Abstract
Infant–caregiver experiences are major contributing factors to neural and behavioral development. Research indicates that epigenetic mechanisms provide a way in which infant–caregiver experiences affect gene activity and other downstream processes in the brain that influence behavioral development. Our laboratory previously demonstrated in a rodent model that exposure to maltreatment alters methylation of DNA associated with the brain-derived neurotrophic factor (bdnf) and reelin genes as well as mRNA of key epigenetic regulatory genes in the medial prefrontal cortex (mPFC). In the current study, we characterized patterns of histone acetylation at bdnf and reelin gene loci after our caregiver manipulations. Using a within-litter design (n = 8–10/group from eight litters), pups were exposed to adverse (maltreatment condition: exposure to a stressed caregiver) or nurturing (cross-foster condition: exposure to a nurturing caregiver) caregiving environments outside the home cage for 30 min daily during the first postnatal week. Remaining pups in a litter were left with the biological mother during each session (providing normal care controls). We then used chromatin immunoprecipitation (ChIP) and quantitative RT-PCR to measure histone 3 lysine 9/14 acetylation associated with bdnf promoters I and IV and the reelin promoter in the adult mPFC. Maltreated females had decreased acetylation at bdnf IV, while neither males nor females exhibited histone acetylation alterations at bdnf I or reelin. These data demonstrate the ability of maltreatment to have long-term consequences on histone acetylation in the mPFC, and provide further evidence of the epigenetic susceptibility of bdnf IV to the quality of infant–caregiver experiences.
Introduction
Early-life stress in the form of caregiver maltreatment is a known factor in the development of psychiatric disorders (Bremner et al., Citation2007; Cicchetti & Toth, Citation2005; Heim & Nemeroff, Citation2001; Neigh et al., Citation2009). Adults who experienced childhood abuse show increased instances of depression, anxiety and post-traumatic stress disorder (PTSD) (Anda et al., Citation2006; Cicchetti & Toth, Citation2005), and rodents and non-human primates that have negative experiences with a caregiver in infancy show neurobiological and behavioral outcomes similar to those observed in humans (Ivy et al., Citation2008, Citation2010; Kaffman & Meaney, Citation2007; Liu et al., Citation1997; McGowan et al., Citation2009). The mechanisms that produce the neurobiological and behavioral outcomes associated with maltreatment are likely varied and complex, but alterations in gene regulation and expression appear to play a crucial role (Liu et al., Citation1997; Meaney, Citation2001; Roceri et al., Citation2004). One way in which gene expression (either basal levels or activity-dependent) can be altered by environmental factors is epigenetic mechanisms, including (but not limited to) DNA methylation and histone acetylation (Blaze & Roth, Citation2012). While DNA methylation is known to generally repress gene transcription, histone acetylation is an activator of transcription, leading to increased mRNA expression.
There is increasing evidence that early-life caregiver experiences can alter central nervous system (CNS) DNA methylation in a genome-wide (Khulan et al., Citation2014; Labonte et al., Citation2012; Naumova et al., Citation2012; Provençal et al., Citation2012) and gene-specific (Blaze et al., Citation2013; Champagne et al., Citation2006; Kundakovic et al., Citation2013; McClelland et al., Citation2011; Murgatroyd et al., Citation2009; Roth et al., Citation2009, Citation2014; Weaver et al., Citation2004) manner. Several brain regions (i.e. hippocampus, cortex, amygdala, hypothalamus) are known to exhibit alterations in methylation of DNA associated with gene loci involved in stress, plasticity and emotion, including the brain-derived neurotrophic factor (bdnf) (Blaze et al., Citation2013; Kundakovic et al., Citation2013; Roth et al., Citation2009, Citation2014), glucocorticoid receptor (GR) (Kundakovic et al., Citation2013; Weaver et al., Citation2004), arginine vasopressin (Murgatroyd et al., Citation2009; Murgatroyd & Spengler, Citation2014) and corticotropin releasing factor (Franklin et al., Citation2010; McClelland et al., Citation2011) genes. For example, our laboratory has shown that exposure to caregiver maltreatment during the first postnatal week results in DNA methylation alterations (at bdnf I, bdnf IV and reelin regulatory regions) that are present in the adult rat medial prefrontal cortex (mPFC) (Blaze et al., Citation2013). Notably, some of these methylation patterns differ from those in the whole PFC (Roth et al., Citation2009), suggesting that subregions of the PFC respond differentially to early-life stress. Human studies have also highlighted this subregion specificity, showing that changes in mPFC structure and activation after early-life stress play a role in sensitivity to stress later in life and changes in cognitive functioning and anxiety (Gorka et al., Citation2014; Hanson et al., Citation2012; van Harmelen et al., Citation2014).
