Abstract
Given the well-documented deleterious health effects, poor sleep has become a serious public health concern and increasing efforts are directed toward understanding underlying pathways. One potential mechanism may be stress and its biological correlates; however, studies investigating the effects of poor sleep on a body’s capacity to deal with challenges are lacking. The current study thus aimed at testing the effects of sleep quality and quantity on cortisol responses to acute psychosocial stress. A total of 73 college-aged adults (44 females) were investigated. Self-reported sleep behavior was assessed via the Pittsburgh Sleep Quality Index and salivary cortisol responses to the Trier Social Stress Test were measured. In terms of sleep quality, we found a significant three-way interaction, such that relative to bad sleep quality, men who reported fairly good or very good sleep quality showed blunted or exaggerated cortisol responses, respectively, while women’s stress responses were less dependent on their self-reported sleep quality. Contrarily, average sleep duration did not appear to impact cortisol stress responses. Lastly, participants who reported daytime dysfunctions (i.e. having trouble staying awake or keeping up enthusiasm) also showed a trend to blunted cortisol stress responses compared to participants who did not experience these types of daytime dysfunctions. Overall, the current study suggests gender-specific stress reactivity dysfunctions as one mechanism linking poor sleep with detrimental physical health outcomes. Furthermore, the observed differential sleep effects may indicate that while the body may be unable to maintain normal hypothalamic-pituitary-adrenal functioning in an acute psychosocial stress situation after falling prey to low sleep quality, it may retain capacities to deal with challenges during extended times of sleep deprivation.
Introduction
Sleep is a naturally-occuring state of decreased consciousness as well as reduced sensory and motor activity (Hays & Stewart, Citation1992). Humans engage in sleep for an average of 7–8 h per 24-h period (Basner et al., Citation2007). Poor sleep behavior occurring over a limited time period induces changes in mood as well as diminishes alertness and cognitive performance. However, the effects of poor sleep behavior are cumulative (Veasey et al., Citation2002). More specifically, chronic poor sleep and chronic insomnia have been linked to elevated risks of negative physiological and mental health outcomes (Spiegel et al., Citation1999; Taylor et al., Citation2003). Given the deleterious health effects of both chronic low sleep quality and chronic sleep restriction, it is crucial to increase our understanding of the pathways underlying these associations.
Several lines of evidence suggest stress and its related physiological changes as a possible link between poor sleep and detrimental health outcomes. Specifically, findings from both animal and human studies point toward bidirectional effects between sleep and the hypothalamic-pituitary-adrenal (HPA) axis (Buckley & Schatzberg, Citation2005; Steiger, Citation2002). HPA axis activity is thereby typically assessed by the circadian rhythm of its end hormone, cortisol (Wust et al., Citation2000), and several studies exist linking variation in diurnal cortisol secretion patterns to sleep behavior (Backhaus et al., Citation2004; Fries et al., Citation2009; Kumari et al., Citation2009; Raikkonen et al., Citation2010; Wust et al., Citation2000). HPA axis dysfunctions, in turn, have consistently been shown to impact physiological and mental health (Chrousos & Gold, Citation1992), thus presenting a pathway contributing to the effects of poor sleep on health (Minkel et al., Citation2014).
Importantly, HPA axis dysfunctions are thought to result from wear and tear due to repeated or chronic activation of the HPA axis (McEwen, Citation1998). As such, changes in acute HPA axis reactivity patterns may precede HPA axis activity dysfunctions and serve as an early warning sign for elevated health risks. However, human studies testing the associations between sleep and HPA axis reactivity in the context of acute stress are scarce and, to date, we are aware of only three studies. One demonstrated that poor-quality sleepers show exacerbated cortisol responses to a physiological stressor in the form of a cold-pressor task (Goodin et al., Citation2012). The second and third studies used a psychosocial stressor in children and adolescents, linking low sleep efficiency to increased cortisol reactivity (Pesonen et al., Citation2012; Raikkonen et al., Citation2010). Thus, it is unknown whether sleep effects on stress responses in adults generalize from physiological stressors to psychosocial stressors, or whether associations between sleep and psychosocial stress responses observed in children and adolescents generalize to adults.
