Abstract
The cortisol awakening response (CAR), a rapid increase in cortisol levels following morning awakening, is an important aspect of hypothalamic–pituitary–adrenocortical axis activity. Alterations in the CAR have been linked to a variety of mental disorders and cognitive function. However, little is known regarding the relationship between the CAR and error processing, a phenomenon that is vital for cognitive control and behavioral adaptation. Using high-temporal resolution measures of event-related potentials (ERPs) combined with behavioral assessment of error processing, we investigated whether and how the CAR is associated with two key components of error processing: error detection and subsequent behavioral adjustment. Sixty university students performed a Go/No-go task while their ERPs were recorded. Saliva samples were collected at 0, 15, 30 and 60 min after awakening on the two consecutive days following ERP data collection. The results showed that a higher CAR was associated with slowed latency of the error-related negativity (ERN) and a higher post-error miss rate. The CAR was not associated with other behavioral measures such as the false alarm rate and the post-correct miss rate. These findings suggest that high CAR is a biological factor linked to impairments of multiple steps of error processing in healthy populations, specifically, the automatic detection of error and post-error behavioral adjustment. A common underlying neural mechanism of physiological and cognitive control may be crucial for engaging in both CAR and error processing.
Introduction
The activity of the hypothalamic–pituitary–adrenocortical (HPA) axis has been demonstrated to have a critical association with cognitive functions (Cavanagh & Allen, Citation2008; Compton et al., Citation2013; van Honk et al., Citation1998). The cortisol awakening response (CAR), the rapid increase in cortisol levels following awakening in the morning, has emerged over the last two decades as an important aspect of HPA axis activity (Pruessner et al., Citation1997; for a review, see Chida & Steptoe, Citation2009). Although the neural mechanisms that regulate the CAR remain unclear, the literature has implicated the roles of the hippocampus, amygdala and prefrontal cortex (PFC) (Fries et al., Citation2009; Lu et al., Citation2013; Ursache et al., Citation2012).
Alterations in the CAR may be related to cognitive function. Increased CAR has been associated with both poorer and better behavioral performance in higher order functions such as working memory and attention-switching (Aas et al., Citation2011; Almela et al., Citation2012; Evans et al., Citation2012; Lind et al., Citation2007; Moriarty et al., Citation2014). The relationship between the CAR and the neurocognitive processing that underlies explicit behavior, however, remains unknown. Furthermore, it remains unclear whether the CAR is related to other aspects of cognition, such as error processing.
Error processing, the ability to recognize and correct errors, is vital for an individual’s behavioral adaptation and survival. Error processing includes a chain of dynamic neurocognitive processes that involve enhanced attention to error, the mobilization of cognitive control and corrective action for subsequent behavior (Gehring et al., Citation1993). Deficits in error processing have been found in individuals with a variety of pathological conditions (Gehring & Knight, Citation2000; Kansal et al., Citation2014; Pizzagalli et al., Citation2006; Van De Voorde et al., Citation2010) and after a single dose of cortisol administration (Hsu et al., Citation2003). Knowledge of the association between the CAR and error processing in healthy individuals not only extends existing understanding of the relationship between the HPA axis activity and cognition but also provides important information about the biological factors linked to altered error processing in the brain.
Previous studies have suggested that the PFC, especially the anterior cingulate cortex (ACC), is critical to error processing (Hester et al., Citation2005). The high-temporal resolution of event-related potentials (ERPs) has been used to identify distinct components of error processing, that is, error-related negativity (Ne/ERN) and error positivity (Pe) (Falkenstein et al., Citation1991). The ERN and Pe may reflect different cognitive aspects of error processing, that is, early and automatic error detection and later and conscious error recognition, respectively (Botvinick et al., Citation2004; Falkenstein et al., Citation1991, Citation2000; Gehring et al., Citation1993; van Veen & Carter, Citation2002).
Behavioral adjustment after error detection has typically been observed in the form of a slowing of response latencies and greater likelihood of correct trials immediately following an error (Rabbitt, Citation1966). This post-error slowing may be indicative of the recruitment of cognitive control processes supported by the PFC (Botvinick et al., Citation2001). When the mechanism of post-error slowing fails to adjust post-error behavior, lower post-error accuracy may occur, reflecting compromised behavioral adjustment (Cavanagh & Allen, Citation2008).