As DNA methylation has been the main focus in early-life stress research, there are few reports of the effects of early-life stress on histone acetylation. Groups that have looked at early stress-induced modifications to histones have used a global approach or specifically targeted histones associated with the GR gene (McGowan et al., Citation2011; Levine et al., Citation2012; Weaver et al., Citation2004). Furthermore, no studies (to date) have investigated histone acetylation at bdnf or reelin regulatory regions after exposure to maltreatment in infancy. Because bdnf and reelin code for proteins important in neural development and plasticity, these genes are widely implicated in various psychiatric disorders (Abdolmaleky et al., Citation2005; Boulle et al., Citation2012; Martinowich et al., Citation2007; Nagahara & Tuszynski, Citation2011; Tissir & Goffinet, Citation2003). BDNF is released from dendrites, axons and terminals in an activity-dependent manner to modulate synaptic strength and neuronal connectivity (Balkowiec & Katz, Citation2002; Martinowich et al., Citation2007). Likewise, cellular release of reelin contributes to synaptic strength and neuronal connectivity via its role in neuronal migration and synapse formation (D'Arcangelo, Citation2014). Changes in mRNA levels of these genes are a known result of early-life stress, and several groups have linked DNA methylation alterations to corresponding changes in mRNA levels (Gross et al., Citation2012; Kundakovic et al., Citation2013; Roth et al., Citation2009). In the current study, we measured histone 3 lysine 9/14 (H3K9/14) acetylation associated with bdnf promoters I and IV and the reelin promoter in the mPFC of adult male and female rats that were subjected to aversive or nurturing caregiving environments during infancy. Based on the susceptibility of the mPFC to early-life stress and environmentally-driven epigenetic changes, along with our previous DNA methylation results, we hypothesized that histone acetylation at bdnf and reelin promoters would be altered in the mPFC of rats subjected to caregiver maltreatment.
Methods
Animals
We obtained male and female outbred Long-Evans rats from Harlan and maintained a breeding colony in a temperature (ranging from 20 ± 1 °C) and light-controlled colony room (12 h light/12 h dark cycle with lights on at 06:00 h). Male and female rats were bred by placing one male and one female rat together in a wire-bottomed cage, and both the male and wire bottom were removed once a sperm plug was detected (indicating successful copulation). Pregnant females were singly housed in standard cages with abundant wood shavings and access to food and water ad libitum. All caregiving manipulations were performed during the light cycle. No primiparous dams were used for experimental litters or stimulus caregivers. Pup day of birth was designated as postnatal day (PN) 0 and litters were culled to 5–6 males and 5–6 females per litter on PN1 before experimental manipulations took place from PN1–7. We used brain tissue from 39 rats derived from a total of eight litters (yielding 8–10 rats per experimental group). All procedures were approved by the University of Delaware Animal Care and Use Committee.
Caregiving manipulations
Caregiver manipulations were performed as previously reported in our laboratory (Blaze & Roth, Citation2013; Blaze et al., Citation2013; Roth et al., Citation2014) and using a protocol adapted from others (Ivy et al., Citation2008; Raineki et al., Citation2010; Roth & Sullivan, Citation2005). Each experimental litter was split into three groups on PN1, with 3–4 male and female pups in each group. Pups were marked numerically each day with a non-toxic permanent marker. For 30 min per day from PN1–7, the maltreatment group was transported into a room and placed in a chamber with a lactating dam that was stressed due to insufficient bedding material and the novel environment of the testing chamber. At the same time, the cross-foster care group was placed with another lactating dam that had plenty of nesting material and was given 1 h to habituate to the chamber before receiving the pups. The remaining pups in the litter comprised the third group (normal maternal care), that were left undisturbed (except for weighing) with the biological mother during the session. After each day’s 30 min exposure session, all pups were returned to the biological mother and the stimulus dams (from the maltreatment and cross-foster conditions) were reunited with their biological litters. Exposure sessions were performed at different times every day to provide an element of unpredictability of stress exposure. Litters were left undisturbed until weaning at PN21–23 and then housed with a same-sex, same-condition littermate until PN90.
Pup-directed behaviors from dams were scored by trained observers via live observations and/or video recordings. Behaviors were tallied in 5-min time bins over the 30 min sessions and averaged across the 7 days. We also recorded 40 kHz ultrasonic (Batbox III, NHBS Ltd., UK) and audible vocalizations for each condition during the exposure sessions, which were both tallied (by trained listeners) based on their presence in 1 min time bins (regardless of duration) and likewise averaged across the seven exposure sessions.