Hence, the current study aimed at investigating effects of sleep habits on acute responses to psychosocial stress in healthy adults. Furthermore, the findings above do not allow any inferences with regard to which sleep dimension may interfere the most with cortisol responses to acute stress. In more detail, measures included “sleep quality” assessed via self-report over the last month (Goodin et al., Citation2012), “sleep efficiency” and “sleep duration” assessed via actigraphy over 7 d (Raikkonen et al., Citation2010), and “sleep problems” such as disorders of initiating sleep and excessive daytime somnolence (Pesonen et al., Citation2012). The current study thus further aimed at exploring the effects of various sleep dimensions, specifically sleep duration, sleep quality, and daytime dysfunctions on cortisol responses to acute psychosocial stress. Lastly, given gender-dependent differences in sleep behavior (Burgard et al., Citation2010) as well as sleep–stress associations (Pesonen et al., Citation2012), gender was considered a moderator of links between sleep habits and cortisol stress responses.
Methods
Participants
A total of 85 undergraduates and community members were assessed. Complete data were available for analyses from 73 participants (44 females; 19.69 ± 2.36 years; body mass index [BMI] = 23.32 ± 4.08 kg/m2). Participants thereby had to be excluded from analyses due to incomplete descriptive information (n = 1), missing Pittsburgh Sleep Quality Index (PSQI) data (n = 2), incomplete cortisol data (n = 5), missing PSQI and cortisol data (n = 1), or abnormally high cortisol values (>2 SD above the mean; n = 3) indicating potential illness or chronic stress.
Participants were recruited via a student subject pool, flyers around campus, and via newspaper advertisements geared toward general community members. Exclusion criteria were habitual smoking, acute diseases (e.g., a flu), current or a history of psychiatric or physiological chronic diseases (e.g., depression, atopic diseases, cardiovascular diseases), medication indicating psychiatric or physiological chronic diseases, or medication interfering with sleep or stress hormone responses (e.g., oral contraceptives; Rohleder et al., Citation2001). The local Institutional Review Board approved the protocol, and participants were compensated either monetarily or with study credits.
Procedure
After a phone screening, eligible participants were scheduled to visit the laboratory on a weekday afternoon to minimize inter-individual differences in basal cortisol levels (Kirschbaum & Hellhammmer, Citation2007). The study protocol was explained and informed consent was obtained. During a subsequent resting period of 30 min allowing participants to acclimate and recover from potential earlier stressors, participants completed self-report demographic and health questionnaires as well as the PSQI. Prior to exposure to a psychosocial stress test protocol (Trier Social Stress Test [TSST]), a pre-stress baseline saliva sample was taken using the Salivette collection device, and participants were lead to a separate testing room to complete the TSST. Upon task completion, participants returned to their study rooms and provided a second saliva sample (1-min post-TSST). Additional saliva samples were taken 10-, 20-, 30-, and 45-min post-TSST. During this time, participants were debriefed and after collection of the last saliva sample, participants received either money or study credit and were free to leave.
Measures and apparatus
Self-report assessments
Sleep behavior. The 19-item PSQI was used to measure sleep behavior over the past month (Buysse et al., Citation1989). An overall sleep quality score is computed (range 0–21) by summing seven subscale scores (range 0–3), each representing responses or combinations of responses to items measuring various dimensions of sleep. Higher PSQI values thereby indicate worse sleep quality. To address the study aim concerning potential sleep dimension-specific effects on cortisol stress responses, subscales aligning with components identified by Cole et al. (Citation2006) as a result of a principal component analysis of the PSQI were assessed (Cole et al., Citation2006). These include “average hours of sleep” (During the past month, how many hours of actual sleep did you get at night?), “sleep quality” (During the past month, how would you rate your sleep quality overall?) and “daytime dysfunction” (During the past month, how often have you had trouble staying awake while driving, eating meals, or engaging in social activity; During the past month, how much of a problem has it been for you to keep up enough enthusiasm to get things done?).
