Abstract
Here we used a 3-dimensional (3D) maze, a modification of the radial maze, to assess the effects of treatment for two weeks with a single daily dose of fluoxetine (20 mg/kg, i.p.) on anxiety in male BALB/c mice. We examined whether anxiolytic effects of fluoxetine can be detected over three daily test sessions. We examined also whether repeated handling associated with chronic treatment interferes with effects of fluoxetine on anxiety responses. The 3D maze comprises nine arms, each connected to an upward inclined bridge radiating from a central platform. In this maze, BALB/c mice cross frequently into the bridges but avoid the arms. This avoidance is used as an index of anxiety. Two separate groups received once a day either saline (SALCH, n = 8) or fluoxetine (FLUCH, n = 8) for 14 days, and up to 30 min before the test during the subsequent 3 days. A third group received saline (SALAC, n = 8) 30 min before the test, once a day for 3 days. SALAC mice did not cross into the arms, and continued this avoidance over 3 sessions. SALCH mice avoided the arms in session 1 whereas FLUCH mice did cross into the arms, and like SALCH mice, increased number of crossings into and time on the arms in subsequent sessions. Fluoxetine evidently had an anxiolytic effect but only in the first session. These results indicate that handling experience decreased fear and anxiety in the mice, which may have masked the anxiolytic effect of fluoxetine in the second and third test sessions.
Introduction
Selective serotonin reuptake inhibitors (SSRIs) have been promoted as alternatives to benzodiazepines; they are commonly held as a first line pharmacological therapy for anxiety disorders and depression. However, their use is surrounded with issues regarding their onset of action (Gelenberg & Chesen, Citation2000; Mitchell, Citation2006; Nierenberg, Citation2001; Posternak & Zimmerman, Citation2005; Taylor et al., Citation2006) as well as their antidepressant and anxiolytic efficacy (Ioannidis Citation2008; Khin et al., Citation2011; Pigott et al., Citation2010; McAllister-Williams, Citation2008). It has been reported that the onset of action of SSRIs can be as long as six weeks after starting treatment (Gelenberg & Chesen, Citation2000; Nierenberg, Citation2001), and that the initial effect of treatment exacerbates anxiety (Gorman et al., Citation1987; Grillon et al., Citation2007). A delayed anxiolytic response to SSRIs has not been replicated by a number of independent groups (Mitchell, Citation2006; Posternak & Zimmerman, Citation2005; Taylor et al., Citation2006) which is possibly due to differences in the diagnosis and/or the baseline level of anxiety. In addition, remission is only achieved in one-third of patients and a large proportion of patients do not experience any response (Ballenger, Citation2004; Huh et al., Citation2011; Katzman, Citation2009). There are also a significant number of patients that withdraw from the treatment due to side effects and/or experience relapse at the end of the treatment period (Ballenger, Citation2004; Huh et al., Citation2011; Katzman, Citation2009).
Animal studies have demonstrated mixed results with the use of SSRIs on anxiety. Some studies reported an anxiogenic effect with acute (Birkett et al., Citation2011; Drapier et al., Citation2007; Gomes et al., Citation2009; Kurt et al., Citation2000; Robert et al., Citation2011; Silva & Brandão, Citation2000; Silva et al., Citation1999) and an anxiolytic effect with chronic (Gomes et al., Citation2009; Kurt et al., Citation2000; Nowakowska et al., Citation2000) treatments, whereas other studies reported an anxiolytic (Griebel et al., Citation1999; Nowakowska et al., Citation1996, Citation2000; Rogóz Skuza, Citation2011) or no effect (Durand et al., Citation1999; Knoll et al., Citation2007; Takeuchi et al., Citation2010) with acute treatments. Some studies reported also anxiogenic (Robert et al., Citation2011; Silva et al., Citation1999) or no effect (Durand et al., Citation1999; Griebel et al., Citation1999; Silva & Brandão, Citation2000; Takeuchi et al., Citation2010) with chronic treatments.
The above conflicting results were mostly obtained in unconditioned tests of anxiety, which have been reported to produce inconsistent results with a wide range of psychoactive compounds (Andrews et al., Citation2014; Cryan & Sweeney, Citation2011; Griebel & Holmes, Citation2013; Miczek & de Wit, Citation2008; Rodgers et al., Citation1995, Citation2002; Thompson et al., Citation2015). One of the major limitations of these tests is that they cannot be used for more than one session in screening for potential anxiolytic candidate drugs. Hence, it is possible that an initial reaction to a drug differs from its effects on further uses (Cole & Pieper, Citation1973; de Wit & Phillips, Citation2012), and may not predict its therapeutic potential. In addition, examination of the effect of SSRIs on anxiety involves administration of the drug for several days; this implies that animals are repeatedly handled when drugs are given by direct administration. This manipulation could affect animal responses to the anxiety test as reported in a number of studies (Andrews & File, Citation1993; Brett & Pratt, Citation1990; Kõks et al., Citation2001; Robert et al., Citation2011; Schmitt & Hiemke, Citation1998). Indeed, some studies reported that handling reduced the anxiolytic effect of diazepam (Brett & Pratt, Citation1990; File et al., Citation1992), chlordiazepoxide (File & Andrews, Citation1992), baclofen and zacopride (Andrews & File, Citation1993) and a corticotropin releasing factor (CRF) antagonist (Adamec et al., Citation1991), and reduced the anxiogenic effect of fluoxetine (Robert et al., Citation2011) and CRF (Adamec et al., Citation1991), or exacerbated the anxiogenic effect of buspirone (Andrews & File, Citation1993). In the present study, we used a 3-dimensional (3D) maze, which is a modified version of the radial arm maze (Ennaceur, Citation2011; Ennaceur et al., Citation2006, Citation2008a), to assess the effects of treatment for two weeks with a single daily dose of fluoxetine (20 mg/kg i.p.) on anxiety in male BALB/c mice. In the 3D maze, mice can be tested for a number of sessions, hence we examined whether the anxiolytic effects of fluoxetine can be detected over three test sessions. We hypothesized that an initial reaction to fluoxetine on a first test session may differ from its effects on subsequent test exposures. However, since chronic treatment involves handling of mice every day, this manipulation may also reduce anxiety, and could be confounded with an anxiolytic effect of fluoxetine. Hence, we also examined whether handling would interfere with the effects of fluoxetine an anxiety responses.