Chromatin immunoprecipitation (ChIP)
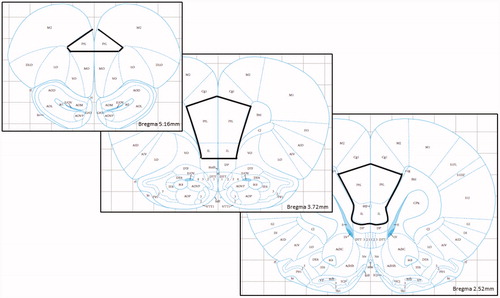
At PN90, rats were killed by decapitation at baseline conditions (i.e. taken from the home cage with minimal disturbance) under light isoflurane anesthesia. Brains were immediately removed, sliced into coronal sections on a 1 mm brain matrix, and flash frozen in 2-methylbutane on untreated slides. Chromatin immunoprecipitation (ChIP) was performed using a modified version of the EMD Millipore ChIP Assay and other published protocols (Bredy et al., Citation2007; Lubin et al., Citation2008). The mPFC (consisting of prelimbic and infralimbic cortex) was dissected on dry ice using stereotaxic coordinates from 2.52 to 5.16 mm anterior to bregma (according to Paxinos & Watson, Citation2007 – see for representation of region of interest) and tissue was immediately cross-linked for 10 min at 37 °C. The tissue was washed twice with an ice-cold protease inhibitor mixture (Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific, San Jose, CA) and 1X PBS) and homogenized in sodium dodecyl sulfate (SDS) lysis buffer (Millipore, Danvers, MA). Chromatin was sheared on ice using the EpiShear probe sonicator (Active Motif, Carlsbad, CA) at 25% amplitude for 12 min (15 s on, 30 s off). Samples were diluted 1:10 with ChIP Dilution Buffer (Millipore) and a small amount of chromatin was saved as input DNA for later analysis. Chromatin was pre-cleared for 45 min using Protein A Agarose/Salmon Sperm DNA (Millipore) and then rotated with acetyl H3K9/14 polyclonal antibody (Epigentek, Farmingdale, NY) overnight at 4 °C. A no-antibody control was also used as a negative control in later PCR reactions. Histone–DNA–antibody complexes were precipitated with Protein A Agarose/Salmon Sperm DNA and then beads were washed with low salt buffer (two washes), high salt buffer (two washes), LiCl (two washes) and 1x Tris-EDTA (TE; three washes) (Millipore). Histone–DNA complexes were eluted with 10% SDS and 1 M NaHCO3. Samples were incubated at 65 °C overnight with 5 M NaCl and RNase A to reverse the cross-link and then DNA was purified using Qiaquick PCR Purification Kit (Qiagen, Valencia, CA) for later amplification with real-time polymerase chain reaction (RT-PCR).
Figure 1. Brain atlas (Paxinos & Watson, Citation2007) representation of region of interest for medial prefrontal cortex dissections (PrL = prelimbic, IL = infralimbic). Reproduced with permission.
Real-time polymerase chain reaction (RT-PCR)
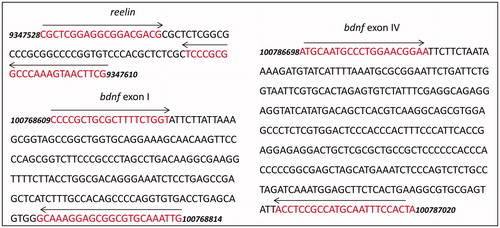
To amplify immunoprecipitated DNA specific to our candidate genes, we used primers specific to the same or nearby region of the bdnf and reelin genes as we have previously used for DNA methylation studies (Blaze et al., Citation2013; Roth et al., Citation2009, Citation2014) (). For bdnf, primer sequences were as follows: bdnf I: 5′-CCCCGCTGCGCTTTTCTGGT-3′ (forward) and 5′-CAATTTGCACGCCGCTCCTTTGC (reverse) and bdnf IV: 5′-ATGCAATGCCCTGGAACGGAA-3′ (forward) and 5′-TAGTGGAAATTGCATGGCGGAGGT-3′ (reverse) (Gupta-Agarwal et al., Citation2012; Huang et al., Citation2002; Lubin et al., Citation2008). For the reelin promoter, primers were 5′-CGCTCGGAGGCGGACGACG-3′ (forward) and 5′-CGAAGTTACTTTGGGCCGCGGGA-3′ (reverse). PCRs were performed in triplicates and product specificity was confirmed with sequencing, melt curve analysis, and gel electrophoresis. Fluorescence for each immunoprecipitated DNA amplicon was normalized to the input DNA fragment (Bredy et al., Citation2007; Lubin et al., Citation2008).
Figure 2. Diagrams of gene targets in the study. Primer pair positions are indicated by the red text and left and right arrows. bdnf, accession NC_005102.4 (primer sequences from Gupta-Agarwal et al., Citation2012; Lubin et al., Citation2008); reelin, accession NC_005103.4.
Methylation-specific real-time PCR (MSP)
To measure DNA methylation associated with acetylated H3K9 in maltreated females, we performed a bisulfite treatment on chromatin-immunoprecipitated DNA and used methylated and unmethylated primer sets to amplify bdnf IV (as previously published, Blaze et al., Citation2013; Lubin et al., Citation2008; Roth et al., Citation2009). Tubulin was used as a reference gene and reactions were run in triplicate, with product specificity confirmed by melt curve analysis and gel electrophoresis.