Stress manipulation
Trier Social Stress Test. Stress was induced by the TSST, a validated and widely used psychosocial laboratory stress protocol consisting of a 5-min preparation period, a 5-min speech in front of a two-member panel, and a 5-min mental arithmetic task (Kirschbaum et al., Citation1993).
Physiological assessment
Cortisol. Saliva samples were stored at −20 °C until study completion, centrifuged for 15 min at 1800 × g, and tested for cortisol levels using a commercially available chemiluminescence assay (IBL, Toronto, Canada). Inter- and intra-assay variability was below 8%.
Analytical plan
First, chi-square and t-tests were computed to assess gender-dependent differences in any of the sleep and cortisol variables. All subsequent analyses controlled for time of day the TSST was administered as well as for BMI due to reported cortisol effects (Champaneri et al., Citation2013). A repeated-measures analysis of covariance (ANCOVA) was computed to test whether the TSST was successful at inducing a cortisol stress response in participants.
To assess how the various sleep dimensions are associated with cortisol stress responses, repeated-measures ANCOVAs examining effects of the overall PSQI scores as well as the above specified PSQI subscales on cortisol stress responses were computed. Furthermore, univariate ANCOVAs examined the effects of sleep dimensions on baseline, pre-TSST cortisol levels. Gender was included as a second between-subject factor in all analyses. The statistic software package SPSS Statistics 21 (IBM Co., Armonk, NY) was used for all analyses and p values <0.05 were considered indicative of a significant effect. Where indicated, Greenhouse–Geisser corrected values are reported.
Results
Preliminary results
As frequencies across the answer categories in each of the subscales were heavily skewed, responses to each subscale were re-categorized either according to the literature or to combine categories with too-low frequencies. More specifically, the overall PSQI score was dichotomized with a score of 5 or lower indicating good sleep (n = 38, 21 women), and scores above 5 indicating poor sleep (n = 35, 23 women). Furthermore, PSQI item 4 asking “During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spent in bed.),” was categorized in line with the current literature (Prather et al., Citation2013; Wright et al., Citation2007) into “below 6 h” (n = 12), “from six until but not including 7 h” (n = 17), and “7 h and above” (n = 44). For all other items or subscales assessed in the current study, see for more details on response frequencies and descriptive statistics. Importantly, frequency distributions for all sleep variables were gender-independent (all ps ≥ 0.46) and age-independent (all ps ≥ 0.22).
Table 1. Categorization of PSQI item scores by gender (mean and SD given for raw data).
As a manipulation check, we next examined cortisol stress responses via repeated-measures ANCOVA (covariates: BMI, TSST start time). Independent of gender (main effect: F(1, 69) = 0.51, p = 0.48, = 0.007; gender × cortisol: F(5, 345) = 2.15, p = 0.11,
= 0.03), cortisol levels changed significantly over time, F(5, 345) = 3.43, p = 0.03,
= 0.05, with peak levels occurring on average 10-min post-TSST followed by a return to baseline by 45-min post-TSST, confirming that the TSST was successful in inducing a cortisol stress response.
Sleep effects on cortisol stress responses
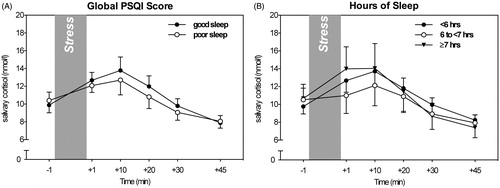
Testing for associations between the dichotomized global PSQI score and cortisol stress responses revealed that good sleepers and poor sleepers did not differ in their cortisol responses to the TSST, F(5, 335) = 0.55, p = .60, = 0.008, nor in their overall cortisol levels, F(1, 67) = 0.12, p = 0.73,
= 0.002. This was true for both genders (all ps ≥ 0.42). Similarly, when assessing average hours of sleep, no differences in overall cortisol levels, F(2, 65) = 0.12, p = .89,
= 0.004, or in cortisol stress responses, F(10, 325) = 0.71, p = 0.60,
= 0.02, were observed among the three groups for either gender (all ps ≥ 0.45; see ).