When exposed to an unfamiliar radial arm maze, rats and mice enter frequently into the proximal segment of an arm of the maze and do not continue into the distal segment. In the 3D maze, these proximal (bridges) and distal (arms) segments are clearly delineated. Animals are observed to reach the end of the first segment, then withdraw and return to the central platform. They seem unable to take a risk, and do not venture far away from the central platform. This avoidance of the distal segment is used as an indicator of fear and anxiety in mice. In previous studies (Ennaceur et al., Citation2006, Citation2008a,Citationb; Ennaceur Citation2011), we demonstrated that BALB/c mice, unlike C57BL/6J and CD-1 mice, did not venture into the arms of the maze when left to explore the maze for the first time. C57BL/6J and CD-1 mice visited several arms on the first and second exposure, respectively, while BALB/c required more than three sessions. We selected for this experiment mice of the BALB/c strain and the administration of three test sessions on the basis of these previous studies, which indicate a high level of anxiety in this strain of mice. In order to control the effect of handling on anxiety behavior, we introduced a group of mice that was handled in exactly the same way as the chronic fluoxetine-treated mice, except this group received saline injections. A third group of mice was not handled and received a single daily dose of saline just before each test session. In this report, handling is used to refer to the gentle removal of a mouse from its cage, the transfer to a beaker in which it is weighed, and the intraperitoneal administration of saline or fluoxetine. Repeated handling would involve habituation to these manipulations but also to the experimenter.
The validity of the open space anxiety tests, which include the 3D maze and the elevated platform with attached slopes, and the validity of the unconditioned tests of anxiety were discussed in a recent review (Ennaceur, Citation2014). The 3D maze offers a completely open space; there is no shelter or a wall against which animals can hide. It is based on our view (Ennaceur, Citation2014) that in anxiogenic conditions, humans and animals face an ambiguous situation. They are (or feel) unable to avoid/escape or approach the perceived threat stimulus (Ennaceur, Citation2014). Hence, a test of anxiety should expose animals to conditions that involve uninformative or ambiguous stimuli, and the outcomes from the choice between these stimuli are uncertain. When exposed to an open space, animals try to escape or explore to find a refuge. This motivation to escape is exploited in the 3D maze to provide measures of anxiety. Hence, escape routes are made available, but the distant segments of these routes (arms) are left inaccessible to immediate or direct sensory perception. The experience of fear from the unfamiliar and open space is therefore complicated by the ambiguity of the choices and the uncertainty of the choice outcomes. Entries into the distal segments of the test environment are used to determine anxiety in animals. As reported above, mice of the BALB/c strain, which are used in the present study, display a high level of anxiety. They make no entries into the arms in the first three test sessions, however in subsequent test exposures they enter into the arms and record more than eight arm visits in the fifth session. In comparison to mice given saline injections, fluoxetine-treated mice are expected to reach a minimum of nine arm visits, and an arm/bridge entries ratio at least equal to 0.5 in fewer test sessions.
Methods
Animals
Twenty-four male BALB/c mice (6–8 weeks old) were purchased from Charles River, UK. They were housed in Perspex cages, four per cage, and maintained under a 12 h:12 h light/dark cycle (light 07:00 h–19:00 h at 180 lux), in temperature (21 ± 2 °C) and humidity (50 ± 5%) controlled conditions. All mice had ad libitum access to standard laboratory chow and water in their home cage. The behavioral experiment was conducted during the light period of the cycle (09:00 h – 15:00 h). Mouse treatment and husbandry were in accordance with approved use of animals in scientific procedures regulated by the Animals (Scientific Procedures) Act 1986, UK. Ethical approval was obtained from the ethics committee at the University of Durham.
Drug treatments
Following at least two weeks acclimatization to the husbandry conditions, mice were randomly assigned to three groups (n = 8 per group). Two groups received an injection of saline while one group received an injection of fluoxetine 20 mg/kg (Griebel et al., Citation1999). The fluoxetine group (FLUCH) with a saline control group (SALCH) received their respective treatments for two weeks, and injections were continued to 30 min before each exposure to the 3D test apparatus during the subsequent three days of testing. The remaining control group (SALAC) only received a saline injection 30 min before each of the three test sessions. In each cage, one or two mice received fluoxetine while the remainder received saline. Fluoxetine was obtained from Tocris (UK), was dissolved in normal saline (0.9% NaCl) and administered by intraperitoneal (ip) injection in a volume of 0.1 ml per 10 g body weight of mice.