Statistical analyses
Specific types of caregiving behaviors (i.e. step on, roughly handle, pup licking) and pup vocalizations (ultrasonic and audible) were each analyzed with one-way ANOVAs, and a two-way ANOVA was used to compare overall nurturing and adverse maternal behaviors across groups. Bonferonni or unpaired t-tests were used for between group comparisons. For RT-PCR data, we used the comparative Ct method (Livak & Schmittgen, Citation2001) to obtain the relative fold change of our experimental (maltreatment or cross-foster care) groups compared to the controls (normal maternal care). Differences in histone acetylation and DNA methylation were analyzed using one- or two-tailed one-sample t-tests (a mean value of 1 would indicate no change in levels in comparison to the normal care control group) and unpaired t-tests (to compare the foster care and maltreatment groups). Male and female samples were run on different 96-well PCR plates that contained a representative sample of each caregiving condition. To help minimize differences between plates within a sex the same lot of SYBR green supermix, RNase free water, and primers were used for all assays. Given that plates did not contain a standard and sexes were run independently, we did not statistically contrast sexes. Outliers that fell more than two standard deviations above or below the mean were excluded (ChIP bdnf I, n = 2, bdnf IV, n = 3; DNA methylation n = 2). We denoted significance at p < 0.05.
Results
Maternal behavior
To alter the caregiving environment of infant rat pups, we used a within-litter design to subject pups to either adverse or nurturing caregiving environments inside (normal care group) or outside (maltreatment or foster care group) of the home cage. Caregiving behaviors that were classified as aversive were stepping on, dragging, dropping, actively avoiding and roughly handling pups. Nurturing behaviors included pup licking, anogenital licking, hovering over pups and nursing. The 30-min exposures were scored in five-minute bins in which behaviors were tallied based upon their presence or not. After scoring, we calculated the percent occurrence of each behavior throughout each exposure session (determined by the number of five-minute bins in which a behavior was observed divided by total number of bins) and then averaged the values across all seven exposure days for each litter (averages are shown in ). A two-way ANOVA confirmed that there was a main effect of type of caregiving behavior (F(1, 42) = 144.8, p < 0.001) and a type of caregiving behavior × infant condition interaction (F(2, 42) = 25.5, p < 0.001). The occurrence of aversive behaviors was significantly greater in the maltreatment group (p < 0.001 versus cross-foster care, p < 0.01 versus normal maternal care). Maltreated pups also experienced significantly less nurturing behavior than pups in the other caregiving conditions (p < 0.001 versus cross-foster care, p < 0.01 versus normal maternal care). Moreover, pups that experienced nurturing care inside or outside of the home cage had similar caregiving experiences (all p values for statistical comparisons >0.05). Percent occurrence of specific aversive and nurturing behaviors is listed in , along with statistical comparisons of each behavior across the three groups.
Table 1. Average percent occurrences of individual adverse and nurturing pup-directed maternal behaviors and pup vocalizations across the seven 30-min exposure sessions.
Infant vocalizations
Infant audible and ultrasonic (40 kHz) vocalizations that occurred within the exposure chambers (the maltreatment and cross-foster care groups) or home cage (the normal care group when littermates were with foster dams) were tallied for each 1 min time bin during the 30-min exposure sessions and averaged across the seven days (). For audible vocalizations, a one-way ANOVA revealed no significant effect of infant condition (F(2, 21) = 3.1, p = 0.07), although mean values indicated that maltreated rats vocalized much more than the other groups. For 40 kHz ultrasonic vocalizations, there was a significant main effect of infant condition (F(2, 21) = 11.2, p < 0.001) such that the maltreated rats emitted significantly more vocalizations than pups in both the normal maternal care (t(14) = 3.5, p < 0.01) and cross-foster care (t(14) = 5.8, p < 0.001) conditions. Literature suggests that 40 kHz ultrasonic vocalizations may serve as distress called from pups (Portfors, Citation2007), indicating they are responding to the stressful environment.
bdnf and reelin H3 acetylation
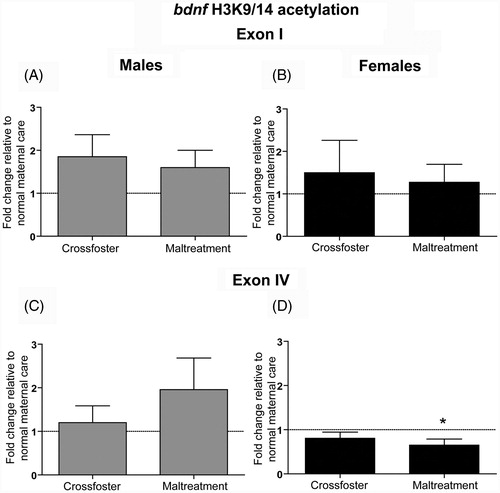
We used ChIP to measure H3K9/14 acetylation in the mPFC of adult (PN90) male and female rats that experienced nurturing care or maltreatment in infancy. We first examined both promoters I and IV of the bdnf gene because these are regions where we have previously found early-life caregiving-induced changes in DNA methylation within the mPFC (Blaze et al., Citation2013). We did not detect any significant changes in bdnf I acetylation in maltreated, or cross-fostered, male or female rats (; all p values for statistical comparisons >0.05). At bdnf IV, there was no significant change in males (), but a one-sample t-test revealed that females who experienced maltreatment during infancy had significantly less acetylation of bdnf IV DNA in comparison to normal care controls (; t(8) = 2.5, p < 0.05).