Figure 1. Neither overall sleep assessed by global PSQI scores (A) nor hours of sleep (B) showed an effect on cortisol stress responses (mean, SE).
Contrarily, examining effects of self-reported sleep quality on cortisol stress responses, a gender-dependent sleep quality main effect, F(2, 65) = 4.07, p = 0.022, = 0.11, as well as a three-way interaction was found, F(10, 325) = 2.71, p = 0.025,
= 0.08. In more detail, relative to stress responses linked to fairly bad/bad sleep quality, men reporting high sleep quality showed the strongest cortisol stress responses, while those reporting fairly good sleep quality showed relatively weak responses. Contrarily, women’s stress responses appeared less dependent on their self-reported sleep quality (see , ).
Figure 2. The impact of sleep quality (SQ) on cortisol stress responses in females (A) and males (B).
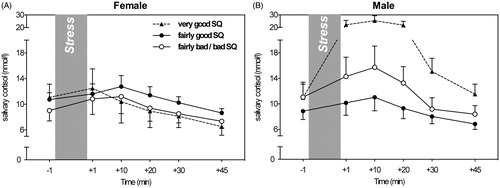
Table 2. Repeated-measures ANCOVAs testing gender-dependent sleep effects on cortisol stress responses (N = 73).
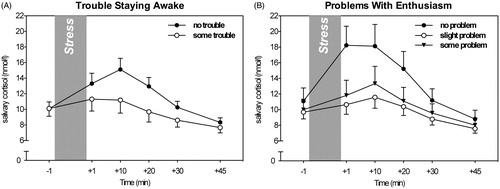
When assessing the role of daytime dysfunctions, a two-way interaction between trouble staying awake and cortisol levels over time emerged. That is, independent of gender, those who had trouble staying awake during the day showed a trend for blunted cortisol stress responses, F(5, 335) = 2.67, p = 0.065, = 0.04 (see , ). Similarly, trouble keeping up enthusiasm was linked to weaker cortisol stress responses, F(10, 325) = 2.31, p = 0.052,
= 0.07 ().
Sleep effects on pre-stress cortisol levels
To distinguish the effects of sleep habits on acute stress responses from more chronic influences on basal cortisol levels, we additionally examined associations between sleep variables and pre-TSST cortisol samples via univariate ANCOVA. Neither dichotomized PSQI scores, nor average hours of sleep, sleep quality, or daytime dysfunctions were linked to baseline cortisol levels (all ps ≥ 0.71; gender interactions: all ps ≥ 0.27).
Discussion
The current study revealed differential effects of sleep dimensions on cortisol responses to acute psychosocial stress. While self-reported average sleep duration did not appear to affect cortisol stress responses, perceived sleep quality impacted cortisol stress responses in a gender-dependent manner. Furthermore, participants who reported having trouble staying awake or keeping up enthusiasm showed blunted cortisol responses compared to participants who did not experience such daytime dysfunctions. Of note, overall sleep behavior assessed by poor and good sleep via PSQI was not associated with cortisol stress responses, supporting the current approach of differentiating between sleep dimensions. In the following, each dimension and its relation to HPA axis reactivity will be discussed.
The self-reported average of hours slept per night over the last month was neither related to cortisol stress responses, nor was it linked to baseline cortisol values. As such, our findings extend previous research on children undergoing the TSST-C that failed to find a link between sleep duration assessed via 7 d of actigraphy and stress responses, to a young adult population and self-reported average sleep duration over the last month (Raikkonen et al., Citation2010). It should be pointed out that research focusing on diurnal cortisol instead of cortisol reactivity in adults suggests a threshold for sleep deprivation effects on HPA axis output (e.g. Leproult et al., Citation1997; Wust et al., Citation2000). Hence, as participants in the current study obtained an average of 6.9 h of sleep per night, their HPA axis ability to respond to challenge may not or not yet be affected by sleep loss (Chrousos & Gold, Citation1992; Lund et al., Citation2010; McEwen, Citation1998).