Apparatus
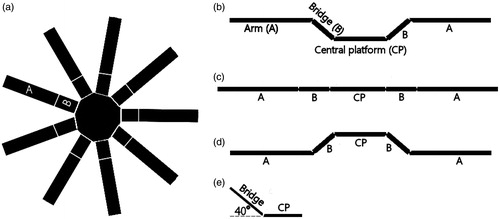
The 3D maze (Grey PVC, 5 mm thick) consisted of nine arms connected to bridges radiating from a central platform. Each arm (35 cm × 11.2 cm) was attached to a bridge (15.2 cm × 11.2 cm), which radiated from a nonagonal shaped central hub. Mice can access an arm by crossing a bridge. The bridges can be level with the arms providing a standard radial maze configuration. They can also be tilted upward or downward providing a maze with raised or lowered arm configurations, respectively () (Ennaceur et al., Citation2008a). All parts of the maze apparatus were unprotected; hence mice were exposed to a completely open space. In the present experiment, the bridge to each arm formed a slope which was inclined upward by about 40°. The maze was surrounded by a heavy pale beige-colored curtain, and it was placed in a sound attenuated room with a masking white noise of 68 dB. The illumination on the surface of the central platform was 180 Lux.
Figure 1. (a) Illustration of the nine arms 3D maze; (b) Raised arm configuration used in the present experiment; (c) Standard arm configuration; (d) Lowered arm configuration; (e) Angle of inclination of the bridge. B = bridge; A = arm; CP = central platform. See pictures of the maze in Ennaceur et al. (Citation2008b).
Test procedure
Behavioral testing was conducted between 09:00 h and 15:00 h. All mice were tested in the maze in three consecutive sessions, one session a day. A mouse was removed from its cage and placed in a 500 ml plastic beaker in which it was weighed. After an i.p. injection of saline or fluoxetine, a mouse was returned to its home cage where it was left for 30 min then transported in the beaker to the test apparatus. The beaker was tilted gently over the center platform of the maze for the release of the mouse which was then free to explore for 12 min. During the test, mice were observed on a screen monitor connected to a video camera suspended above the test arena. Using an in-house computer program (EventLog) we recorded in sequential order the start and end of each entry to the different parts of the maze. This record provided a variety of measures including frequency, latency, duration and the sequence order of each visit to the bridges and arms of the maze. An entry onto a bridge or an arm was recorded whenever a mouse crossed with all four paws the line that delimits these areas. After each test the surface of the platform was cleaned to minimize the effects of lingering olfactory cues. Any feces and urine on the maze were removed with paper towels, then cleaned with ethanol, and left to dry before the introduction of the next mouse.
Statistical analysis
This was performed using Statistica (v. 6, 1998, Statsoft, Tulsa, OK). All data are presented as mean and standard error of the mean (±SEM). Differences among group mean values for each measurement were tested for significance with two-way ANOVA repeated measures followed up with Newman–Keuls post-hoc comparisons. Results were considered significant with p < 0.05.
Results
Arm entries
There were significant differences between groups in the latency to first entry and in the number and duration of entries (F2,21 = 9.74 and 11.75 respectively, p < 0.001). There were also significant differences between sessions (F2,42 = 29.44 and 35.37 respectively, p < 0.001) and significant interactions between groups and sessions (F4,42 = 5.63 and 13.00 respectively, p < 0.001).
In chronic saline-treated mice, the latency of first crossing onto an arm () was significantly greater in session 1 than in sessions 2 (p < 0.03) and 3 (p < 0.001), and in session 2 compared to session 3 (p < 0.008). In fluoxetine-treated mice, the latency was significantly greater in sessions 1 and 2 compared to session 3 (p < 0.03 and p < 0.05, respectively). There were no significant differences between sessions 1 and 2 (p > 0.10).
Figure 2. Post-hoc comparisons between groups (Student Newman–Keuls). Each data point represents the mean ± s.e.m of each group in each test session. SALAC = Saline acute (n = 8); SALCH = Saline chronic (n = 8); FLUCH = Fluoxetine chronic (n = 8); s1 to s3 = session 1 to session 3. (a) *FLUCH compared to SALCH and SALAC in s1 (p < 0.02); •SALAC compared to SALCH and FLUCH in s2 (p < 0.02) and s3 (p < 0.0002); (b) In s1, *FLUCH compared to SALAC (p < 0.02) and SALCH (p < 0.01); in s2, •SALAC compared to SALCH (0.05) and FLUCH (0.03); in s3, •SALAC compared to SALCH (p < 0.0001) and FLUCH (p < 0.0003), ♣FLUCH compared to SALCH (p < 0.04); (c) In s1, *FLUCH compared to SALAC (0.02) and to SALCH (p < 0.008); in s2, •SALAC compared to SALCH (0.03) and FLUCH (p < 0.05); in s3, ♦SALAC compared to SALCH (0.0002) and FLUCH (p < 0.0007), ♣SALCH compared to FLUCH (p < 0.07).
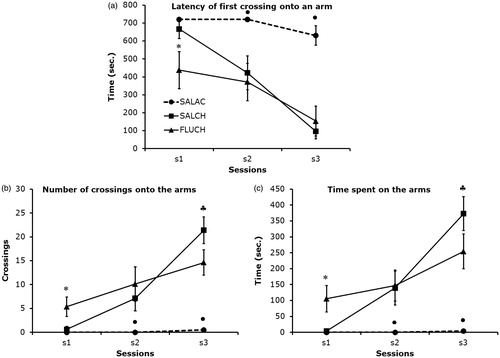
The latency to first entry onto an arm () was significantly greater in both acute and chronic saline-treated mice compared to fluoxetine-treated mice in session 1 (p < 0.02), and it was significantly greater in acute saline treated mice compared to chronic saline and fluoxetine treated mice in sessions 2 (p < 0.02) and 3 (p < 0.001). There were no significant differences between chronic saline and fluoxetine treated mice in sessions 2 and 3 (p > 0.10).
In chronic saline-treated mice, the number () and duration () of entries into the arms were significantly lower in session 1 compared to sessions 2 (p < 0.03) and 3 (p < 0.001), and in session 2 compared to session 3 (p < 0.006). In fluoxetine-treated mice, arm entries and duration were significantly lower in session 1 compared to sessions 2 (p < 0.05) and 3 (p < 0.01), and in session 2 compared to session 3 for the duration of entries (p < 0.03) but not for the number of entries (p > 0.10).