Figure 3. Adult (PN90) H3K9/14 acetylation at brain-derived neurotrophic factor (bdnf) promoters I and IV in the medial prefrontal cortex (mPFC). One-sample (to compare to normal care controls) and two-tailed unpaired t-tests indicate that neither (A) males nor (B) females showed any differences in H3K9/14 acetylation at bdnf I. At bdnf IV, (C) males showed no significant changes, but a one-sample t-test revealed that (D) females that experienced maltreatment had less acetylation in comparison to normal care control females (*p < 0.05). Error bars represent SEM, n = 8–10/group.
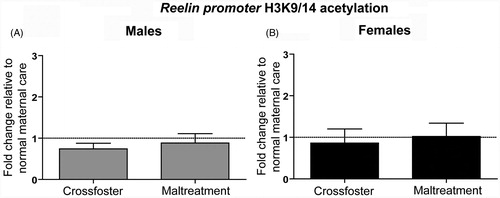
Using the same methods, we measured H3K9/14 acetylation at the reelin promoter in the adult rat mPFC since we have also found changes in reelin methylation in rats from our caregiver manipulation paradigm (Blaze et al., Citation2013). Interestingly, there were no significant changes in acetylation of DNA associated with the reelin promoter in the mPFC of males or females exposed to either the maltreatment or cross-foster care (; all p values >0.05).
bdnf DNA methylation
We have previously shown increased bdnf IV DNA methylation in maltreated females (Blaze et al., Citation2013). To replicate this observation and confirm the inverse relationship between histone acetylation and DNA methylation, we bisulfite-converted H3K9 ChIP DNA from the maltreated females and measured DNA methylation at bdnf IV. A one-tailed one-sample t-test confirmed that maltreated females had greater bdnf IV methylation in comparison to normal care controls (mean of 1.954 fold-change, ±0.46 SEM; t(6) = 2.1, p < 0.05).
Discussion
While DNA methylation alterations have been consistently linked to caregiving conditions, histone acetylation has not been well-characterized. The current study was designed to assess histone acetylation in the mPFC of adult rats exposed to various caregiving conditions. Our main finding here is that female rats that experienced maltreatment had less histone H3K9/14 acetylation associated with bdnf IV DNA in the mPFC, which corresponded to greater bdnf IV methylation. As early-life stress is known to induce changes in mPFC structure and activation, conferring sensitivity to stress later in life and changes in cognitive function and anxiety (Gorka et al., Citation2014; Hanson et al., Citation2012; van Harmelen et al., Citation2014), our data suggest both histone and DNA methylation alterations could be mechanisms underlying these long-term effects. DNA methylation involves the addition of methyl groups to cytosines, and usually represses transcription via the binding of methyl-CpG binding protein (MeCP2) and other co-repressors or by interfering with transcription factor binding. Conversely, histone acetylation is characterized by the addition of acetyl groups to lysine residues on histone tails, a reaction that is catalyzed by histone acetyltransferases (HATs) and can be reversed by histone deacetylases (HDACs) (Strahl & Allis, Citation2000). Histone acetylation loosens the chromatin complex and makes it more accessible for transcription factor binding, thus increasing gene expression.
We previously reported altered methylation of DNA associated with exons I and IV of the bdnf gene in the mPFC of male and female rats that experienced early-life maltreatment (Blaze et al., Citation2013). Specifically, in comparison to controls, females that experienced maltreatment had greater methylation of bdnf IV and less methylation of bdnf I, while males had less methylation of bdnf I. Based upon these findings we predicted that histone acetylation associated with the bdnf gene would likewise be altered in rats exposed to maltreatment. Female data for bdnf IV from the present study were consistent with our prediction, showing less histone H3K9/14 acetylation associated with bdnf IV DNA in the mPFC. Notably, this corresponds to our observation of higher methylation in this group in our previous (Blaze et al., Citation2013) and current study, supporting the typically observed negative correlation between DNA methylation and histone acetylation (Cedar & Bergman, Citation2009; Eden et al., Citation1998; Irvine et al., Citation2002; Weaver et al., Citation2004). Other studies have found experience-induced changes in histone acetylation associated with this same locus of the bdnf gene. For example, Fuchikami et al. (Citation2009) revealed decreased H3 acetylation at bdnf IV in the adult rat hippocampus after a single bout of immobilization stress (Fuchikami et al., Citation2009). Conversely, prenatal cocaine exposure increased H3 acetylation at bdnf IV (Kabir et al., Citation2014). Communal nesting in infancy and fear conditioning in adulthood have too been shown to increase bdnf IV H3 (Branchi et al., Citation2011, Bredy et al., Citation2007; Lubin et al., Citation2008; Takei et al., Citation2011) and H4 (Bredy et al., Citation2007; Takei et al., Citation2011) acetylation.
In a previous study, we also saw significant changes in reelin promoter methylation in our cross-foster care group (males showed an increase, females showed a decrease), with a trending increase in maltreated males (Blaze et al., Citation2013). Additionally, our laboratory has previously reported decreased reelin mRNA in the mPFC of males and females that were maltreated (Blaze et al., Citation2013). Other groups have shown that early-life maternal separation produces alterations in adult reelin mRNA levels in the hippocampus (Gross et al., Citation2012; Zhang et al., Citation2013). We thus predicted that we would see changes to reelin promoter histone acetylation. In the current study, however, we did not find any changes in reelin H3 acetylation in males or females from either group.