The present study aims to investigate the relationship between individual differences in the CAR and the distinct cognitive steps of error processing using both behavioral and ERP methods. Because the PFC is one of the main brain regions supporting error processing and because it plays a critical role in regulating cortisol negative feedback in the HPA-axis system (Fries et al., Citation2009), we predicted that relatively higher CAR levels would be associated with altered ERP indexes of error processing and a decreased ability to adjust post-error behavior.
Methods
Participants
In consideration of sex differences in the modulation of HPA axis activity (Kudielka & Kirschbaum, Citation2005), only male participants were recruited for this preliminary study. All recruited students were in school at the time and were experiencing regular course study and exams. Eighty-seven volunteer students from a medical university in China responded to advertisements, and 63 were ultimately recruited. The inclusion criteria included the following: no history of serious physiological illness, no history of psychiatric disorder including moderate or severe depression, no current illness, no current periodontitis, no current medication use within two days of participation in the study, no irregular sleep/wake cycles, and no excessive nicotine consumption (more than five cigarettes a day). The participants were also assessed with the Life Events Scale (Tennant & Andrews, Citation1976) to exclude those with major life stressors. The data from three participants were discarded because there were fewer than six artifact-free false alarm trials for the ERN/Pe analysis (Olvet & Hajcak, Citation2009). Thus, the data from 60 participants (age 22.53 ± 1.02) were included in the final analysis. This study reports a subset of the results obtained from other studies that addressed the relationship between long-term stress and cortisol response/cognition (Duan et al., Citation2013). The final sample was composed of 40 participants who were preparing for a major academic examination and were expecting the exam to begin 11–25 days later, as well as 20 participants who were not expecting the exam. Students chose to prepare or not to prepare for this major exam according to their own academic plan at the time. The variables of exam status and long-term stress were accounted for in the analyses of the current study (described below). This experiment was approved by the Ethics Committee of Human Experimentation at the Institute of Psychology, Chinese Academy of Sciences. All participants provided written informed consent and were compensated for their participation.
General procedures
After arriving at the laboratory, the participants were seated in a normally lit room and completed questionnaires. After being fitted with scalp electrodes to measure electroencephalogram (EEG) activity, the participants completed the Go/No-go task (see descriptions below) while behavioral and EEG data were collected. After the experiment, the participants received a detailed instruction packet that described the method of saliva collection over the next two days. Saliva samples were collected immediately after awakening in the morning on two consecutive days, and the participants were asked to return the saliva samples to the laboratory as soon as possible (see below for a full description of the saliva sampling protocol).
Go/No-go task
The Go/No-go task is one of the most commonly used paradigms in the study of error-processing (Falkenstein et al., Citation2000). Two letters (“O” and “X”) were presented one at a time in the center of the screen with a visual angle of approximately 2.5° vertically and 2.2° horizontally. After an initial practice block of 20 stimuli was completed, two experimental blocks each consisting of 240 stimuli (probability of 20% No-go and 80% Go) were completed with 1- to 2-min breaks between blocks. The stimuli were presented for 150 ms with a random interstimulus interval of 1200–1500 ms. During each trial, one of the two letters was presented, and either a response (Go) or the withholding of a response (No-go) was required. The participants were asked to respond as quickly as possible on Go trials by pressing a button on the keyboard with the index finger of their dominant hand. The consecutive presentation of two No-go trials was avoided. The association between the stimuli and Go/No-go responses was counterbalanced across participants.
Questionnaires
Long-term psychological stress was assessed with Cohen’s Perceived Stress Scale (PSS) (10-item version) (Cohen & Williamson, Citation1988). We also collected the state anxiety level (Spielberger et al., Citation1983) before the experimental task and the sleeping status of the previous night during salivary sampling after awakening. Sleeping status was assessed by a sleeping questionnaire that included two parts. The first part asked participants “What time did you wake up this morning and how long did you sleep last night”. The awakening time and sleeping duration were averaged between the two days. The second part included seven items rating sleeping quality, and each item had five response options: (1) how did you sleep (very poorly, 1, to very well, 5); (2) did you feel refreshed upon waking (not at all, 1, to completely, 5); (3) how deeply did you sleep last night (very lightly, 1, to very deeply, 5); (4) did you sleep for the entire time allocated for sleep (woke up much earlier, 1, to slept for the whole night, 5); (5) how easy was it for you to wake up (very easy, 1, to very difficult, 5); (6) how easily did you fall asleep last night (very easily, 1, to very difficult, 5); and (7) how many dreams did you have last night (no dreams, 1, to many dreams, 5). The scores of items 6 and 7 were inverted. The sleeping quality score was calculated by adding the scores for each item on each night and then averaging the total scores of the two nights.