The number () and duration () of arm entries were significantly lower in both acute and chronic saline treated mice compared to fluoxetine treated mice in session 1 (p < 0.02), and significantly lower in acute saline treated mice compared to chronic saline and fluoxetine-treated mice in sessions 2 (p < 0.05) and 3 (p < 0.001). Chronic saline-treated mice had a lower number of entries compared to fluoxetine-treated mice in session 3 (p < 0.04) whereas the duration of entries was not significantly different (p < 0.07).
Bridge entries
There were no significant differences between groups in the latency to first entry, number or duration of bridge entries (F2,21 ≤ 2.81, p > 0.10) (). There were, however, significant differences between sessions (F2,42 = 8.03, p < 0.001) and significant interactions between groups and sessions (F4,42 = 7.01, p < 0.002) in the duration of bridge entries (). Repeated exposures to the test decreased the time spent on the bridges in the chronic saline treated mice. The duration of entries was significantly lower in sessions 2 and 3 compared to session 1 (p < 0.001), and in session 3 compared to session 2 (p < 0.008).
Figure 3. Post-hoc comparisons between groups (Student Newman–Keuls). Each data point represents the mean ± s.e.m of each group in each test session. SALAC = Saline acute (n = 8); SALCH = Saline chronic (n = 8); FLUCH = Fluoxetine chronic (n = 8); s1 to s3 = session 1 to session 3. (a) No significant differences between groups (p > 0.10); (b) *SALCH compared to SALAC and FLUCH in s1 (p < 0.02) and •SALCH compared to SALAC in s3 (p < 0.05); (c) *FLUCH compared to SALAC and SALCH in s1 (p < 0.02); •SALAC compared to SALCH and FLUCH in s2 (p < 0.05) and s3 (p < 0.001).
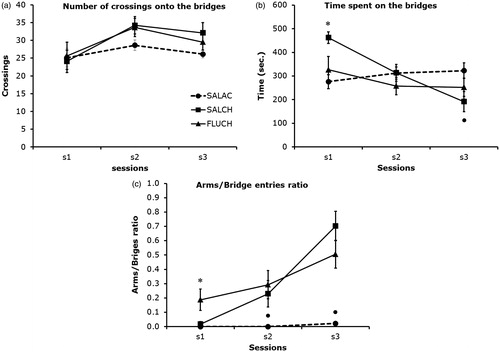
Chronic saline-treated mice spent more time on the bridges compared to both acute saline and chronic fluoxetine-treated mice in session 1 (p < 0.02) but they spent less time on the bridges compared to acute saline treated mice in session 3 (p < 0.05).
Arm/Bridge entries ratio
There were significant differences between groups (F2,21 = 10.34, p < 0.001), between sessions (F2,42 = 36.17, p < 0.001) and significant interactions between groups and sessions (4,42 = 11.35, p < 0.001). Repeated exposures to the test increased the arm/bridge ratio between sessions in both chronic saline and fluoxetine-treated mice (). The ratio was significantly greater in session 3 compared to sessions 1 (p < 0.005) and 2 (p < 0.05), and in session 2 compared to session 1 (p < 0.03).
In session 1, there were no significant differences between acute and chronic saline-treated mice (p > 0.10). However, the ratio was significantly lower in these two groups compared to fluoxetine-treated mice (p < 0.02). The ratio remained significantly lower in the acute saline treated mice compared to chronic saline and fluoxetine treated mice in sessions 2 (p < 0.05) and 3 (p < 0.001).
Discussion
In the present study, acute saline-treated mice that were exposed for the first time to the 3D maze did not cross into the arms, and continued to avoid the arms over three consecutive sessions, which confirms our previous reports (Ennaceur, Citation2011; Ennaceur et al., Citation2006, Citation2008a). However, chronic saline-treated mice avoided the arms, but only in the first session, whereas chronic fluoxetine-treated mice did cross onto the arms in the first session, and as with the chronic saline-treated mice, their number of crossings into and time spent on the arms increased in subsequent sessions. The results for chronic saline treated mice indicate clearly that repeated handling during daily injection did reduce anxiety responses to the 3D maze, and the anxiolytic effect of fluoxetine was confounded by this manipulation. The initial response of chronic saline-treated mice to the test discriminated between the effects of these two manipulations on anxiety, as hypothesized.
It is unlikely that fluoxetine had a facilitation effect on locomotion that would have led mice to venture onto the arms; the effect of chronic saline-treatment was not different from that of fluoxetine. In addition, there were no differences between groups in the latency to first entry and number of entries onto the bridges, which indicate that all groups of mice were mobile, able to explore and climb the inclined bridges. Furthermore, it is unlikely that a drug which induces hyperactivity or impulsivity would facilitate an increase in the number of arm entries, such that the arm/bridge entries ratio is progressively increased in subsequent test exposures. In a comparable open space anxiety test (Ennaceur et al., Citation2010), different doses of amphetamine increased more than three-fold the number of crossings on the surface of the platform in BALB/c mice, but none of the treated mice crossed on to the slopes attached on two opposite sides of the platform. We expect that amphetamine treatment of mice exposed to the 3D maze would increase the number of bridge entries without facilitating entries onto the arms. This will be examined in a future experiment.