Contrary to self-reported sleep duration, we observed associations between self-reported sleep quality and cortisol stress responses. This finding expands previous findings on physiological stress effects to a psychosocial stressor (Goodin et al., Citation2012), suggesting that sleep quality affects the body’s HPA axis independently of type of stressor. Importantly, in the current study, this effect differed for men and women, such that relative to fairly good sleep, men who reported poor-quality sleep showed exaggerated cortisol stress responses. Contrarily, women reporting poor-quality sleep showed slightly reduced responses. As such, the current findings in men are in line with those reported in the study on children mentioned above by Raikkonen et al. (Citation2010), as well as with previous findings demonstrating that women who were poor sleepers as measured by nighttime awakenings via actigraphy showed blunted responses to an acute cognitive stressor, i.e. the Stroop test (Wright et al., Citation2007). It should be noted that women’s cortisol responses generally appeared less dependent on sleep quality, as well as less strong overall, compared to men’s responses. This suggests that formerly reported effects of sex hormones on stress responsivity may outweigh potential effects of perceived sleep quality (Kirschbaum et al., Citation1992). Lastly, although not a frequent questionnaire response, men who reported very good sleep quality showed exaggerated cortisol stress responses. One potential pathway contributing to this pattern of sleep effects on stress responsivity may be differences in emotion regulation strategies employed by men and women. Between the two predominant types of emotion regulation strategies, reappraisal and suppression, suppression is employed more frequently by men than by women (Gross & John, Citation2003). Interestingly, using suppression as an emotion regulation strategy has been linked to exaggerated cortisol responses (Lam et al., Citation2009), while using a more accepting emotion-approaching way of emotion regulation employed before sleep has been associated with better sleep quality than a more top-down analytical evaluative approach (Vandekerckhove et al., Citation2012). Therefore, it is possible that differences in emotion regulation strategy use may influence sleep quality or report thereof and thus contribute to the gender-dependent relationship between sleep quality and cortisol.
Lastly, we assessed the effects of daytime dysfunctions experienced over the last month on cortisol stress responses. We found that those who reported trouble staying awake during the day or problems keeping up enough enthusiasm to get things done showed a trend toward lower cortisol responses to the TSST. Interestingly, daytime sleepiness has recently been reported to be associated with poor health outcomes in diabetes self-management (Chasens et al., Citation2013). As such, our findings suggest that daytime dysfunctions may be another way in which poor sleep contributes to an organism’s impaired ability to cope with challenges and eventually, to negative health outcomes.
Contrary to sleep quality, we found no gender-dependent differences in the link between daytime dysfunctions and acute stress measures. This may be due to differences in factors motivating men’s and women’s self-report behavior. When examining self-report measures of sleep quality, men state that they do not sleep as poorly as women (Vitiello et al., Citation2004) though the majority of studies indicate that men have lower quality sleep than women (Ancoli-Israel et al., Citation2003). Thus, while men may not accurately self-report their sleep quality, it may be socially more acceptable for men to self-report daytime dysfunctions.
From a broader perspective, the current study observed differential stress effects for sleep quality and sleep quantity. When exposed to chronic sub-optimal sleep duration, the HPA axis appears to maintain its ability to perform appropriately in an acute psychosocial stress situation. On the other hand, perceptions of chronic poor sleep quality had immediate effects. It could be speculated that the body may retain compensatory capacities to deal with challenges during times of being taxed with the physiological effects of non-severe sleep deprivation. Self-reported poor sleep quality, however, may represent a more integrated measure capturing various aspects and consequences of poor sleep habits, and thus a circumstance in which an organism is no longer able to adequately respond to challenges.