ERP recordings and preprocessing
During the Go/No-go task, an EEG was recorded at 64 scalp sites using Ag/AgCl electrodes fastened to an elastic cap (Neuroscan Inc., Charlotte, NC) and placed according to the international 10–20 system, with an online reference to the left mastoid and off-line algebraic re-reference to the average of the left and right mastoids. Vertical electro-oculogram and horizontal electro-oculogram data were recorded via two pairs of electrodes, one placed above and below the left eye and another placed 10 mm from the outer canthi of each eye. All interelectrode impedance was maintained at <5 kΩ. The signals were amplified with a 0.05 to 100 Hz bandpass filter and digitized at 1000 Hz.
The EEG data were processed by Scan 4.3 software (Neuroscan). Ocular artifacts were removed from the EEG signal using a regression procedure implemented in the Neuroscan software. The data were digitally filtered with a 30 Hz lowpass filter and were epoched into periods of 1000 ms (including the 400 to 200 ms pre-response time (RT) as the baseline) time-locked to the onset of the button press. Trials with various artifacts were rejected, with a criterion of ±100 µV.
Salivary cortisol sampling and cortisol analysis
Saliva samples were collected using Salivette collection devices (Sarstedt, Germany). On two consecutive school days after EEG measurement, saliva samples were collected immediately upon awakening (sample 1), 15 min (sample 2), 30 min (sample 3) and 60 min (sample 4) thereafter, resulting in four samples per day and a total of eight samples per individual. Participants were asked to wake up between 06:00 and 08:00 on both days and were asked to stay in bed until all four saliva samples were obtained. However, they were allowed to read quietly or listen to music as well as to go to the bathroom if necessary. To avoid contaminating the saliva, participants were asked not to brush their teeth, drink, eat or smoke before completing the saliva sampling procedures. They were also required to refrain from alcohol and nicotine consumption as well as excessive exercise on the day before saliva sampling. The participants were asked to complete the sleeping questionnaires and record when each sample was taken to assure adherence to the timing protocol. The participants were told the importance of adhering to the sampling instructions and were asked to follow the instructions carefully, and each participant was given a copy of the instruction sheet to increase compliance. The participants were instructed to return the completed samples to the laboratory, where the samples were kept frozen (−20 °C) until assaying. After thawing and centrifuging the samples at 3200 rpm for 10 min, the samples were analyzed by an electrochemiluminescence immunoassay (ECLIA, Cobas e601, Roche Diagnostics, Mannheim, Germany) with a sensitivity of 0.5 nmol/L (lower limit) and a standard range of 0.5–1750 nmol/L. Intra- and inter-assay variations were below 10%.
First, the values for the two days were averaged for each of the four time points, and then the CAR was computed. The CAR was computed as the area under the curve with respect to the increase (AUCi) (Pruessner et al., Citation2003). This measure of CAR is very commonly used in the literature and it emphasizes changes over time and is related to the sensitivity of the HPA axis (Chida & Steptoe, Citation2009; Pruessner et al., Citation2003). To test the results from AUCi, we also computed another common used measure of CAR, the absolute increase in cortisol (AINC), which is computed as the difference of maximal value of “15, 30, or 60 min post-awakening” minus the awakening value (Chida & Steptoe, Citation2009).
Data analysis
The behavioral data, including the RT of the correct hit trials, the miss rate in the Go trials and the rate of false alarms or commission errors in the No-go trials were analyzed. Trials with an RT below 50 ms and above 1000 ms were excluded from the average of correct hit trials. We specifically focused on post-error behavioral performance. The post-error condition refers to the Go trial after a false alarm trial. As a control condition, we also analyzed the post-correct condition, which refers to the Go trial after a correct hit trial. Both the miss rate and the RT of the correct hit were calculated separately for the post-error and post-correct conditions.
For the ERP data, only error trials of the No-go condition (i.e. trials with false alarms) were averaged for the Ne/ERN and Pe components. FCz and Cz were selected to measure the Ne/ERN and Pe, respectively, where the maximum amplitude was observed for each component. The peak amplitude and latency of the Ne/ERN component were measured 0–100 ms after a false alarm response. The mean amplitude of the Pe component was measured 200–400 ms after a false alarm response.