In the present experiment, fluoxetine evidently produced an anxiolytic effect but this was seen only in the first 3D maze exposure. This result indicates that repeated handling experience during the chronic treatment period did affect anxiety responses, by decreasing fear and anxiety in the mice, and this may have masked the anxiolytic effect of fluoxetine in the second and third test sessions. Handling experience, however, did not prevent an initial spontaneous anxiety response in chronic saline treated mice. Exposure to novelty (3D maze) appears to facilitate the “return of fear” which can be accounted for by the dishabituation phenomenon. Dishabituation refers to the fast recovery of a response (fear and anxiety) that has undergone habituation. This response can be transient and occurs after the presentation of a novel stimulus (Rachman, Citation1989; Thompson & Spencer, Citation1966).
Handling experience has been reported to decrease anxiety in rats and mice (Andrews & File, Citation1993; Boix et al., Citation1988; Brett & Pratt, Citation1990; Costa et al., Citation2012; Robert et al., Citation2011; Schmitt & Hiemke, Citation1998), and it evidently prevents the anxiolytic effects of benzodiazepines and antidepressant drugs (Andrews & File, Citation1993; Boix et al., Citation1988; Brett & Pratt, Citation1990; Kõks et al., Citation2001; Robert et al., Citation2011). This is puzzling as one would expect that habituation to handling which has been shown to reduce fear and anxiety can only facilitate, rather than oppose, the anxiolytic effect of a drug. Our results suggest that handling did not prevent but rather masked the anxiolytic effect of fluoxetine; mice treated with this drug continued to venture onto the arms of the 3D maze. Further experiments are needed to demonstrate whether an anxiogenic drug would prevent the effect of handling and/or prevent habituation to repeated exposures to the 3D maze.
In the 3D maze, anxious mice (BALB/c) do not cross onto an arm in the first test session and the least anxious mice (C57BL/6J and CD-1) make a few crossings. However, in subsequent sessions the number of crossings does progressively increase though this takes much longer for BALB/c compared to C57BL/6J and CD-1 mice (Ennaceur, Citation2011; Ennaceur et al., Citation2006). Hence, in the 3D maze, the anxiolytic effect of a drug should not be expected to decrease with repeated exposures to the test; it can be masked, however, by the progressive improvement in the performance of the control group. This behavioral profile of mice exposed to the 3D maze differs considerably from that observed in the current unconditioned tests of anxiety.
In the plus-maze, animals are reported to display an aversion to the open-arms from the second minute of a test session, and this aversion is increased further throughout the test session, and in subsequent sessions (Arabo et al., Citation2014; Casarrubea et al., Citation2013; Espejo, Citation1997; Holmes & Rodgers, Citation1998; Rosa et al., Citation2000; Treit et al., Citation1993). In addition, a single previous experience of the plus-maze or light/dark box has been reported to reduce or abolish the effects of both anxiolytic and anxiogenic drugs (Dawson et al., Citation1994; Escarabajal et al., Citation2003; Holmes & Rodgers, Citation2003; Rodgers & Shepherd, Citation1993). Furthermore, this persistent aversion to the open-arms and this “one-trial tolerance” has been reported for both anxious and less anxious strains of mice and rats. Numerous interpretations have been provided to account for these behaviors, but none have considered the possibility that the current unconditioned tests of anxiety promote a natural preference for a protected and/or an unlit space over risk taking (Ennaceur, Citation2014). A number of studies suggest that, in a natural or experimental open field environment, the primary function of the behavior of mice and rats is to optimize security (Alstott et al., Citation2009; Whishaw et al., Citation2006; Yaski & Eilam, Citation2007). Hence, whether impulsivity, curiosity or attempt to find an escape route would have led animals initially to make a few entries into the open and/or lit space, these entries can only decline within and between sessions. The prevalence of security and safety provided by the enclosed spaces is likely to reduce or eliminate the incentive to explore the other parts of a test apparatus which are lit and/or unprotected. Indeed, in our previous studies, when a refuge was provided during the test, both anxious (BALB/c) and less anxious (C57/BL6J and CD-1) strains of mice did not venture onto the arms of the 3D maze (Ennaceur et al., Citation2008a) or onto the steep slopes attached to an elevated platform (Michalikova et al., Citation2010); they spent most of the time inside the refuge. These results are supported by other studies, which suggest that the behavior of rats and mice in a novel environment is directed toward optimizing safety (Alstott et al., Citation2009; Whishaw et al., Citation2006; Yaski & Eilam, Citation2007). Rats and mice, like other predated animals in the wild, are most likely to experience anxiety when they are in the open than when they are hiding in a burrow. The interpretation of the behavior of rodents in the current unconditioned tests of anxiety suggests the opposite; avoidance of the open/lit space is considered indicative of high anxiety though most, if not all, authors describe the selection of the protected/unlit space as a natural preference response. It has been difficult to challenge this paradox. The anxiety construct validity of the current unconditioned tests is defended on the basis that these tests involve a conflict, which has not been demonstrated behaviorally. Where is the objective evidence that animals are interested in visiting the open/lit space? Even if animals “appear” interested, why would staying in the protected/unlit space indicate anxiety rather than a sense of safety and security? A behavioral test is meant to provide an unequivocal measure of the observed response to the test; hence, no disagreements should occur between independent observers on the measurement of a specific behavioral response. In the 3D maze, animals that express high anxiety through avoidance of the arms in the first test sessions do visit the arms after a number of exposures to the test or, as shown in this report, after habituation to handling and to fluoxetine. The motivation to explore the arms is evident with both low and high anxiety strains as the number of entries increase and exceed nine arm visits with further exposures, and the number of bridge entries decrease to the point where arm/bridge entries ratio is very close or equal to 1 (Ennaceur, Citation2011; Ennaceur et al., Citation2006, Citation2011). In the plus-maze, however, if animals made a few entries into the open arms, these entries decline to a floor level in a subsequent exposure whether they represent a strain of animals with low or high anxiety (Arabo et al., Citation2014; Espejo, Citation1997; Holmes & Rodgers, Citation1998; Treit et al., Citation1993), and whether they received a saline or a drug injection (Dawson et al., Citation1994; Escarabajal et al., Citation2003; File et al., Citation1992; Holmes & Rodgers, Citation1998; Rodgers & Shepherd, Citation1993). In addition, the above evidence from the 3D maze and the elevated platform, in which a refuge was available to animals, does not support the anxiety conflict hypothesis attributed to the plus-maze (Ennaceur et al., Citation2008a; Michalikova et al., Citation2010).