While H3 acetylation data for bdnf IV are consistent with our previous and current methylation findings in maltreated female rats, we did not find any changes in acetylation at bdnf I or reelin in males or females in either of our caregiving conditions. Multiple environmental factors, including drug administration and stress in adulthood, are known to alter H3 acetylation at these same bdnf (Fuchikami et al., Citation2009; Lubin et al., Citation2008; Schmidt et al., Citation2012) and reelin loci (Kundakovic et al., Citation2007; Mitchell et al., Citation2005; Sui et al., Citation2012). It is possible that stress early in life may exert differential effects on bdnf I and reelin histone acetylation. It is also possible that histone acetylation is focused at other lysine residues on H3 or perhaps on H4, which we did not examine here.
Finally, many studies in the field of behavioral epigenetics have been conducted under the framework that environmentally-driven increases in DNA methylation and decreases in histone acetylation will correlate with sustained decreases in basal levels of gene expression. Due to methodological limitations (i.e. needing sufficient tissue for ChIP), we were unable to dedicate any tissue for RNA extractions and thus did not measure bdnf or reelin gene expression in the current study. We have previously reported lower levels of bdnf mRNA (bdnf IV-containing transcripts) in the PFC (as a whole) of maltreated rats (Roth et al., Citation2009). In our prior study focused on the mPFC we did not find a change in total bdnf mRNA levels, however we did not examine just bdnf IV-containing transcripts (Blaze et al., Citation2013). In that same study we did find a significant decrease in reelin gene expression in maltreated females. Though concomitant changes in gene expression are one way to envision functional significance of epigenetic changes, an emerging view in the field is that discordance between epigenetic changes and basal gene expression might reflect a form of CNS plasticity that serves to direct gene transcription upon subsequent stimulation (Baker-Andresen et al., Citation2013).
Future directions
Other studies have shown global changes in H3 and H4 acetylation (i.e. not looking at a specific gene locus) following either early- or later-life stress. For example, maternal separation in infancy alters H4 acetylation in the forebrain neocortex (Levine et al., Citation2012). Moreover, rats that were exposed to social defeat in adulthood have histone acetylation changes in several stress-related brain regions (Hinwood et al., Citation2011; Kenworthy et al., Citation2014). Further, our laboratory has shown that maltreated males (and females, though not statistically significant) also had lower levels of hdac1 mRNA levels compared to control males (Blaze & Roth, Citation2013). Because epigenetic changes to histones are present on a global level in other models, it may be useful to take a global approach to investigate histone acetylation in our model. Conversely, it may also be useful to look more specifically at other lysine residues on histones 3 and 4 as well as HDACs bound to acetylated histones at the various bdnf regulatory regions or reelin promoter.
Other histone modifications are also known to be affected by stress. In adults, both social defeat (Tsankova et al., Citation2006) and acute or chronic restraint stress (Hunter et al., Citation2009) alter histone methylation, both globally and at bdnf IV. Further, aberrant histone methylation has been associated with multiple psychiatric disorders (Akbarian & Huang, Citation2009; Huang et al., Citation2007). Less is known regarding histone methylation after early-life stress (Kao et al., Citation2012), but it is likely that our model elicits changes in histone methylation. It will be important in future studies to investigate repressive chromatin marks, such as H3K9 methylation, to see if these are likewise affected.
Finally, an emerging theme in the behavioral epigenetics literature is that of cell-type specificity. It would be useful to know the phenotype of cells affected by early-life maltreatment, since current methodologies (including ours here in this study) often use a heterogeneous population of cells including neurons and glia. Because methylation patterns have been shown to differ between these cell types (Li et al., Citation2014), future studies should distinguish epigenetic patterns between cell-populations in brain samples.
Conclusions
Although it is widely known that early-life stress exerts deleterious effects on brain function and mental health, the pursuit of mechanisms for these effects is ongoing. Using a rodent model, we have characterized changes in histone acetylation associated with bdnf and reelin DNA following exposure to caregiver maltreatment during the first week of life. Results indicate that adult females who experienced maltreatment in infancy have less bdnf IV histone acetylation, while there were no changes in bdnf I or reelin histone acetylation in either males or females. Together with our previous work, maltreated females appear to be especially vulnerable to epigenetic changes at bdnf IV. Additional research is needed to characterize how early-life stress, including maltreatment, affects histone acetylation at different loci and how these changes are reflected in downstream processes, from gene expression to behavior. Investigating epigenetic modifications to histones will also provide insights for the utility of pharmaceuticals with epigenetic targets (i.e. HDAC inhibitors) to reverse the negative effects of early-life stress (Abel & Zukin, Citation2008; Szyf, Citation2009).
Acknowledgements
We thank Hannah Evans, Samantha Jones, Hillary Porter, Brittany Rider, Lisa Scheuing, Angela Maggio, Sarah Pingar and Kristyn Borrelli for their help in generating animals, behavior coding and gel electrophoresis.