Simultaneous multivariate regression analyses were conducted to examine the associations between the CAR and error processing. The AUCi (or AINC), age, exam status (i.e. whether the participant was preparing for an exam), PSS, state anxiety and sleeping status (awakening time, sleeping duration and sleep quality) were treated as independent variables and the ERPs or Go/No-go behavioral measures were treated as dependent variables, focusing on the relationship between the AUCi (or AINC) and ERP/post-error behavioral effects. The post-error behavioral effects were used as an index of behavioral adjustment after making an error and were defined as the difference in behavioral performance between the post-error and post-correct conditions. Thus, we first performed a repeated measures analysis (post-error vs. post-correct condition) to examine the post-error effect on both the miss rate and the RT. If there was a significant post-error effect on any behavioral index (miss rates or RTs), we treated the difference of this index between the post-error and post-correct conditions as dependent variables in the multivariate regression analysis. To determine whether the CAR, not the raw cortisol levels at one of the four time points, was driving this association, we conducted the same multivariate regression analyses treating the cortisol levels at the four time points after awakening as independent variables. To investigate whether the CAR was associated with other cognitive measures, we conducted multivariate regression analyses that treated the false alarm rate for No-go trials, the miss rate/hit RTs for Go trials or the miss rate/hit RTs in the post-correct condition as dependent variables. The distribution of the cortisol data was examined by the Kolmogorov–Smirnov test, and a natural log transformation was applied to the cortisol data, which were not normally distributed. All reported p values are two-tailed.
Results
Descriptive data
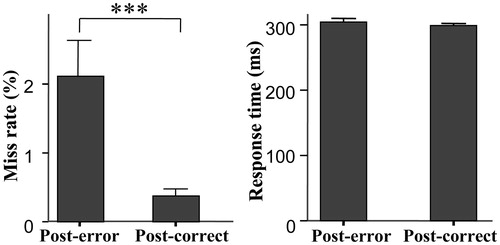
For the behavioral data, there was an average of 23 false alarm trials (range: 6–56) of the No-go condition and the false alarm rate was 24.0 ± 11.5%. Additionally, there was an average of three miss trials (range: 0–23) for the Go condition; the miss rate was 0.9 ± 1.3%, and the hit RT for all Go trials was 294 ± 25 ms. The post-error condition had a significantly higher miss rate than the post-correct condition (F(1,59) =12.79, p = 0.001, partial eta squared (PES) = 0.18; 2.1 ± 4.0% vs. 0.4 ± 0.8%), but the difference between the hit RTs of the post-error and post-correct conditions was not significantly different (F(1,59) = 1.83, p > 0.05; 305 ± 42 vs. 299 ± 26 ms) (). Thus, the post-error behavioral effect was computed as the miss rates increased from the post-correct condition to the post-error condition. This post-error miss rate increase was used to reflect the ability for behavioral adjustment for further regression analyses (i.e. higher post-error miss rate increases, lower abilities for behavioral adjustment).
Figure 1. Comparison of behavioral performance between the post-correct and post-error condition (mean values and standard errors). The left panel shows that the post-error condition had a significantly higher miss rate than the post-correct condition. Post-error: post-error condition; post-correct: post-correct condition; ***p ≤ 0.001.
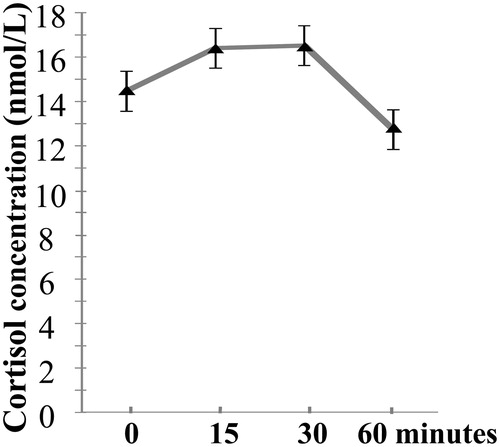
The cortisol levels at each sampling point between the two days were significantly correlated (rs = 0.30–0.53, ps < 0.05), indicating some degree of intraindividual stability across day 1 and day 2. presents the average cortisol data after awakening over two days and the standard error of the mean. There were significant correlations between the cortisol levels in the four samples (r = 0.42–0.82, p ≤ 0.001), except for the correlation between 0 and 60 min after awakening (r = 0.18, p > 0.05). The mean AUCi was 0.83 ± 4.65 nmol/L, and the mean AINC was 2.65 ± 5.18 nmol/L. The AUCi and AINC were highly correlated with each other (r = 0.95, p < 0.001).