The present experiment comprised a chronic saline treated group but did not include a chronic handled untreated group; this might be considered as a limitation to our results. In the present study, handling experience, as a result of systemic injection, reduced anxiety compared to acute saline injections given to controls; a similar result was observed in the plus-maze with rats (Robert et al., Citation2011). It seems unlikely that a different outcome would have been obtained with chronic handled untreated mice. Such a group would have been necessary if handling associated with the injection procedure did increase anxiety. In such a case, we would have to distinguish whether the handling experience or the intraperitoneal injection led to that effect. If we consider that needle insertion in the peritoneum can be stressful, then it seems unlikely that such intervention every day over two weeks would lead to a reduction in anxiety response. Rats injected with saline chronically were found to demonstrate reduced number of crossings and rearing in an open-field (Izumi et al., Citation1997) and reduced open arm entries in the plus-maze (Adamec et al., Citation1991; Mòdol et al., Citation2013; Kofman et al., Citation2000) compared to untreated and unhandled rats. Other studies reported no differences between chronic saline injected and chronic handled rats (Cloutier & Newberry, Citation2008) and between chronic saline injected and non-treated mice (Rodgers et al., Citation1997) in the plus-maze. In addition, handling habituated animals are less anxious than non-handled animals (Andrews & File, Citation1993; Andrews et al., Citation1992; Boix et al., Citation1988), and both handling habituation with or without saline injection were found to block (probably mask) the anxiolytic effect of diazepam (Andrews et al., Citation1992; Boix et al., Citation1988), baclofen and zacopride (Andrews & File, Citation1993) and the anxiogenic effect of fluoxetine (Robert et al., Citation2011) and CRF (Adamec et al., Citation1991) in the plus-maze. All these studies do not comprise the full experimental design that would control for the separate effect of chronic systemic injection and habituation to handling. Providing animals with the drug in their drink or food can overcome this issue of handling; however, this option does not control for the drug dose received by each individual animal. A better alternative is to deliver a drug using an osmotic mini-pump. There are few behavioral pharmacology studies that use this technology.
The present study confirms the consistency of the behavioral profile of BALB/c mice in the 3D maze. It also demonstrates that repeated handling, as a result of daily systemic injection, reduced anxiety in BALB/c mice, and that such an effect could have masked the anxiolytic effect of fluoxetine. This sensitivity of the 3D maze, however, remains to be challenged in independent laboratories. The open space anxiety tests, which comprise the 3D maze and the elevated platform with slopes, were developed to overcome the methodological shortcomings of the current unconditioned tests of anxiety. Hence, it may not be expected that the present findings will be replicated in findings obtained in the latter. However, it remains to be seen whether the 3D maze can be used to predict the anxiolytic effects of novel drug compounds.
Acknowledgments
We are very grateful to Professor John Russell for his constructive comments and helpful suggestions, which contributed significantly in improving the quality of the present report.
Declaration of interest
The authors report no conflicts of interest.
References
- Adamec RE, Sayin U, Brown A. (1991). The effects of corticotrophin releasing factor (CRF) and handling stress on behavior in the elevated plus-maze test of anxiety. J Psychopharmacol 5:175–86
- Alstott J, Timberlake W. (2009). Effects of rat sex differences and lighting on locomotor exploration of a circular open field with free-standing central corners and without peripheral walls. Behav Brain Res 96:214–19
- Andrews N, File SE. (1993). Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. Eur J Pharmacol 235:109–12
- Andrews N, Zharkovsky A, File SE. (1992). Acute handling stress downregulates benzodiazepine receptors: reversal by diazepam. Eur J Pharmacol 210:247–51
- Andrews NA, Papakosta M, Barnes NM. (2014). Discovery of novel anxiolytic agents – the trials and tribulations of pre-clinical models of anxiety. Neurobiol Dis 61:72–8
- Arabo A, Potier C, Ollivier G, Lorivel T, Roy V. (2014). Temporal analysis of free exploration of an elevated plus-maze in mice. J Exp Psychol Anim Learn Cogn 40:457–66
- Ballenger JC. (2004). Remission rates in patients with anxiety disorders treated with paroxetine. J Clin Psychiatry 65:1696–707
- Birkett MA, Shinday NM, Kessler EJ, Meyer JS, Ritchie S, Rowlett JK. (2011). Acute anxiogenic-like effects of selective serotonin reuptake inhibitors are attenuated by the benzodiazepine diazepam in BALB/c mice. Pharmacol Biochem Behav 98:544–51
- Boix F, Fernández Teruel A, Tobeña A. (1988). The anxiolytic action of benzodiazepines is not present in handling-habituated rats. Pharmacol Biochem Behav 31:541–6
- Brett RR, Pratt JA. (1990). Chronic handling modifies the anxiolytic effect of diazepam in the elevated plus-maze. Eur J Pharmacol 178:135–8