Declaration of interest
The authors report no conflicts of interest. This work was supported by a grant from the National Institute of General Medical Sciences (1P20GM103653).
References
- Abdolmaleky HM, Thiagalingam S, Wilcox M. (2005). Genetics and epigenetics in major psychiatric disorders. Am J Pharmacogenomics 5:149–60
- Abel T, Zukin RS. (2008). Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol 8:57–64
- Akbarian S, Huang H-S. (2009). Epigenetic regulation in human brain—focus on histone lysine methylation. Biol Psychiatry 65:198–203
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. (2006). The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci 256:174–86
- Baker-Andresen D, Ratnu VS, Bredy TW. (2013). Dynamic DNA methylation: a prime candidate for genomic metaplasticity and behavioral adaptation. Trends Neurosci 36:3–13
- Balkowiec A, Katz DM. (2002). Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci 22:10399–407
- Blaze J, Roth TL. (2012). Epigenetic mechanisms in learning and memory. WIRES Cogn Sci 4:105–15
- Blaze J, Roth TL. (2013). Exposure to caregiver maltreatment alters expression levels of epigenetic regulators in the medial prefrontal cortex. Int J Dev Neurosci 31:804–10
- Blaze J, Scheuing L, Roth TL. (2013). Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Dev Neurosci 35:306–16
- Boulle F, Van Den Hove DLA, Jakob SB, Rutten BP, Hamon M, Van Os J, Lesch KP, et al. (2012). Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry 17:584–96
- Branchi I, Karpova NN, D’andrea I, Castrén E, Alleva E. (2011). Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett 495:168–72
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. (2007). Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem 14:268–76
- Bremner JD, Elzinga B, Schmahl C, Vermetten E. (2007). Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res 167:171–86
- Cedar H, Bergman Y. (2009). Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. (2006). Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147:2909–15
- Cicchetti D, Toth SL. (2005). Child maltreatment. Annu Rev Clin Psychol 1:409–38
- D'Arcangelo G. (2014). Reelin in the years: controlling neuronal migration and maturation in the mammalian brain. Adv Neurosci 2014:1–9
- Eden S, Hashimshony T, Keshet I, Cedar H, Thorne AW. (1998). DNA methylation models histone acetylation. Nature 394:842
- Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. (2010). Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 68:408–15
- Fuchikami M, Morinobu S, Kurata A, Yamamoto S, Yamawaki S. (2009). Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int J Neuropsychopharmacol 12:73–82
- Gorka A, Hanson J, Radtke S, Hariri A. (2014). Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biol Mood Anxiety Disord 4:12
- Gross CM, Flubacher A, Tinnes S, Heyer A, Scheller M, Herpfer I, Berger M, et al. (2012). Early life stress stimulates hippocampal reelin gene expression in a sex-specific manner: evidence for corticosterone-mediated action. Hippocampus 22:409–20
- Gupta-Agarwal S, Franklin AV, Deramus T, Wheelock M, Davis RL, Mcmahon LL, Lubin FD. (2012). G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. J Neurosci 32:5440–53
- Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. (2012). Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci 32:7917–25
- Heim C, Nemeroff CB. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49:1023–39
- Hinwood M, Tynan RJ, Day TA, Walker FR. (2011). Repeated social defeat selectively increases ΔFosB expression and histone H3 acetylation in the infralimbic medial prefrontal cortex. Cereb Cortex 21:262–71
- Huang H-S, Matevossian A, Whittle C, Kim SY, Schumacher A, Baker SP, Akbarian S. (2007). Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci 27:11254–62
- Huang Y, Doherty JJ, Dingledine R. (2002). Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci 22:8422–8
- Hunter RG, Mccarthy KJ, Milne TA, Pfaff DW, Mcewen BS. (2009). Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proc Natl Acad Sci USA 106:20912–17
- Irvine RA, Lin IG, Hsieh C-L. (2002). DNA methylation has a local effect on transcription and histone acetylation. Mol Cell Biol 22:6689–96
- Ivy AS, Brunson KL, Sandman C, Baram TZ. (2008). Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154:1132–42
- Ivy AS, Rex CS, Chen Y, Dubé C, Maras PM, Grigoriadis DE, Gall CM, et al. (2010). Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J Neurosci 30:13005–15
- Kabir ZD, Kennedy B, Katzman A, Lahvis GP, Kosofsky BE. (2014). Effects of prenatal cocaine exposure on social development in mice. Dev Neurosci 36:338–46
- Kaffman A, Meaney MJ. (2007). Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry 48:224–44
- Kao G-S, Cheng L-Y, Chen L-H, Tzeng W-Y, Cherng CG, Su C-C, Wang C-Y, Yu L. (2012). Neonatal isolation decreases cued fear conditioning and frontal cortical histone 3 lysine 9 methylation in adult female rats. Eur J Pharmacol 697:65–72
- Kenworthy CA, Sengupta A, Luz SM, Ver Hoeve ES, Meda K, Bhatnagar S, Abel T. (2014). Social defeat induces changes in histone acetylation and expression of histone modifying enzymes in the ventral hippocampus, prefrontal cortex, and dorsal raphe nucleus. Neuroscience 264:88–98