Figure 2. Cortisol data after awakening, averaged over two days. The x-axis represents the time points of saliva sampling, and the y-axis represents the averaged raw cortisol levels across two days. The error bars represent the standard error of the mean.
There was a significant positive correlation between ERN latency and the post-error miss rate increase (r = 0.27, p < 0.05). There were no other significant correlations between ERP measures and behavior (ps > 0.05). The correlations between the number of the false alarm trials and the AUCi or AINC/each of these ERP measures did not achieve significance (ps > 0.05).
The mean PSS score was 16.37 with a standard deviation (SD) of 3.59; the mean awakening time was 07:12 (SD = 34 min); the mean sleep duration was 7.26 h (SD = 49 min); the mean sleep quality was 26.36 (SD =3.49); and the mean state anxiety score was 40.05 (SD = 8.82). The exam group had a significantly higher PSS (F(1,58) = 6.65, p = 0.01, PES: 0.10), an earlier awakening time (F(1,58) = 13.82, p < 0.001, PES: 0.19), a shorter sleep duration (F(1,58) = 18.54, p < 0.001, PES: 0.24), lower sleep quality (F(1,58) = 9.55, p < 0.01, PES: 0.14), and a higher state anxiety (F(1,58) = 7.75, p < 0.01, PES: 0.12) than the non-exam group.
The association between the CAR and ERN/Pe
shows that the false alarm trials elicited clear ERN and Pe results compared with the correct hit trials. We focused our multivariate regression analyses on the false alarm trials. The results showed that all of the independent variables predicted 16.8% of the variance in ERN latency (R2 = 0.168, F(8,51) = 1.29, p = 0.27), and only AUCi had a significantly positive association with ERN latency (t = 2.07, p < 0.05) (). (left) illustrates the scatter plot of the bivariate correlation between AUCi and ERN latency without controlling for the other independent variables (r = 0.24, p = 0.06). Multivariate regression also showed that the AUCi was not significantly correlated with the ERN or Pe amplitude and that none of the individual cortisol levels at the four time points after awakening had a significant association with ERN latency (ps > 0.05).
Figure 3. ERPs time-locked to the false alarms and correct hits. The topographic maps show the scalp distributions of the peak of the Ne/ERN component and the mean amplitude of the Pe component (200–400 ms). Ne/ERN, Error-related negativity; Pe, error positivity.
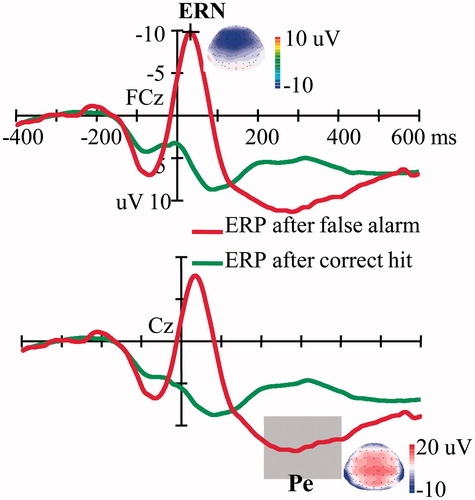
Figure 4. Scatter plots showing the bivariate correlation between the AUCi and the peak latency of the measured ERN (left) and the miss rate increase (post-error minus post-correct condition) (right) (n = 60). There were many “floor” values in the right panel (0% change); thus, we performed the correlation analysis again without these floor values (n = 33), and similar results were achieved: r = 0.49, p < 0.01. AUCi, The cortisol area under the curve with respect to the increase; ERN, error-related negativity.
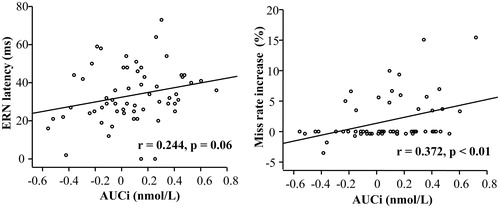
Table 1. Results of the multivariate regression analyses with ERN latency as the dependent variable and the AUCi, age, exam, PSS, awakening time, sleep quality, sleeping duration and state anxiety treated as independent variables (n = 60).
The results for AINC showed a similar pattern as AUCi. The multivariate regression analysis results showed that all of the independent variables predicted 17.7% of the variance in ERN latency (R2 = 0.177, F(8,51) = 1.37, p = 0.23), and only AINC had a significantly positive association with ERN latency (t = 2.20, p < 0.05). Bivariate correlation analysis also showed that AINC was positively correlated with ERN latency (r = 0.26, p < 0.05). Multivariate regression showed that the AINC was not significantly correlated with the ERN or Pe amplitude (ps > 0.05).