- Casarrubea M, Roy V, Sorbera, F, Magnusson MS, Santangelo A, Arabo A. (2013). Temporal structure of the rat’s behavior in elevated plus maze test. Behav Brain Res 237:290–9
- Cloutier S, Newberry RC. (2008). Use of a conditioning technique to reduce stress associated with repeated intra-peritoneal injections in laboratory rats. Appl Anim Behav Sci 112:158–73
- Cole JM, Pieper WA. (1973). The effects of N,N-dimethyltryptamine on operant behavior in squirrel monkeys. Psychopharmacologia 29:107–12
- Costa R, Tamascia ML, Nogueira MD, Casarini DE, Marcondes FK. (2012). Handling of adolescent rats improves learning and memory and decreases anxiety. J Am Assoc Lab Anim Sci 51:548–53
- Cryan JF, Sweeney FF. (2011). The age of anxiety: role of animal models of anxiolytic action in drug discovery. Br J Pharmacol 164:1129–61
- Dawson GR, Crawford SP, Stanhope KJ, Iversen SD, Tricklebank MD. (1994). One-trial tolerance to the effects of chlordiazepoxide on the elevated plus-maze may be due to locomotor habituation, not repeated drug exposure. Psychopharmacology (Berlin) 113:570–2
- de Wit H, Phillips TJ. (2012). Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev 36:1565–76
- Drapier D, Bentué-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, Reymann JM. (2007). Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res 176:202–9
- Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormède P, Chaouloff F. (1999). Effects of repeated fluoxetine on anxiety-related behaviours, central serotonergic systems, and the corticotropic axis in SHR and WKY rats. Neuropharmacology 38:893–907
- Ennaceur A. (2011). Omission of the habituation procedure in the acquisition of a working memory task – evidence from Balb/c, C57BL/6J, and CD-1 mice. Behav Brain Res 223:203–10
- Ennaceur A. (2014). Tests of unconditioned anxiety – pitfalls and disappointments. Physiol Behav 135:55–71
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. (2008a). Are benzodiazepines really anxiolytic? Evidence from a 3D maze spatial navigation task. Behav Brain Res 188:136–53
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. (2008b). Detailed analysis of the behavior and memory performance of middle-aged male and female CD-1 mice in a 3D maze. Behav Brain Res 187:312–26
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. (2010). Distinguishing anxiolysis and hyperactivity in a novel open space anxiety test. Behav Brain Res 207:84–98
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. (2011). MK-801 increases the baseline level of anxiety in mice introduced to a spatial memory task without prior habituation. Neuropharmacology 61:981–91
- Ennaceur A, Michalikova S, van Rensburg R, Chazot PL. (2006). Models of anxiety: responses of mice to novelty and open spaces in a 3D maze. Behav Brain Res 174:9–38
- Escarabajal MD, Torres C, Flaherty CF. (2003). The phenomenon of one-trial tolerance to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze test is abolished by previous administration of chlordiazepoxide or buspirone. Life Sci 73:1063–74
- Espejo EF. (1997). Effects of weekly or daily exposure to the elevated plus-maze in male mice. Behav Brain Res 87:233–8
- File SE, Andrews N, Wu PY, Zharkovsky A, Zangrossi H. (1992). Modification of chlordiazepoxide’s behavioural and neurochemical effects by handling and plus-maze experience. Eur J Pharmacol 218:9–14
- Gelenberg AJ, Chesen CL. (2000). How fast are antidepressants? J Clin Psychiatry 61:712–21
- Gomes KS, de Carvalho-Netto EF, Monte KC, Acco B, Nogueira PJ, Nunes-de-Souza RL. (2009). Contrasting effects of acute and chronic treatment with imipramine and fluoxetine on inhibitory avoidance and escape responses in mice exposed to the elevated T-maze. Brain Res Bull 78:323–7
- Gorman JM, Liebowitz MR, Fyer AJ, Goetz D, Campeas RB, Fyer MR, Davies SO, Klein DF. (1987). An open trial of fluoxetine in the treatment of panic attacks. J Clin Psychopharmacol 7:329–32
- Griebel G, Cohen C, Perrault G, Sanger DJ. (1999). Behavioral effects of acute and chronic fluoxetine in Wistar-Kyoto rats. Physiol Behav 67:315–20
- Griebel G, Holmes A. (2013). 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov 12:667–687
- Grillon C, Levenson J, Pine DS. (2007). A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology 32:225–31
- Holmes A, Rodgers RJ. (1998). Responses of Swiss-Webster mice to repeated plus-maze experience: further evidence for a qualitative shift in emotional state? Pharmacol Biochem Behav 60:473–88
- Holmes A, Rodgers RJ. (2003). Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol 459:221–30
- Huh J, Goebert D, Takeshita J, Lu BY, Kang M. (2011). Treatment of generalized anxiety disorder: a comprehensive review of the literature for psychopharmacologic alternatives to newer antidepressants and benzodiazepines. Prim Care Companion CNS Disord 13:PCC.08r00709
- Ioannidis JPA. (2008). Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials. Philos Ethics Humanit Med 3:14
- Izumi J, Washizuka M, Hayashi-Kuwabara Y, Yoshinaga K, Tanaka Y, Ikeda Y, Kiuchi Y, Oguchi K. (1997). Evidence for a depressive-like state induced by repeated saline injections in Fischer 344 rats. Pharmacol Biochem Behav 57:883–8
- Katzman MA. (2009). Current considerations in the treatment of generalized anxiety disorder. CNS Drugs 23:103–20
- Khin NA, Chen YF, Yang Y, Yang P, Laughren TP. (2011). Exploratory analyses of efficacy data from major depressive disorder trials submitted to the US Food and Drug Administration in support of new drug applications. J Clin Psychiatry 72:464–72