- Khulan B, Manning JR, Dunbar DR, Seckl JR, Raikkonen K, Eriksson JG, Drake AJ. (2014). Epigenomic profiling of men exposed to early-life stress reveals DNA methylation differences in association with current mental state. Transl Psychiatry 4:e448
- Kundakovic M, Chen Y, Costa E, Grayson DR. (2007). DNA methyltransferase inhibitors coordinately induce expression of the human reelin and glutamic acid decarboxylase 67 genes. Mol Pharm 71:644–53
- Kundakovic M, Lim S, Gudsnuk K, Champagne FA. (2013). Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry 4:78
- Labonte B, Suderman M, Maussion G, Navaro L, Yerko V, Mahar I, Bureau A, et al. (2012). Genome-wide epigenetic regulation by early-life trauma. Arch Gen Psychiatry 69:722–31
- Levine A, Worrell TR, Zimnisky R, Schmauss C. (2012). Early life stress triggers sustained changes in histone deacetylase expression and histone H4 modifications that alter responsiveness to adolescent antidepressant treatment. Neurobiol Dis 45:488–98
- Li X, Baker-Andresen D, Zhao Q, Marshall V, Bredy TW. (2014). Methyl CpG Binding Domain Ultra-Sequencing: a novel method for identifying inter-individual and cell-type-specific variation in DNA methylation. Genes Brain Behav 13:721–31
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, et al. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 277:1659–62
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–8
- Lubin FD, Roth TL, Sweatt JD. (2008). Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci 28:10576–86
- Martinowich K, Manji H, Lu B. (2007). New insights into BDNF function in depression and anxiety. Nat Neurosci 10:1089–93
- McClelland S, Korosi A, Cope J, Ivy A, Baram TZ. (2011). Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol Learn Mem 96:79–88
- McGowan PO, Sasaki A, D'alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–8
- McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, Szyf M. (2011). Broad epigenetic signature of maternal care in the brain of adult rats. PLoS One 6:e14739
- Meaney MJ. (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24:1161–92
- Mitchell CP, Chen Y, Kundakovic M, Costa E, Grayson DR. (2005). Histone deacetylase inhibitors decrease reelin promoter methylation in vitro. J Neurochem 93:483–92
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, et al. (2009). Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci 12:1559–66
- Murgatroyd C, Spengler D. (2014). Polycomb binding precedes early-life stress responsive DNA methylation at the AVP enhancer. PLoS One 9:e90277
- Nagahara AH, Tuszynski MH. (2011). Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10:209–19
- Naumova OY, Lee M, Koposov R, Szyf M, Dozier M, Grigorenko EL. (2012). Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Dev Psychopathol 24:143–55
- Neigh GN, Gillespie CF, Nemeroff CB. (2009). The neurobiological toll of child abuse and neglect. Trauma Violence Abuse 10:389–410
- Paxinos G, Watson C. (2007). The rat brain in stereotaxic coordinates. 6th ed. Boston: Academic Press/Elsevier
- Portfors C. (2007). Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Ass Lab Anim Sci 46:28–34
- Provençal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, Bennett AJ, et al. (2012). The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J Neurosci 32:15626–42
- Raineki C, Moriceau S, Sullivan RM. (2010). Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry 67:1137–45
- Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA. (2004). Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry 55:708–14
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. (2009). Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65:760–9
- Roth TL, Matt S, Chen K, Blaze J. (2014). Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Dev Psychobiol 56:1755–63
- Roth TL, Sullivan RM. (2005). Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry 57:823–31
- Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha J-HJ, Pierce RC, Sadri-Vakili G. (2012). Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem 120:202–9
- Strahl BD, Allis CD. (2000). The language of covalent histone modifications. Nature 403:41–5
- Sui L, Wang Y, Ju LH, Chen M. (2012). Epigenetic regulation of reelin and brain-derived neurotrophic factor genes in long-term potentiation in rat medial prefrontal cortex. Neurobiol Learn Mem 97:425–40
- Szyf M. (2009). Epigenetics, DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol 49:243–63
- Takei S, Morinobu S, Yamamoto S, Fuchikami M, Matsumoto T, Yamawaki S. (2011). Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. J Psychiatr Res 45:460–8
- Tissir F, Goffinet AM. (2003). Reelin and brain development. Nat Rev Neurosci 4:496–505
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–25
- van Harmelen A-L, van Tol M-J, Dalgleish T, van der Wee NJA, Veltman DJ, Aleman A, Spinhoven P, et al. (2014). Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci 9:2026–33
- Weaver IC, Cervoni N, Champagne FA, D'alessio AC, Sharma S, Seckl JR, Dymov S, et al. (2004). Epigenetic programming by maternal behavior. Nat Neurosci 7:847–54
- Zhang J, Qin L, Zhao H. (2013). Early repeated maternal separation induces alterations of hippocampus reelin expression in rats. J Biosci 38:27–33