The association between the CAR and post-error behavior
As reported above, there is a significantly higher miss rate for the post-error condition than for the post-correct condition; thus, we computed the post-error miss rate increases by subtracting the post-correct miss rates from the post-error miss rates. Multivariate regression analyses showed that all independent variables predicted 26.8% of the post-error miss rate increase (R2 = 0.268, F(8,51) = 2.33, p < 0.05), and only AUCi had a significantly positive association with the post-error miss rate increase (t = 2.63, p = 0.01) (). (right) presents the scatter plot of the bivariate correlation between AUCi and the post-error miss rate increase without controlling for the other independent variables (r = 0.37, p < 0.01). Multivariate regression analyses did not show significant associations between AUCi and any of the other behavioral indices (including the false alarm rate for No-go trials, the miss rate/hit RT for the Go trials and the miss rate/hit RT in the post-correct condition) (ps > 0.05). The multivariate regression analyses also did not find that any of the individual cortisol levels at the four time points after awakening had a significant association with the miss rate increase (ps > 0.05).
Table 2. Results of the multivariate regression analyses with the post-error miss rate increase as the dependent variable and the AUCi, age, exam, PSS, awakening time, sleep quality, sleeping duration and state anxiety treated as independent variables (n = 60).
The results for AINC showed a similar pattern as AUCi. Multivariate regression analyses showed that all independent variables predicted 26.4% of the post-error miss rate increase (R2 = 0.264, F(8,51) = 2.29, p < 0.05), and only AINC had a significantly positive association with the post-error miss rate increase (t = 2.58, p = 0.01). Bivariate correlation analysis also showed that AINC had a significant correlation with post-error miss rate increase (r = 0.39, p < 0.01). Multivariate regression analyses did not show significant associations between AINC and any of the other behavioral indexes (including the false alarm rate for No-go trials, the miss rate/hit RT for the Go trials and the miss rate/hit RT in the post-correct condition) (ps > 0.05).
Discussion
The present study investigated the relationship between the CAR and error processing using a Go/No-go paradigm and ERP methods. The results revealed that individuals with higher CAR showed both a delayed ERN/Ne latency and a higher post-error miss rate increase.
Our results revealed that individual differences in the CAR (measured using both AUCi and AINC) were significantly positively associated with ERN latency. Furthermore, none of the single cortisol values at the four time points after awakening had a significant association with ERN latency, demonstrating that it is the CAR that drives this association between cortisol release within one hour after awakening and ERN latency. The insignificant correlation between the number of the false alarm trials and the CAR/ERN latency suggests that these results were not contaminated by the number of the false alarm trials. Previous studies have revealed a delayed ERN in individuals with mental disorders (Johannes et al., Citation2001; Sokhadze et al., Citation2010). The results of the present study suggest that higher HPA activity in the morning may have an association with decreased speed of the PFC-mediated error monitoring in healthy individuals.
Critically, we also observed that individual differences in the CAR were positively associated with higher post-error miss rate increases, reflecting compromised behavioral adjustment capabilities (Cavanagh & Allen, Citation2008). Interestingly, cortisol levels at any single time point after awakening did not have a significant association with the post-error miss rate increase. Multivariate regression analyses did not show a significant association between the CAR and the other behavioral indexes, such as the false alarm rate, and the post-correct miss rate/hit RT, suggesting that the association between the CAR and behavior was specific to post-error behavior and not to the general accuracy or speed of responding. Previous studies have demonstrated reduced post-error accuracy in individuals with PFC damage (Gehring & Knight, Citation2000) and several psychopathological disorders (Cavanagh & Allen, Citation2008; Pizzagalli et al., Citation2006; Van De Voorde et al., Citation2010). Our data suggest that higher HPA activity after awakening in the morning is associated with impaired post-error behavioral adjustment in healthy population.
The strength of our current design is the use of a high-temporal resolution technique in combination with thoughtful analytic methods that allow us to address how CAR is associated with distinct neurocognitive components in error processing. Error detection and post-error behavioral adjustment are the two major steps of error processing. Error detection may play a role in directing attentional resources to error and then triggering subsequent behavioral adjustments (Ladouceur et al., Citation2007; Tops & Boksem, Citation2011). Our results, which show an association between ERN latency and post-error miss rate increase (i.e. more sensitive ERN in terms of speed and better post-error behavior), provide evidence to support the relationship of these two cognitive components within error processing. Our finding that higher CAR is associated with both delayed error monitoring and poorer post-error behavioral adjustment suggests that HPA activity after awakening in the morning has an association with multiple steps of error processing not only with the final behavioral output of error processing but also with one of the neurocognitive steps before the final behavioral adjustment.