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA Jr. (2007). Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther 323:838–45
- Kofman O, Einat H, Cohen H, Tenne H, Shoshana C. (2000). The anxiolytic effect of chronic inositol depends on the baseline level of anxiety. J Neural Transm 107:241–53
- Kõks S, Beljajev S, Koovit I, Abramov U, Bourin M, Vasar E. (2001). 8-OH-DPAT, but not deramciclane, antagonizes the anxiogenic-like action of paroxetine in an elevated plus-maze. Psychopharmacology (Berlin) 153:365–72
- Kurt M, Arik AC, Celik S. (2000). The effects of sertraline and fluoxetine on anxiety in the elevated plus-maze test in mice. J Basic Clin Physiol Pharmacol 11:173–80
- McAllister-Williams RH. (2008). Do antidepressants work? A commentary on “Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration” by Kirsch et al. Evid Based Ment Health 11:66–8
- Michalikova S, van Rensburg R, Chazot PL, Ennaceur A. (2010). Anxiety responses in Balb/c, c57 and CD-1 mice exposed to a novel open space test. Behav Brain Res 207:402–17
- Miczek KA, de Wit H. (2008). Challenges for translational psychopharmacology research—some basic principles. Psychopharmacology (Berlin) 199:291–301
- Mitchell AJ. (2006). Two-week delay in onset of action of antidepressants: new evidence. Br J Psychiatry 188:105–6
- Mòdol L, Darbra S, Vallèe M, Pallarès M. (2013). Alteration of neonatal Allopregnanolone levels affects exploration, anxiety, aversive learning and adult behavioural response to intrahippocampal neurosteroids. Behav Brain Res 241:96–104
- Nierenberg AA. (2001). Do some antidepressants work faster than others? J Clin Psychiatry 15:22–5
- Nowakowska E, Kus K, Chodera A, Rybakowski J. (2000). Behavioural effects of fluoxetine and tianeptine, two antidepressants with opposite action mechanisms, in rats. Arzneimittelforschung 50:5–10
- Nowakowska E, Chodera A, Kus K. (1996). Anxiolytic and memory improving activity of fluoxetine. Pol J Pharmacol 48:255–60
- Pigott HE Leventhal AM, Alter GS, Boren JJ. (2010). Efficacy and effectiveness of antidepressants: current status of research. Psychother Psychosom 79:267–79
- Posternak MA, Zimmerman M. (2005). Is there a delay in the antidepressant effect? A meta-analysis. J Clin Psychiatry 66:148–58
- Rachman S. (1989). The return of fear: review and prospect. Clin Psychol Rev 9:147–68
- Robert G, Drapier D, Bentué-Ferrer D, Renault A, Reymann JM. (2011). Acute and chronic anxiogenic-like response to fluoxetine in rats in the elevated plus-maze: modulation by stressful handling. Behav Brain Res 220:344–8
- Rodgers RJ, Cole JC, Aboualfa K, Stephenson LH. (1995). Ethopharmacological analysis of the effects of putative ‘anxiogenic' agents in the mouse elevated plus-maze. Pharmacol Biochem Behav 52:805–13
- Rodgers RJ, Cutler MG, Jackson JE. (1997). Behavioural effects in mice of subchronic buspirone, ondansetron and tianeptine. II. The elevated plus-maze. Pharmacol Biochem Behav 56:295–303
- Rodgers RJ, Davies B, Shore R. (2002). Absence of anxiolytic response to chlordiazepoxide in two common background strains exposed to the elevated plus-maze: importance and implications of behavioural baseline. Genes Brain Behav 1:242–51
- Rodgers RJ, Shepherd JK. (1993). Influence of prior maze experience on behaviour and response to diazepam in the elevated plus-maze and light/dark tests of anxiety in mice. Psychopharmacology (Berlin) 113:237–42
- Rogóz Z, Skuza G. (2011). Anxiolytic-like effects of olanzapine, risperidone and fluoxetine in the elevated plus-maze test in rats. Pharmacol Rep 63:1547–52
- Rosa VP, Vandresen N, Calixto AV, Kovaleski DF, Faria MS. (2000). Temporal analysis of the rat's behavior in the plus-maze: Effect of midazolam. Pharmacol Biochem Behav 67:177–82
- Schmitt U, Hiemke C. (1998). Strain differences in open-field and elevated plus-maze behavior of rats without and with pretest handling. Pharmacol Biochem Behav 59:807–11
- Silva MT, Alves CR, Santarem EM. (1999). Anxiogenic-like effect of acute and chronic fluoxetine on rats tested on the elevated plus-maze. Braz J Med Biol Res 32:333–9
- Silva RC, Brandão ML. (2000). Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: an ethological analysis. Pharmacol Biochem Behav 65:209–16
- Takeuchi T, Owa T, Nishino T, Kamei C. (2010). Assessing anxiolytic-like effects of selective serotonin reuptake inhibitors and serotonin-noradrenaline reuptake inhibitors using the elevated plus maze in mice. Methods Find Exp Clin Pharmacol 32:113–21
- Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. (2006). Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry 63:1217–23
- Thompson RF, Spencer WA. (1966). Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73:16–43
- Thompson T, Grabowski-Boase L, Tarantino LM. (2015). Prototypical anxiolytics do not reduce anxiety-like behavior in the open field in C57BL/6J mice. Pharmacol Biochem Behav 133:7–17
- Treit D, Menard J, Royan C. (1993). Anxiogenic stimuli in the elevated plus-maze. Pharmacol Biochem Behav 44:463–9
- Whishaw IQ, Gharbawie OA, Clark BJ, Lehmann H. (2006). The exploratory behavior of rats in an open environment optimizes security. Behav Brain Res 171:230–9
- Yaski O, Eilam D. (2007). The impact of landmark properties in shaping exploration and navigation. Anim Cogn 10:415–28

