglia activation and proinflammatory cytokine production are attenuated by minocycline administration. Anti-inflammatory effects are accompanied by restoration of hippocampal GluR1 (Ser845, Ser831) phosphorylation, hippocampal LTP and spatial memory.
Supplementary data available online
Figure S1. The distance covered before and after escape latency trail.
Figure S2. Morris water maze schematic diagram.
Figure S3. Lead exposure induced microglia activation.
Declaration of interest
This work was supported by grants from the National Key Technology R&D Program (2009BAI85B04); National Basic Research Program of China (973 Program, #2012CB525002); National Natural Science Foundation of China (#81172621, #30901173); Program for New Century Excellent Talents in University; Science and Technology Innovation Projects of Shaanxi Province (#2011KTCL03-19). This work was also supported by Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT); Program of Public Welfare, Ministry of Health, China (No. 200902006). The authors have no financial or other interests with regard to the article that represent a conflict of interest. None of the funding organizations listed above had any role in the design or conduct of the study, the data analysis, in the preparation of the manuscript or in the decision to submit the work for publication.
References
- Alfarez DN, Joels M, Krugers HJ. (2003). Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci 17:1928–34
- Alkadhi KA, Alzoubi KH, Srivareerat M, Tran TT. (2011). Chronic psychosocial stress exacerbates impairment of synaptic plasticity in beta-amyloid rat model of Alzheimer's disease: prevention by nicotine. Curr Alzheimer Res 8:718–31
- Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. (2002). Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol 52:54–61
- Barger SW, Basile AS. (2001). Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem 76:846–54
- Barnum CJ, Blandino P, Jr Deak T. (2008). Social status modulates basal IL-1 concentrations in the hypothalamus of pair-housed rats and influences certain features of stress reactivity. Brain Behav Immun 22:517–27
- Barria A, Derkach V, Soderling T. (1997). Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole- propionate-type glutamate receptor. J Biol Chem 272:32727–30
- Bian Y, Pan Z, Hou Z, Huang C, Li W, Zhao B. (2012). Learning, memory, and glial cell changes following recovery from chronic unpredictable stress. Brain Res Bull 88:471–6
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. (2005). Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174:7268–77
- Bird CM, Burgess N. (2008). The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci 9:182–94
- Blandino P Jr, Barnum CJ, Deak T. (2006). The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol 173:87–95
- Blandino P, Jr Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. (2009). Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun 23:958–68
- Bliss TV, Collingridge GL. (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–9
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. (2008). Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33:320–31
- Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, et al. (2011). Caspase signalling controls microglia activation and neurotoxicity. Nature 472:319–24
- Carty ML, Wixey JA, Colditz PB, Buller KM. (2008). Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. Int J Dev Neurosci 26:477–85
- Chu YX, Zhang Y, Zhang YQ, Zhao ZQ. (2010). Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav Immun 24:1176–89
- Cooke SF, Bliss TV. (2006). Plasticity in the human central nervous system. Brain 129:1659–73
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56
- De Leonibus E, Costantini VJ, Castellano C, Ferretti V, Oliverio A, Mele A. (2003). Distinct roles of the different ionotropic glutamate receptors within the nucleus accumbens in passive-avoidance learning and memory in mice. Eur J Neurosci 18:2365–73
- Diz-Chaves Y, Pernia O, Carrero P, Garcia-Segura LM. (2012). Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J Neuroinflammation 9:71
- Dunn AJ, Swiergiel AH. (2005). Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav 81:688–93
- Ehlert U, Gaab J, Heinrichs M. (2001). Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol 57:141–52
- Eichenbaum H. (2000). A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1:41–50
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. (2007). Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun 21:47–59
- Frigo DE, Vigh KA, Struckhoff AP, Elliott S, Beckman BS, Burow ME, McLachlan JA. (2005). Xenobiotic-induced TNF-alpha expression and apoptosis through the p38 MAPK signaling pathway. Toxicol Lett 155:227–38
- Gecz J. (2010). Glutamate receptors and learning and memory. Nat Genet 42:925–6
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, OC J, Castanon N, et al. (2008). Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology 33:2341–51
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. (2008). Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry 13:717–28
- Goshen I, Yirmiya R. (2009). Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 30:30–45
- Gouirand AM, Matuszewich L. (2005). The effects of chronic unpredictable stress on male rats in the water maze. Physiol Behav 86:21–31
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. (2005). Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav 84:697–706
- Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. (2002). Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia 37(3):229–40
- Hinwood M, Morandini J, Day TA, Walker FR. (2012). Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex 22:1442–54
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. (2013). Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex 23(8):1784–97
- Jiao J, Xue B, Zhang L, Gong Y, Li K, Wang H, Jing L, Xie J, Wang X. (2008). Triptolide inhibits amyloid-beta1-42-induced TNF-alpha and IL-1beta production in cultured rat microglia. J Neuroimmunol 205:32–6