The mechanism behind the association between the CAR and cognition is poorly understood. On the one hand, the CAR may have an effect on error processing. Both the acute and long-term negative effects of cortisol/stress on cognition, including error processing, have been demonstrated in the literature (Hsu et al., Citation2003; Liston et al., Citation2009). It is more probable, however, that the same neural functions that mediate the CAR may also mediate error processing. Error processing has long been believed to rely on the PFC and ACC (Bediou et al., Citation2012; Botvinick et al., Citation2001; Ladouceur et al., Citation2007). Studies in both animals and human have also suggested the role of the PFC and ACC in the regulation of the HPA axis, such as negative feedback (Diorio et al., Citation1993; MacLullich et al., Citation2006; Sullivan & Gratton, Citation2002; Teves et al., Citation2004; for a review, see Dedovic et al., Citation2009). Compton et al.’s results (Citation2013) suggest that common neural processing may be crucial for engaging in both error processing and cortisol reactivity during a task. Although it is unknown whether the roles of the PFC and ACC in regulating HPA axis activity in general are identical to their roles in regulating the CAR, a recent result, namely, reduced cingulate gyrus volume associated with enhanced CAR (Lu et al., Citation2013), supports this hypothesis. Therefore, decreased function in the PFC and ACC may underlie the same mechanism as both higher CAR and impaired error processing.
Our research has some limitations that should be mentioned. First, our study focuses on a highly selective group of male university students (most of them under exam stress). The association between the CAR and error processing observed in this study may not be generalizable to other samples. Second, no causality can be attributed to the observed association between the CAR and error processing. Third, we did not collect pre-test cortisol samples. Without controlling the pre-test cortisol level, it is unclear whether the CAR-error processing relationship is confounded by the acute cortisol level at the moment of cognitive testing. However, we collected and controlled the state anxiety level before the Go/No-go task, which may provide an alternative assessment of the pre-test stress level. Finally, although we repeatedly emphasized the importance of compliance with the timing of saliva collection, we did not use electronic devices to monitor awakening and the collection time of these samples.
In conclusion, our finding suggests that HPA activity after awakening in the morning is associated with multiple steps of error processing; specifically, individuals with an increased CAR show slowed automatic error detection, as reflected by delayed ERN latency, and impaired post-error behavioral adjustment, as reflected by higher post-error miss rate increases. Common mechanisms of PFC activity underlying HPA activity and error processing may be crucial for control of both of these processes and thus may provide a common site for intervention in disorders of stress physiology and error processing.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by the NSF China (81371203, 91124003, 31100734) and the Basic Project of National Science and Technology of China (No. 2009FY110100).
References
- Aas M, Dazzan P, Mondelli V, Toulopoulou T, Reichenberg A, Di Forti M, Fisher HL, et al. (2011). Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol Med 41:463–76
- Almela M, van der Meij L, Hidalgo V, Villada C, Salvador A. (2012). The cortisol awakening response and memory performance in older men and women. Psychoneuroendocrinology 37:1929–40
- Bediou B, Koban L, Rosset S, Pourtois G, Sander D. (2012). Delayed monitoring of accuracy errors compared to commission errors in ACC. Neuroimage 60:1925–36
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. (2001). Conflict monitoring and cognitive control. Psychol Rev 108:624–52
- Botvinick MM, Cohen JD, Carter CS. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–46
- Cavanagh JF, Allen JJB. (2008). Multiple aspects of the stress response under social evaluative threat: an electrophysiological investigation. Psychoneuroendocrinology 33:41–53
- Chida Y, Steptoe A. (2009). Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol 80:265–78
- Cohen S, Williamson GM. (1988). Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. Social psychology of health. Newbury: Sage Publications Inc. p 31–67
- Compton RJ, Hofheimer J, Kazinka R. (2013). Stress regulation and cognitive control: evidence relating cortisol reactivity and neural responses to errors. Cogn Affect Behav Neurosci 13:152–63
- Dedovic K, D’Aguiar C, Pruessner JC. (2009). What stress does to your brain: a review of neuroimaging studies. Can J Psychiat-Rev Can Psychiat 54:6–15