Overall, the current study suggests psychosocial stress and its endocrine effects as one mechanism linking poor sleep quality and daytime dysfunctions with detrimental physical health outcomes. More specifically, both blunted and exaggerated cortisol reactivity have been shown to be insalubrious (Andrews & Walker, Citation1999; Buske-Kirschbaum et al., Citation2003) and as such may be one physiological link underlying the well-documented detrimental health effects of poor sleep (Spiegel et al., Citation1999; Taylor et al., Citation2003). For example, blunted cortisol responses, such as seen in participants reporting high levels of daytime dysfunctions, may represent an increased risk of diseases associated with a lack of immune inhibition, including asthma (Buske-Kirschbaum et al., Citation2003; Fagan et al., Citation2001). Contrarily, men’s exaggerated cortisol responses observed in the context of self-reported very good sleep quality may put them at risk of problems related to chronically elevated cortisol, such as insulin resistance and hypertension (Andrews & Walker, Citation1999).
The findings of the current study have to be interpreted in light of several limitations. First, the study was conducted on a sample dominated by university students, thus raising generalizability concerns. However, college students have been shown to exhibit erratic sleep schedules (Lund et al., Citation2010), making this population an interesting target group for sleep-related research. Second, without measures corroborating the effectiveness of the employed stress protocol, it is difficult to differentiate between blunted cortisol stress responses as an indicator of HPA dysfunction versus blunted cortisol stress responses being the result of lack of a stress experience in the first place. Hence, future studies should include self-report measures of stress and cardiovascular stress response measures. Lastly, we observed small to medium effect sizes for links between sleep quality dimension measures (self-reported sleep quality, daytime dysfunctions) and cortisol measures (Cohen, Citation1988), cautioning against overinterpretation of the findings. However, given the complexity of factors determining an individual’s response to psychosocial stress exposure, as well as considering the differential pattern across sleep dimensions, these effect sizes support sleep quality as one important determinant of cortisol stress responsivity.
Conclusions
Results of the current study indicate that perceptions of sleep quality and daytime dysfunction have consequences for the body’s ability to respond to challenges. As such, the current study implicates stress and the associated physiological changes in HPA axis reactivity as one gender-dependent pathway linking poor sleep with negative health outcomes. However, the lack of sleep duration effects suggests that the body may retain some degree of resilience. Future studies will have to investigate at what point the costs of counteracting sleep deprivation contribute to sleep quality-related HPA axis dysfunctions and thus negative health outcomes.
Declaration of interest
The authors report no conflicts of interest. This work was supported by the NIGMS “Brain-Body-Behavior Interface in Learning and Development across the Lifespan” training grant T32GM084907 (S.B.L., D.G.).
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. (2003). The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26(3):342–92
- Andrews RC, Walker BR. (1999). Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 96(5):513–23
- Backhaus J, Junghanns K, Hohagen F. (2004). Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology 29(9):1184–91
- Basner M, Fomberstein KM, Razavi FM, Banks S, William JH, Rosa RR, Dinges DF. (2007). American time use survey: sleep time and its relationship to waking activities. Sleep 30(9):1085–95
- Buckley TM, Schatzberg AF. (2005). On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 90(5):3106–14
- Burgard SA, Ailshire JA, Hughes MN. (2010). Gender and sleep duration among American adults. Population Studies Center: Research Report No. 09-693. Ann Arbor: University of Michigan
- Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. (2003). Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosom Med 65(5):806–10
- Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Diez Roux A, et al. (2013). Diurnal salivary cortisol is associated with body mass index and waist circumference: the multiethnic study of atherosclerosis. Obesity 21(1):E56–63