- Keblesh JP, Dou H, Gendelman HE, Xiong H. (2009). 4-Aminopyridine improves spatial memory in a murine model of HIV-1 encephalitis. J Neuroimmune Pharmacol 4:317–27
- Kelly MP, Adamowicz W, Bove S, Hartman AJ, Mariga A, Pathak G, Reinhart V, et al. (2014). Select 3′,5′-cyclic nucleotide phosphodiesterases exhibit altered expression in the aged rodent brain. Cell Signal 26:383–97
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, et al. (2013). Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4:e525
- Kohman RA, Bhattacharya TK, Kilby C, Bucko P, Rhodes JS. (2013). Effects of minocycline on spatial learning hippocampal neurogenesis and microglia in aged and adult mice. Behav Brain Res 242:17–24
- Kopp BL, Wick D, Herman JP. (2013). Differential effects of homotypic vs. heterotypic chronic stress regimens on microglial activation in the prefrontal cortex. Physiol Behav 122:246–52
- Kwon MS, Seo YJ, Lee JK, Lee HK, Jung JS, Jang JE, Park SH, Suh HW. (2008). The repeated immobilization stress increases IL-1beta immunoreactivities in only neuron, but not astrocytes or microglia in hippocampal CA1 region, striatum and paraventricular nucleus. Neurosci Lett 430(3):258–63
- Lai AY, Swayze RD, El-Husseini A, Song C. (2006). Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J Neuroimmunol 175:97–106
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, et al. (2003). Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112:631–43
- Lisman J, Raghavachari S. (2006). A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE 2006:re11
- Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J. (2009). Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotox Res 16:42–9
- Lopez JF, Akil H, Watson SJ. (1999). Neural circuits mediating stress. Biol Psychiatr 46:1461–71
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, et al. (1998). The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 10:313–18
- McEwen BS, Sapolsky RM. (1995). Stress and cognitive function. Curr Opin Neurobiol 5:205–16
- Miller AH, Maletic V, Raison CL. (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatr 65:732–41
- Nadel L, MacDonald L. (1980). Hippocampus: cognitive map or working memory? Behav Neural Biol 29:405–9
- Nair A, Bonneau RH. (2006). Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol 171:72–85
- O'Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. (2003). Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res 991:123–32
- Ossowska G, Danilczuk Z, Klenk-Majewska B, Czajkowski L, Zebrowska-Lupina I. (2004). Antidepressants in chronic unpredictable mild stress (CUMS)-induced deficit of fighting behavior. Pol J Pharmacol 56:305–11
- Parkhurst CN, Gan WB. (2010). Microglia dynamics and function in the CNS. Curr Opin Neurobiol 20:595–600
- Perry VH, Nicoll JA, Holmes C. (2010). Microglia in neurodegenerative disease. Nat Rev Neurol 6:193–201
- Poucet B, Alvernhe A, Hok V, Renaudineau S, Sargolini F, Save E. (2010). The hippocampus and the neural code of spatial memory. Biol Aujourdhui 204:103–12
- Riedel G, Wetzel W, Reymann KG. (1996). Comparing the role of metabotropic glutamate receptors in long-term potentiation and in learning and memory. Prog Neuropsychopharmacol Biol Psychiatr 20:761–89
- Rolls ET. (2010). A computational theory of episodic memory formation in the hippocampus. Behav Brain Res 215:180–96
- Sama DM, Mohmmad Abdul H, Furman JL, Artiushin IA, Szymkowski DE, Scheff SW, Norris CM. (2012). Inhibition of soluble tumor necrosis factor ameliorates synaptic alterations and Ca2+ dysregulation in aged rats. PLoS one 7:e38170
- Sanderson DJ, Good MA, Seeburg PH, Sprengel R, Rawlins JN, Bannerman DM. (2008). The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory. Prog Brain Res 169:159–78
- Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. (1998). Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatr 172:527–32
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. (1996). Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93:3908–13
- Sugama S. (2009). Stress-induced microglial activation may facilitate the progression of neurodegenerative disorders. Med Hypotheses 73(6):1031–4
- Tanaka S, Kondo H, Kanda K, Ashino T, Nakamachi T, Sekikawa K, Iwakura Y, et al. (2011). Involvement of interleukin-1 in lipopolysaccaride-induced microglial activation and learning and memory deficits. J Neurosci Res 89:506–14
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. (2001). Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21(8):2580–8
- Tomas-Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL. (2004). Minocycline reduces the lipopolysaccharide-induced inflammatory reaction, peroxynitrite-mediated nitration of proteins, disruption of the blood-brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis 16:190–201
- van Rossum D, Hanisch UK. (2004). Microglia. Metab Brain Dis 19:393–411
- Vilhardt F. (2005). Microglia: phagocyte and glia cell. Int J Biochem Cell Biol 37:17–21
- Walker FR, Nilsson M, Jones K. (2013). Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets 14(11):1262–76
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. (2012). Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci: The official journal of the Society for Neuroscience 32:15124–32
- Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M. (2001). Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci 4:164–9
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. (1999). A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA 96:13496–500
- Zhang M, Zheng C, Quan M, An L, Yang Z, Zhang T. (2011). Directional indicator on neural oscillations as a measure of synaptic plasticity in chronic unpredictable stress rats. Neurosignals 19:189–97
- Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J. (2009). Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci 107:156–64
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, et al. (2005). Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47:859–72