- Diorio D, Viau V, Meaney MJ. (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13:3839–47
- Duan HX, Yuan YR, Zhang L, Qin SZ, Zhang K, Buchanan TW, Wu JH. (2013). Chronic stress exposure decreases the cortisol awakening response in healthy young men. Stress 16:630–7
- Evans P, Hucklebridge F, Loveday C, Clow A. (2012). The cortisol awakening response is related to executive function in older age. Int J Psychophysiol 84:201–4
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. (1991). Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78:447–55
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. (2000). ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol 51:87–107
- Fries E, Dettenborn L, Kirschbaum C. (2009). The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 72:67–73
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. (1993). A neural system for error detection and compensation. Psychol Sci 4:385–90
- Gehring WJ, Knight RT. (2000). Prefrontal-cingulate interactions in action monitoring. Nat Neurosci 3:516–20
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. (2005). Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage 27:602–8
- Hsu FC, Garside MJ, Massey AE, McAllister-Williams RH. (2003). Effects of a single dose of cortisol on the neural correlates of episodic memory and error processing in healthy volunteers. Psychopharmacology 167:431–42
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, Munte TF, et al. (2001). Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Res Neuroimaging 108:101–10
- Kansal V, Patriciu I, Kiang M. (2014). Illness insight and neurophysiological error-processing deficits in schizophrenia. Schizophr Res 156:122–7
- Kudielka BM, Kirschbaum C. (2005). Sex differences in HPA axis responses to stress: a review. Biol Psychol 69:113–32
- Ladouceur CD, Dahl RE, Carter CS. (2007). Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev Sci 10:874–91
- Lind K, Edman A, Nordlund A, Olsson T, Wallin A. (2007). Increased saliva cortisol awakening response in patients with mild cognitive impairment. Dement Geriatr Cogn Disord 24:389–95
- Liston C, McEwen BS, Casey BJ. (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA 106:912–17
- Lu SJ, Gao WJ, Wei ZG, Wu WW, Liao M, Ding YQ, Zhang ZJ, et al. (2013). Reduced cingulate gyrus volume associated with enhanced cortisol awakening response in young healthy adults reporting childhood trauma. PLoS One 8:6
- MacLullich AMJ, Ferguson KJ, Wardlaw JM, Starr JM, Deary IJ, Seckl JR. (2006). Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic–pituitary–adrenal axis regulation in healthy elderly men. J Clin Endocrinol Metab 91:1591–4
- Moriarty AS, Bradley AJ, Anderson KN, Watson S, Gallagher P, McAllister-Williams RH. (2014). Cortisol awakening response and spatial working memory in man: a U-shaped relationship. Hum Psychopharmacol 29:295–8
- Olvet DM, Hajcak G. (2009). The stability of error-related brain activity with increasing trials. Psychophysiology 46:957–61
- Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. (2006). Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: a 128-channel EEG study. Hum Brain Mapp 27:185–201
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28:916–31
- Pruessner JC, Wolf OT, Hellhammer DH, BuskeKirschbaum A, vonAuer K, Jobst S, Kaspers F, et al. (1997). Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61:2539–49
- Rabbitt PMA. (1966). Errors and error correction in choice-response tasks. J Exp Psychol 71:264–72
- Sokhadze E, Baruth J, El-Baz A, Horrell T, Sokhadze G, Carroll T, Tasman A, et al. (2010). Impaired error monitoring and correction function in autism. J Neurother 14:79–95
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. (1983). Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press
- Sullivan RM, Gratton A. (2002). Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology 27:99–114
- Tennant C, Andrews G. (1976). A scale to measure the stress of life events. Aust N Z J Psychiatry 10:27–32
- Teves D, Videen TO, Cryer PE, Powers WJ. (2004). Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci USA 101:6217–21
- Tops M, Boksem MAS. (2011). Cortisol involvement in mechanisms of behavioral inhibition. Psychophysiology 48:723–32
- Ursache A, Wedin W, Tirsi A, Convit A. (2012). Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology 37:1270–6
- Van De Voorde S, Roeyers H, Wiersema JR. (2010). Error monitoring in children with ADHD or reading disorder: an event-related potential study. Biol Psychol 84:176–85
- van Honk J, Tuiten A, van den Hout M, Koppeschaar H, Thijssen J, de Haan E, Verbaten R. (1998). Baseline salivary cortisol levels and preconscious selective attention for threat. A pilot study. Psychoneuroendocrinology 23:741–7
- van Veen V, Carter CS. (2002). The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci 14:593–602