- Chasens ER, Korytkowski M, Sereika SM, Burke LE. (2013). Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. Diabetes Educ 39(1):74–82
- Chrousos G, Gold PW. (1992). The concepts of stress and stress system disorders. JAMA 267(9):1244–52
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates
- Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. (2006). Validation of a 3-factor scoring model for the pittsburgh sleep quality index in older adults. Sleep 29(1):112–16
- Fagan JK, Scheff PA, Hryhorczuk D, Ramakrishnan V, Ross M, Persky V. (2001). Prevalence of asthma and other allergic diseases in an adolescent population: association with gender and race. Ann Allergy Asthma Immunol 86(2):177–84
- Fries E, Dettenborn L, Kirschbaum C. (2009). The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 72(1):67–73
- Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. (2012). Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol 91(1):36–41
- Gross JJ, John OP. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 85(2):348–62
- Hays RD, Stewart AL. (1992). Sleep measures. In: Stewart AL, Ware JE, editors. Measuring functioning and well-being: the medical outcomes study approach. Durham, NC: Duke University Press. p 235–59
- Kirschbaum C, Hellhammmer DH. (2007). Salivary cortisol. In: Fink G, editor. Encyclopedia of stress. Vol. 3. 2nd ed. Oxford: Academic Press. p 405–9
- Kirschbaum C, Pirke KM, Hellhammer DH. (1993). The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28(1–2):76–81
- Kirschbaum C, Wüst S, Hellhammer D. (1992). Consistent sex differences in cortisol responses to psychological stress. Psychosom Med 54(6):648–57
- Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. (2009). Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab 94(12):4801–9
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. (2009). Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology 34(9):1355–62
- Leproult R, Copinschi G, Buxton O, Van Cauter E. (1997). Sleep loss results in an elevation of cortisol levels the next evening. Sleep 20(10):865–70
- Lund HG, Reider BD, Whiting AB, Prichard JR. (2010). Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health 46(2):124–32
- McEwen BS. (1998). Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci 840(1):33–44
- Minkel J, Moreta M, Muto J, Htaik O, Jones C, Basner M, Dinges D. (2014). Sleep deprivation potentiates hpa axis stress reactivity in healthy adults. Health Psychol 33(11):1430–4
- Pesonen AK, Kajantie E, Heinonen K, Pyhala R, Lahti J, Jones A, Matthews KA, et al. (2012). Sex-specific associations between sleep problems and hypothalamic-pituitary-adrenocortical axis activity in children. Psychoneuroendocrinology 37(2):238–48
- Prather AA, Epel ES, Cohen BE, Neylan TC, Whooley MA. (2013). Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: findings from the Heart and Soul Study. J Psychiatr Res 47(9):1228–35
- Raikkonen K, Matthews KA, Pesonen AK, Pyhala R, Paavonen EJ, Feldt K, Jones A, et al. (2010). Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab 95(5):2254–61
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. (2001). Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med 63(6):966–72
- Spiegel K, Leproult R, Van Cauter E. (1999). Impact of sleep debt on metabolic and endocrine function. Lancet 354(9188):1435–9
- Steiger A. (2002). Sleep and the hypothalamo-pituitary-adrenocortical system. Sleep Med Rev 6(2):125–38
- Taylor DJ, Lichstein KL, Durrence HH. (2003). Insomnia as a health risk factor. Behav Sleep Med 1(4):227–47
- Vandekerckhove M, Kestemont J, Weiss R, Schotte C, Exadaktylos V, Haex B, Verbraecken J, Gross JJ. (2012). Experiential versus analytical emotion regulation and sleep: breaking the link between negative events and sleep disturbance. Emotion 12(6):1415–21
- Veasey S, Raymond R, Barzansky B, Rosen I, Owens J. (2002). Sleep loss and fatigue in residency training, a reapprasial. JAMA 288(9):1117–24
- Vitiello MV, Larsen LH, Moe KE. (2004). Age-related sleep change: gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. J Psychosom Res 56(5):503–10
- Wright CE, Valdimarsdottir HB, Erblich J, Bovbjerg DH. (2007). Poor sleep the night before an experimental stress task is associated with reduced cortisol reactivity in healthy women. Biol Psychol 74(3):319–27
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. (2000). The cortisol awakening response – normal values and confounds. Noise Health 2(7):79–88